Abstract
Background: Current treatment guidelines for type 2 diabetes (T2D) recommend individualized intensification of therapy for glycated hemoglobin (A1C) ≥ 7% in most patients. The purpose of this investigation was to explore the ability of an electronic medical record (EMR) to identify glycemic intensification strategies among T2D patients receiving pharmacologic therapy. Methods: Patient records between 2005 and 2011 with documentation of A1C and active prescriptions for any diabetes medications were queried to identify potential candidates for intensification based on A1C ≥ 7% while on 1-2 oral diabetes medications (ODM). Patients with follow-up A1C values within 1 year of index A1C were grouped according to intensification with insulin, GLP-1 receptor agonists (GLP-1RA), a new class of ODM, or no intensification. Changes in A1C and continuation of intensification therapy were determined. Results: A total of 4921 patients meeting inclusion criteria were intensified with insulin (n = 416), GLP-1RA (n = 68), ODM (n = 1408), or no additional therapy (n = 3029). Patients receiving insulin had higher baseline (9.3 ± 2.0 vs 8.3 ± 1.2 vs 8.3 ± 1.3 vs 7.6 ± 1.0%, P < .0001) and follow-up A1C (8.1 ± 1.6 vs 7.5 ± 1.2 vs 7.6 ± 1.3 vs 7.2 ± 1.1%, P < .0001) despite experiencing larger absolute A1C reductions (−1.2 ± 2.1 vs −0.8 ± 1.4 vs −0.7 ± 1.4 vs −0.3 ± 1.1%, P < .0001). Patients receiving GLP-1RA were more obese at baseline (BMI: 33.6 ± 7.1 vs 37.7 ± 6.1 vs 33.7 ± 6.8 vs 32.9 ± 7.1 kg/m2, P < .0001) and follow-up (BMI: 33.9 ± 7.3 vs 36.6 ± 6.1 vs 33.8 ± 7.0 vs 32.4 ± 7.0 kg/m2, P < .0001) despite experiencing more absolute weight reduction. Insulin was the most and GLP-1RA the least likely therapy to be continued. Conclusions: An EMR allows identification of prescribing practices and compliance with T2D treatment guidelines. Patients receiving intensification of glycemic medications had baseline A1C >8% suggesting that treatment recommendations are not being followed.
Keywords: electronic health records, hospital information systems, hypoglycemic agents, type 2 diabetes mellitus
Metformin is recommended as the preferred initial pharmacologic agent for treatment of type 2 diabetes (T2D), provided there are no contraindications to its use.1-4 When glycemic goals are not achieved with metformin alone, an individualized approach to intensification of therapy is recommended. Glycemic control is defined as achieving glycated hemoglobin (A1C) levels of ≤ 6.5 to 7% in the majority of individuals with T2D, provided that this can be safely achieved with minimal risk for hypoglycemia and weight gain.1-3 For older adult patients with coexisting comorbidities, these A1C targets can be modified to goals of <8 to 8.5% depending on the severity of the associated chronic illnesses or anticipated life expectancy.5,6
The degree to which these recommendations for therapeutic intensification are implemented in outpatient clinical settings is not known.7 There are several classes of glucose lowering agents available for therapeutic intensification in T2D. These include insulin secretogogues, thiazolidinediones (TZD), dipeptidyl peptidase-4 (DPP-4) inhibitors, alpha glucosidase inhibitors, noninsulin injectable therapies, and insulin. There are no studies demonstrating the superiority of 1 agent over another for therapeutic intensification.1,8-10 The National Institutes of Health–sponsored study Glycemia Reduction Approaches in Diabetes (GRADE) Study is currently recruiting patients with T2D for a study that will compare glycemic outcomes with a sulfonylurea, DPP-4 inhibitor, a glucagon-like peptide 1 receptor agonist (GLP-1RA), or long-acting insulin added to metformin to determine if there are any advantages to 1 particular approach. Results from this study will not be available for several years.11
Until these results are available, the emergence of the electronic medical record (EMR) for documentation of patient care provides the opportunity to identify patterns of pharmacologic intensification among patient populations with T2D. The EMR can also provide information on glycemic control over time.12,13 We report the results of a query into a large university-based EMR system that sought to identify patients with T2D with evidence of current therapy with 1 or 2 oral diabetes medications (ODM) who were candidates for intensification based on A1C values above 7%. The purpose of this investigation was to determine the ability of an EMR to identify patterns of therapeutic intensification of diabetes medications and associated changes in glycemic control.
Methods
This study was approved by the Institutional Review Board at the University of Pittsburgh. For this study we identified patients with T2D from the University of Pittsburgh Medical Center (UPMC) general and specialty outpatient clinics between June 2005 and November 2011. UPMC is an integrated health system that operates more than 20 academic, community, and specialty hospitals and 400 outpatient sites and employs more than 3500 physicians. The UPMC EMR data repository includes administrative and clinical data forwarded from the health system’s clinical, administrative, and financial databases.14 The EMR includes patient demographics, office visits, medication lists, laboratory results, and charges from both inpatient and outpatient settings throughout the health system (EpicCare, Epic Systems Corp, Verona, WI; PowerChart, Cerner Corporation, Kansas City, MO). An interface (dbMotion, Inc, Pittsburgh, PA) connects and shares key information between systems.
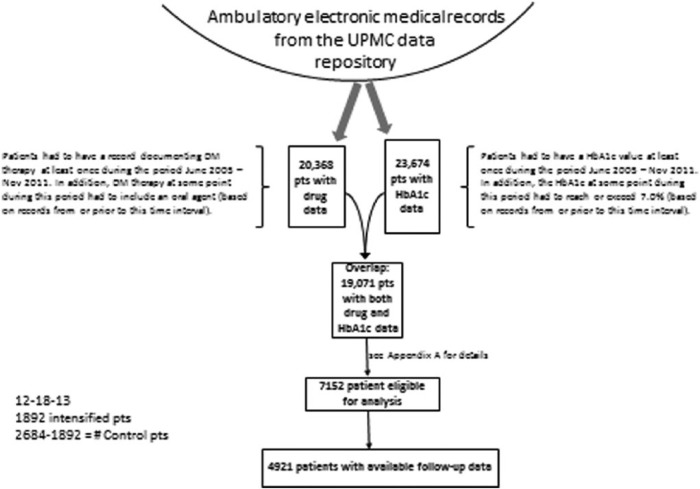
Ambulatory EMRs from the UPMC data repository were searched to identify any patient visit that listed an ODM as an active medication. Simultaneously, all patients with an A1C during this time period were also identified (Figure 1). Patients identified as having A1C values ≥ 7.0% while on therapy with 1 or 2 ODM were grouped according to evidence of therapeutic intensification with any insulin therapy, a GLP-1RA (exenatide, liraglutide), an ODM in a class different from what was already being taken, or no intensification. Chart review was performed on all patients identified as receiving GLP-1RA to determine accuracy of the database query to verify the prescribing of this group of agents.
Figure 1.
Scheme for identification of subjects meeting inclusion criteria for the study. Ambulatory EMRs from the UPMC data repository were searched to identify any patient visit that listed an ODM as an active medication between June 2005 and November 2011. Simultaneously, all patients with an A1C during this time period were also identified.
The first record following documentation of an A1C ≥ 7% with current prescriptions for ODM was identified as the index date, and the A1C at that time was identified as the baseline A1C. Patients identified as taking insulin, a GLP-1 RA, or a third ODM within a 6-month period prior to the index A1C were excluded (n = 36). Patients intensified with ≥ 2 agents at the time of the index A1C were also excluded (Appendix A).
Glycemic outcomes following intensification were defined as percentage reductions in A1C, the percentage of patients achieving A1C < 7%, and the percentage of patients who achieved ≥0.5% reductions in A1C at a median time point of 8 months following therapeutic intensification.
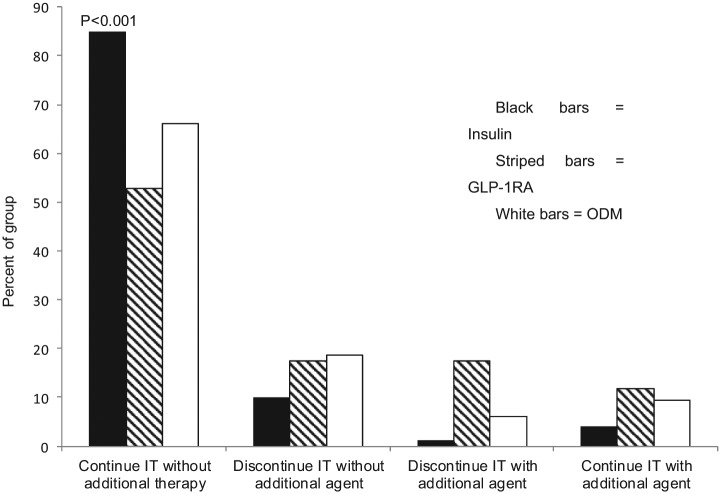
We also sought to determine the percentage of patients receiving intensification who remained on the assigned therapy without the addition of an agent from another group at 1 year. Patients were further grouped according to evidence of remaining on the intensification drug with the addition of ≥1 additional agent(s) or discontinuation of the intensification drug with or without the start of another agent (Figure 2).
Figure 2.
Percentage of patients continuing intensified therapy (IT) at 1 year with insulin (black bars), GLP-1RA (hatched bars), or a third ODM. Data are presented as the percentage of patients remaining on the assigned IT without the addition of an agent from another group or with the addition of ≥ 1 additional agent(s); or discontinuation of the IT with or without the start of another agent.
Additional measures included changes in BMI (kg/m2), systolic (SBP) and diastolic (DBP) blood pressure, lipid parameters (HDL, non-HDL cholesterol, log transformed triglycerides), and renal function (serum creatinine). Changes in lipid parameters were defined as differences in mean concentrations obtained at baseline and follow-up, and changes in the percentage of patients with non-HDL cholesterol < 130 mg/dl and triglycerides < 150 mg/dl. Changes in renal function were defined as the average annual increase in serum creatinine, the percentage of patients experiencing ≥50% increase in serum creatinine, and the percentage of patients with an initial serum creatinine ≤ 2.0 mg/dl who progressed to a value >2.0 mg/dl.
Statistics
Comparisons were conducted among the 3 groups receiving therapeutic intensification and between the intensified and nonintensified subjects. Categorical variables were compared with chi-square tests and continuous variables were compared with either ANOVA or t tests, depending, respectively, on whether more than 2 or exactly 2 groups were being compared. Adjusted changes in continuous variables, such as A1C and BMI, were analyzed with linear regression models adjusting for baseline differences in age, sex, race (black vs nonblack), baseline A1C, BMI, systolic and diastolic blood pressure, triglycerides (log scale), HDL, non-HDL, and creatinine. The number of subjects with baseline and follow-up data varied according to each measured variable (Appendix B). To adjust for variables with missing values, the missing values were replaced by an arbitrary numeric value (0), and a separate indicator variable (0/1) was included in the model, where the numeric value of 1 represented the records with missing values. Through this technique, all records were kept in the regression models.
Results
There were 7152 patients identified who met inclusion criteria (Figure 1). Of these, 4921 patients had both baseline and follow-up A1C measurements (Table 1). The majority of patients did not receive therapeutic intensification. These patients were older and more metabolically healthy with lower BMI, A1C, and triglyceride values and higher HDL cholesterol than the intensified groups (Tables 1 and 2).
Table 1.
Baseline Demographic Characteristics of Study Groups.
| Variable | Insulin (n = 416) | GLP-1RA (n = 68) | ODM (n = 1408) | Control (n = 3029) | Added therapy groups (P value)a | Added vs no added therapy (P value)b |
|---|---|---|---|---|---|---|
| Female, % | 53.7 | 58.2 | 47.1 | 50.2 | .024 | .39 |
| Race, % | <.0001 | .47 | ||||
| White | 72.5 | 91.0 | 80.2 | 77.6 | ||
| African American | 24.3 | 3.0 | 14.9 | 17.0 | ||
| Other | 2.0 | 4.5 | 1.9 | 2.2 | ||
| Unknown | 1.2 | 1.5 | 2.9 | 3.2 | ||
| Age, yearsc | 61.6, 14.2 | 56.2, 12.2 | 62.7, 12.4 | 66.3, 13.0 | .0001 | <.0001 |
| Lipid-lowering drugsd | ||||||
| Statins | 66.4 | 56.1 | 72.0 | 65.2 | <.0001* | |
| Other | 26.9 | 24.2 | 27.9 | 22.1 | .0003* | |
| Antihypertensives %d | ||||||
| Ace inhibitors | 52.8 | 56.1 | 52.7 | 47.2 | .003* | |
| ARBs | 17.8 | 21.2 | 22.0 | 21.8 | .31* | |
| Other | 72.8 | 62.1 | 67.8 | 66.2 | .045* | |
Chi-square for categorical variables, ANOVA for continuous variables.
Chi-square for categorical variables, t test for continuous variables.
Data are mean ± SD.
Most recent visit preintensification (but not older than 1 year) or at time of intensification.
4-group comparison.
Table 2.
Changes in Clinical Measures and A1C According to Intensification Strategy.
| Variable | Insulin (n = 416) | GLP-1RA (n = 68) | ODM (n = 1408) | No added therapy (n = 3029) | Added therapy groups (P value)a | Added vs no added therapy (P value)b |
|---|---|---|---|---|---|---|
| BMI, kg/m2c | ||||||
| n (% of total) | 338 (81.3) | 58 (85.3) | 1168 (83) | 1878 (62) | ||
| Baseline | 33.6, 7.1 | 37.7, 6.1 | 33.7, 6.8 | 32.9, 7.1 | <.0001 | <.0001 |
| Follow-up | 33.9, 7.3 | 36.6, 6.1 | 33.8, 7.0 | 32.4, 7.0 | .02 | <.0001 |
| ΔBMI | 0.29, 2.26 | −1.19, 1.88 | 0.06, 2.05 | −0.48, 2.19 | <.0001 | <.0001 |
| A1C %c | ||||||
| n (% of total) | 416 (100) | 68 (100) | 1408 (100) | 3029(100) | ||
| Baseline | 9.30, 2.01 | 8.26, 1.15 | 8.27, 1.29 | 7.56, 0.97 | <.0001 | <.0001 |
| Follow-up | 8.13, 1.62 | 7.46, 1.25 | 7.57, 1.34 | 7.22, 1.11 | <.0001 | <.0001 |
| ΔA1C | −1.18, 2.14 | −0.80, 1.41 | −0.70, 1.39 | −0.34, 1.11 | <.0001 | <.0001 |
| A1C ≥ 0.5%( %) | 59.6 | 52.9 | 54.8 | 36.6 | .20 | <.0001 |
| Follow-up A1C < 7.0% (%) | 22.6 | 39.7 | 36.2 | 49.2 | <.0001 | <.0001 |
| SBP, mmHgc | ||||||
| n (% of total) | 367 (88.2) | 63 (92.6) | 1311 (93.1) | 2199 (73) | ||
| Baseline | 130, 18 | 131, 12.5 | 131.8, 15.7 | 132.7, 17 | .24 | .03 |
| Follow-up | 130.2, 17 | 128.3, 13 | 131.8, 16 | 131, 16.7 | .16 | .62 |
| ΔSystolic BP | −0.06, 19.5 | −2.37, 13.1 | −0.38, 17.6 | −1.83, 18.8 | .63 | .01 |
| DBP, mmHgc,d | ||||||
| Baseline | 75.9, 11.7 | 75.9, 9.0 | 77.6, 10.0 | 77.2, 10.0 | .02 | .84 |
| Follow-up | 76.8, 10.2 | 76.2, 7.9 | 77.0, 9.9 | 75.8, 10.3 | .81 | .0009 |
| ΔDBP, mmHg | 0.82, 11.5 | 0.32, 8.7 | −0.58, 10.7 | −1.39, 11.0 | .08 | .001 |
| TG, mg/dLc | ||||||
| n (% of total) | 286 (68.6) | 51 (75.0) | 1125 (79.9) | 1985 (66) | ||
| Baseline (log) | 5.11, 0.58 | 5.42, 0.69 | 5.12, 0.54 | 5.03, 0.53 | .0006 | <.0001 |
| Follow-up (log) | 5.04, 0.57 | 5.29, 0.68 | 5.03, 0.52 | 4.98, 0.53 | .003 | .002 |
| Δ(log) TG | −0.07, 0.51 | −0.13, 0.49 | −0.09, 0.41 | −0.05, 0.39 | .65 | .005 |
| <150 mg/dL(%) | ||||||
| Baseline | 45.2 | 23.5 | 42.8 | 50.2 | .02 | <.0001 |
| Follow-up | 48.6 | 25.5 | 49.0 | 53.4 | .005 | .002 |
| HDL-C, mg/dLc | ||||||
| n (% of total) | 293 (70.4) | 52 (76.5) | 1143 (81.2) | 2021 (67) | ||
| Baseline | 42.0, 14.5 | 41.2, 11.4 | 44.4, 12.6 | 45.4, 13.3 | .006 | .0003 |
| Follow-up | 42.3, 12.0 | 41.3, 10.2 | 43.9, 12.9 | 44.9, 13.0 | .08 | .001 |
| ΔHDL-C | 0.34, 10.3 | 0.12, 6.6 | −0.52, 7.7 | −0.56, 7.9 | .26 | .40 |
| Non-HDL-C, mg/dLc | ||||||
| n (% of total) | 285 (68.5) | 52 (76.5) | 1117 (79.3) | 1954 (64) | ||
| Baseline | 130, 45.5 | 135, 45.6 | 129.1, 40.5 | 127.4, 39 | .55 | .13 |
| Follow-up | 127.3, 45 | 135, 57.5 | 123.5, 37.2 | 123.6, 39 | .06 | .43 |
| ΔNon-HDL-C | −2.9, 45.7 | −0.4, 39.7 | −5.6, 34.4 | −3.8, 32.9 | .38 | .39 |
| Non-HDL-C < 130 mg/dL, % | ||||||
| Baseline | 55.4 | 53.8 | 57.0 | 59.7 | .82 | .07 |
| Follow-up | 60.4 | 55.8 | 62.8 | 62.8 | .48 | .63 |
| Creat, mg/dLc | ||||||
| n (% of total) | 371 (89.2) | 59 (86.8) | 1293 (91.8) | 2399 (79) | ||
| Baseline | 1.12, 0.90 | 0.99, 0.34 | 0.99, 0.31 | 1.05, 0.42 | <.0001 | .07 |
| Follow-up | 1.18, 1.01 | 1.01, 0.41 | 1.02, 0.38 | 1.06, 0.53 | <.0001 | .52 |
| ΔCreatinine | 0.06, 0.41 | 0.02, 0.16 | 0.025, 0.24 | 0.018, 0.3 | .096 | .13 |
Chi-square for categorical variables, ANOVA for continuous variables.
Chi-square for categorical variables, t test for continuous variables.
Data are mean ± SD unless otherwise specified.
Amount of available data is identical to systolic blood pressure.
Among the 1892 patients receiving therapeutic intensification, the addition of an ODM in a different class was the most frequently used and addition of a GLP-1RA the least frequently used strategy. The insulin group had a higher percentage of African American patients with higher baseline A1C and creatinine than those intensified with GLP-1RA or ODM (Tables 1 and 2). Those intensified with an additional ODM were older and more likely to be male, with higher HDL than the insulin or GLP-1RA groups (Tables 1 and 2). The GLP-1RA group was younger, more obese, more likely to be female and white, and more likely to be receiving ACE inhibitors. Those receiving GLP-1RA had a lower percentage of patients on lipid-lowering therapy, which may have accounted for their higher TG and lower HDL than the other groups (Tables 1 and 2).
Patients intensified with insulin were the most likely to remain on this therapy at 1 year following escalation of glycemic therapy (Figure 2). Relative to insulin therapy, the unadjusted odds ratio for continuing GLP-1RA was 0.20 (confidence interval [CI], 0.12-0.35) and for ODM was 0.35 (CI, 0.26-0.46) (P < .0001). The significance of these odds ratios persisted when adjusting for baseline A1C (P < .0001) alone or in combination with age, gender, race, or BMI (P < .0001).
Reductions in A1C were observed in all groups receiving therapeutic intensification, with the greatest absolute reduction occurring in those receiving insulin (Table 2). This group had the highest percentage of patients who experienced A1C reductions of ≥ 0.5% (P = ns), but persisted as having the highest mean A1C and the lowest percentage of patients with A1C values < 7.0%. Patients intensified with GLP-1RA experienced more weight loss than other groups (Table 2). No group differences were observed for changes in SBP, DBP, HDL, or non-HDL. Those receiving GLP-1RA maintained higher TG with the smallest percentage of patients having TG values < 150 mg/dl. Those receiving insulin had higher serum creatinine at baseline and follow-up, without any group differences in the mean change for this measure. A larger percentage of patients intensified with insulin experienced ≥50% increases in serum creatinine (insulin vs GLP-1RA vs ODM vs control: 5.0 vs 0.0 vs 1.8 vs 1.9%, respectively) and in the percentage of patients with follow-up creatinine values > 2.0 mg/dl in those with baseline values ≤ 2 mg/dl (1.9 vs 0.0 vs 0.9 vs 0.8%).
When compared to the intensified groups, patients who received no additional glucose lowering agents experienced lower absolute reductions in A1C with a lower percentage of patients experiencing A1C reductions of ≥ 0.5% (Table 2). However, this group had the lowest mean A1C values at follow-up with more patients achieving values < 7%. The nonintensified group also maintained a lower BMI, with lower TG and higher HDL (Table 2).
When controlling for baseline differences in age, sex, race, A1C, BMI, SBP and DBP, lipids, or creatinine between the groups receiving and not receiving therapeutic intensification, the finding of more weight loss in patients receiving GLP-1RA remained significant (Table 3).
Table 3.
Adjusted Changes in Clinical Measures in Intensified Compared to Nonintensified Groups.
| Insulin | GLP-1 | +1 ODM | P | |
|---|---|---|---|---|
| ΔA1C | 0.17 (0.04, 0.29) | −0.09 (−0.36, 0.18) | 0.05 (−0.03, 0.12) | .09 |
| ΔBMI | 0.78 (0.52, 1.05) | −0.57 (−1.11, −0.02) | 0.50 (0.34, 0.66) | <.0001 |
| ΔSystolic BP | 0.41 (−1.46, 2.28) | −0.56 (−4.41, 3.29) | 1.28 (0.19, 2.37) | .45 |
| ΔDiastolic BP | 0.94 (−0.14, 2.07) | −0.48 (−2.77, 1.81) | 0.55 (−0.10, 1.20) | .48 |
| ΔTriglyceride (log) | 0.029 (−0.022, 0.080) | 0.031 (−0.07, 0.136) | −0.003 (−0.032, 0.026) | .40 |
| ΔHDL | 0.05 (−0.97, 1.06) | −0.13 (−2.22, 1.97) | −0.14 (−0.73, 0.44) | .93 |
| ΔNon-HDL | 3.30 (−0.92, 7.52) | 6.25 (−2.34, 14.8) | −0.52 (−2.94, 1.90) | .075 |
| ΔCreatinine | 0.043 (0.007, 0.079) | 0.009 (−0.070, 0.09) | 0.007 (−0.014, 0.03) | .15 |
For each outcome, the table shows the “excess change,” defined as the change in each intensification group that exceeds the change in the control group, adjusted for baseline characteristics (age, sex, race [black vs nonblack], baseline A1C, BMI, systolic and diastolic blood pressure, triglycerides [log scale], HDL, non-HDL, and creatinine). Data are shown as means with 95% confidence intervals.
Discussion
These results support the ability of the EMR to provide information regarding prescribing practices and intensification strategies among patients with T2D meeting A1C criteria for additional glucose lowering therapies. We observed that the majority of patients do not receive intensification of their diabetes therapy despite documentation of A1C ≥ 7%. The mean A1C levels among patients who received additional therapy exceeded 8%, which is well above the threshold recommended for the majority of patients.1,2 There are several potential explanations for this observation that cannot be easily captured from an EMR. These include reinforcement of nonpharmacologic interventions with lifestyle modifications, patient or physician reluctance to add additional medications, or concerns regarding hypoglycemia. A1C values > 7% but < 8% in the nonintensified group support the suggestion that physicians and other care providers may be reluctant to advance therapy in this group of patients, particularly since almost 50% of patients in the nonintensified group had A1C values < 7% at follow-up (Table 2).15
The findings in this report also suggest that current guidelines for glycemic management are not being followed in clinical practice.1,3,4,16 The mean A1C > 9% in the group intensified with insulin supports prior studies demonstrating clinical inertia among care providers for intensifying therapy when patients are already taking diabetes medications.17,18 These values exceed even what is recommended for older adults with significant comorbidities, where an upper limit for A1C values is defined as 8.5%, as levels above this are associated with increased risk for diabetes-related complications and mortality.5,19-22
This report adds to the available literature exploring the use of large EMR databases to investigate questions related to the clinical care of patients with diabetes.10,23-26 There is no protocol-based implementation strategy for glycemic management in this population, meaning that this investigation reflects individual rather than protocol-driven practice. This provides data that extend beyond those limited to 1 particular insurance plan, where therapeutic interventions and data collection strategies may be more homogeneous.10
An EMR provides the opportunity to investigate clinical questions relating to compliance with care guidelines, tracking population changes in metabolic measures, as well as determining changes in other clinical parameters according to a specific therapeutic strategy.27 An EMR can also serve as an adjunct to the randomized controlled clinical trial for clinical research and quality improvement initiatives.8,10,28-31
It is not surprising that insulin was the strategy most likely to be continued at 1 year, as insulin is often used only when all other treatment strategies are exhausted or contraindicated.29 What is surprising is the observation that approximately 40% of patients intensified with GLP-1RA had discontinued therapy at 1 year (Figure 2). This percentage approximates the percentage of subjects reporting gastrointestinal side effects with these agents in other studies.8 The absolute reasons for discontinuation of any of the medications in this report are likely embedded within clinical notes and are not necessarily amenable to data extraction methods employed. Future investigations using more sophisticated word and phrase recognition software may help in identifying reasons for discontinuation of a particular medication in the future.32,33
There are several limitations to this study. One is that the EMRs used in this study may not include all medical information on participants as individual patients are free to obtain their medical care from a variety of sources. Similar to other reports using EMR data, there is no standardization of visit frequency for this patient population.10,25,26 Another limitation is that the prescribing of a medication does not necessarily mean that it was taken by a patient.34 EMR data have been demonstrated as often being prone to error and incomplete.35 To address this, chart review was performed on patients identified as receiving GLP-1RA to verify the prescribing of this group of agents.
The number of subjects intensified with a GLP-1RA was very small in this study, creating disproportionate comparisons with the other groups. However, the infrequent use of GLP-1RA using the criteria specified for this investigation is also informative, suggesting that the clinical use of these agents may fall outside current recommended guidelines.1,2 It is possible that GLP-1RA are being prescribed to subjects who may have lower A1C values, or who have failed 3 or more ODM, populations that were not included in this study.23 The population for this study was limited to those receiving no more than 2 ODM as it was felt that the use of 3 or more ODM would prompt a higher likelihood of intensification with insulin. It is also possible that insurance coverage for these newer and more expensive GLP-1RA may have influenced prescribing patterns and patient willingness to comply with treatment, information that is not easily extracted from an EMR.
In prior studies using a commercial EMR (GE Centricity) to investigate therapeutic strategies in T2D, a larger percentage of the population received prescriptions for GLP-1RA.23,25 The inclusion criteria in these earlier studies differed from what was used in this report in that inclusion was independent of baseline A1C and included patients new to pharmacologic therapy or already taking ≥4 ODM. These differences likely accounted for the larger percentage of subjects. Similar to the current study, baseline A1C levels were higher in those assigned to insulin therapy, while BMI was higher in those assigned to GLP-1RA.23,25
Changes in traditional cardiovascular risk factors were examined as part of this investigation. With the exception of more weight loss in those intensified with GLP-1 agents, no significant group differences were observed in changes in any of these measures when controlling for baseline differences in age, sex, race, A1C, BMI, SBP and DBP, lipids, or creatinine (Table 3). While it is difficult to draw any conclusions from these observations given the very small number of patients who received GLP-1RA agents, the fact that the majority of patients in each group were receiving antihypertensive and lipid-lowering therapies may have contributed.
Conclusions
In conclusion, this report supports the ability of an EMR to identify prescribing practices and compliance with current guidelines in a population of patients already receiving pharmacologic therapy for T2D. Further investigations into the ability of an EMR to identify clinical outcomes, including diabetes-related complications, are planned.
Appendix A
Criteria for Inclusion in the Analysis
The circumstance targeted by the patient selection is that of an unsatisfactory glycemic control, defined as A1C ≥ 7.0%, while the patient is on diabetes therapy consisting of 1 or 2 oral agents, and no additional therapy. In addition, selected patients were not receiving insulin or GLP-1RA therapy at the time of the index date.
A number of requirements were followed in an effort to enhance the validity of the treatment assignments and control group designation:
The addition of the intensification therapy could not occur at a time point > 9 months following the index A1C, guaranteeing that no baseline A1C value is older than 9 months.
The baseline A1C could not be obtained less than 2 months following the receipt of a prescription for 1 or 2 ODM prior to inclusion in an intensification group. It can therefore be assumed that the measured A1C at baseline reflects the glycemic benefit of the prior oral diabetes therapy.
While patients were required to have no history of insulin or GLP-1RA therapy to be included in an intensification group, exceptions were allowed for cases in which the drug was used (or prescribed) for a time period of <6 months, and the last recorded use (or prescription) was >6 months prior to the index date.
Patients whose therapy is simultaneous intensified with ≥2 strategies (eg, insulin plus an ODM in a different class) were not included in the analysis.
Appendix B
Data Available for the Tables in the Manuscript: n and Percentage of Total (Maximum Possible)
| Insulin group |
GLP-1RA |
+1 ODM |
Control |
|||||
|---|---|---|---|---|---|---|---|---|
| Total | n = 416 | n = 68 | n = 1408 | n = 3029 | ||||
| Table 1 | ||||||||
| Female | 404 | 97.1 | 67 | 98.5 | 1400 | 99.4 | 2730 | 90.1 |
| Race | 404 | 97.1 | 67 | 98.5 | 1400 | 99.4 | 2730 | 90.1 |
| Age | 404 | 97.1 | 67 | 98.5 | 1399 | 99.4 | 2729 | 90.1 |
| BMI | 372 | 89.4 | 65 | 95.6 | 1257 | 89.3 | 2110 | 69.7 |
| A1C | 416 | 100.0 | 68 | 100.0 | 1408 | 100.0 | 3029 | 100.0 |
| BP | 385 | 92.5 | 65 | 95.6 | 1333 | 94.7 | 2279 | 75.2 |
| Triglyceride | 341 | 82.0 | 56 | 82.4 | 1266 | 89.9 | 2303 | 76.0 |
| HDL | 346 | 83.2 | 57 | 83.8 | 1277 | 90.7 | 2332 | 77.0 |
| Non-HDL | 335 | 80.5 | 57 | 83.8 | 1249 | 88.7 | 2248 | 74.2 |
| Creatinine | 395 | 95.0 | 63 | 92.6 | 1349 | 95.8 | 2523 | 83.3 |
| Table 2 | ||||||||
| BMIa | 338 | 81.3 | 58 | 85.3 | 1168 | 83.0 | 1878 | 62.0 |
| A1C | 416 | 100.0 | 68 | 100.0 | 1408 | 100.0 | 3029 | 100.0 |
| BP | 367 | 88.2 | 63 | 92.6 | 1311 | 93.1 | 2199 | 72.6 |
| Triglyceride | 286 | 68.6 | 51 | 75.0 | 1125 | 79.9 | 1985 | 65.5 |
| HDL | 293 | 70.4 | 52 | 76.5 | 1143 | 81.2 | 2021 | 66.7 |
| Non-HDL | 285 | 68.5 | 52 | 76.5 | 1117 | 79.3 | 1954 | 64.5 |
| Creatinine | 371 | 89.2 | 59 | 86.8 | 1293 | 91.8 | 2399 | 79.2 |
If more than 1 follow-up BMI exists for a given patient, the 1 closest to 9 months is used.
Footnotes
Abbreviations: ACE-I, angiotensin converting enzyme inhibitors; A1C, glycated hemoglobin; ARB, angiotensin receptor blockers; BMI, body mass index; CI, confidence interval; creat, creatinine; DBP, diastolic blood pressure; EMR, electronic medical record; GLP-1RA, glucagon-like peptide 1 receptor agonists; ODM, oral diabetes medications; SBP, systolic blood pressure; TG, triglycerides; T2D, type 2 diabetes; UPMC, University of Pittsburgh Medical Center.
Authors’ Note: Some of the data from this study have been previously presented in abstract form at the annual meetings of the American Diabetes Association and the Diabetes and Cardiovascular Disease EASD Study Group.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MTK, MB, ML, JK, TO, and LS received salary support for their role in this funded project from Sanofi-Aventis. MTK has also served as a consultant with Sanofi-Aventis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided through an investigator-initiated award from Sanofi-Aventis.
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2014;37:S14-S80. [DOI] [PubMed] [Google Scholar]
- 3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327-336. [DOI] [PubMed] [Google Scholar]
- 4. Qaseem A, Humphrey LL, Sweet DE, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218-231. [DOI] [PubMed] [Google Scholar]
- 5. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154:554-559. [DOI] [PubMed] [Google Scholar]
- 7. Davidson MB. How our current medical care system fails people with diabetes: lack of timely, appropriate clinical decisions. Diabetes Care. 2009;32:370-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559-569. [DOI] [PubMed] [Google Scholar]
- 9. Joshi PH, Kalyani RR, Blumenthal RS, Donner TW. Cardiovascular effects of noninsulin, glucose-lowering agents: need for more outcomes data. Am J Cardiol. 2012;110:32B-42B. [DOI] [PubMed] [Google Scholar]
- 10. Reed M, Huang J, Graetz I, Brand R, Hsu J, Fireman B, Jaffe M. Outpatient electronic health records and the clinical care and outcomes of patients with diabetes mellitus. Ann Intern Med. 2012;157:482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) is a long-term study of different treatments for type 2 diabetes. 2014. Available at: http://www.nih.gov/news/health/jun2013/niddk-2003.htm.
- 12. Brownstein JS, Murphy SN, Goldfine AB, et al. Rapid identification of myocardial infarction risk associated with diabetes medications using electronic medical records. Diabetes Care. 2010;33:526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cimino JJ. Improving the electronic health record—are clinicians getting what they wished for? JAMA. 2013;309:991-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zgibor JC, Orchard TJ, Saul M, et al. Developing and validating a diabetes database in a large health system. Diabetes Res Clin Pract. 2007;75:313-319. [DOI] [PubMed] [Google Scholar]
- 15. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535-1540. [DOI] [PubMed] [Google Scholar]
- 16. Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963-1972. [DOI] [PubMed] [Google Scholar]
- 17. Davis J, Chavez B, Juarez DT. Adjustments to diabetes medications in response to increases in hemoglobin a1c: an epidemiologic study. Ann Pharmacother. 2014;48:41-47. [DOI] [PubMed] [Google Scholar]
- 18. Lovshin JA, Zinman B. Diabetes: Clinical inertia—a barrier to effective management of T2DM. Nat Rev Endocrinol. 2013;9:635-636. [DOI] [PubMed] [Google Scholar]
- 19. Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481-489. [DOI] [PubMed] [Google Scholar]
- 20. Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med. 2008;359:1618-1620. [DOI] [PubMed] [Google Scholar]
- 21. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [DOI] [PubMed] [Google Scholar]
- 22. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morgan CL, Poole CD, Evans M, Barnett AH, Jenkins-Jones S, Currie CJ. What next after metformin? A retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:4605-4612. [DOI] [PubMed] [Google Scholar]
- 25. Pawaskar M, Li Q, Hoogwerf BJ, et al. Metabolic outcomes of matched patient populations initiating exenatide BID vs. insulin glargine in an ambulatory care setting. Diabetes Obes Metab. 2012;14:626-633. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Q, Rajagopalan S, Mavros P, et al. Differences in baseline characteristics between patients prescribed sitagliptin versus exenatide based on a US electronic medical record database. Adv Ther. 2010;27:223-232. [DOI] [PubMed] [Google Scholar]
- 27. Chen S-Y, Siu K, Kovacs B, et al. Clinical and economic outcomes associated with National Kidney Foundation guideline-concordant oral antidiabetic drug treatment among type 2 diabetes patients with chronic kidney disease. Curr Med Res Opin. 2012;28:493-501. [DOI] [PubMed] [Google Scholar]
- 28. Grant RW, Buse JB, Meigs JB, University Health System Consortium Diabetes Benchmarking Project T. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28:337-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grant RW, Wexler DJ, Watson AJ, et al. How doctors choose medications to treat type 2 diabetes: a national survey of specialists and academic generalists. Diabetes Care. 2007;30:1448-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2006;64:277-281. [DOI] [PubMed] [Google Scholar]
- 32. Turchin A, Kohane IS, Pendergrass ML. Identification of patients with diabetes from the text of physician notes in the electronic medical record. Diabetes Care. 2005;28:1794-1795. [DOI] [PubMed] [Google Scholar]
- 33. Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32:1153-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ekedahl A, Brosius H, Jonsson J, Karlsson H, Yngvesson M. Discrepancies between the electronic medical record, the prescriptions in the Swedish national prescription repository and the current medication reported by patients. Pharmacoepidemiol Drug Saf. 2011;20:1177-1183. [DOI] [PubMed] [Google Scholar]
- 35. Yasnoff WA, Sweeney L, Shortliffe EH. Putting health IT on the path to success. JAMA. 2013;309:989-990. [DOI] [PMC free article] [PubMed] [Google Scholar]