Abstract
Objectives:
The objectives were to evaluate the current prevalence of lipoatrophy at insulin injection sites in young patients with type 1 diabetes.
Methods:
Standardized examination of insulin injection sites in all 678 patients with type 1 diabetes treated in 2013 in our outpatient clinic were conducted. In case of lipoatrophy photo documentation and standardized interview with parents and patients were performed.
Methods:
We identified a total of 16 patients (43.8% male) with lipoatrophy (overall prevalence 2.4%). The current mean age (±SD) of the affected patients was 14.4 ± 3.9 years, age and diabetes duration at onset of lipoatrophy were 11.5 ± 3.8 years and 5.4 ± 3.6 years, respectively. All patients were using analogs at the onset of lipoatrophy. In all, 14 of 16 patients (87.5%) were on insulin pump compared with 52% without lipoatrophy (P = .0018). The use of steel needle and Teflon catheter was equal between the pump patients. Concomitant autoimmune diseases were present in 37.5% of the patients (thyroiditis: n = 3, thyroiditis and celiac disease: n = 2, celiac disease: n = 1) compared with 15.0% in those without lipoatrophy (P = .0128).
Conclusions:
Lipoatrophy was present in young patients treated with modern insulins and pumps; however, the prevalence was relatively low as expected with the use of modern insulins. Our data may support the hypothesis that a constant mechanical element such as a subcutaneous catheter may trigger the development of lipoatrophy, particularly in those patients with more than 1 autoimmune disease.
Keywords: lipoatrophy, CSII, catheters, insulin analogs, injection sites
Long-term insulin use can be associated with local subcutaneous fat abnormalities in patients with diabetes. Local fat accumulation, lipohypertrophy, is still a frequent problem associated with long diabetes duration and nonrotation of the injection sites.1 Local fat loss, lipoatrophy, is clinically characterized by visible cutaneous depression and palpable atrophy of subcutaneous fat tissue at the injection site. Due to the use of purified human insulin preparations this condition has dramatically decreased since the 1950s, when 10-55% of patients treated with nonpurified bovine/porcine insulin preparations were troubled.2 In 1957, Renold et al reported a lipoatrophy prevalence of 44% in 342 pediatric patients.3
More recently, some data on the presence of insulin-induced localized lipoatrophy were published, indicating a rerising in the prevalence of this diabetes-related complication.4 Particularly, lipoatrophy has been reported in pediatric patients treated with insulin analogs and pumps.5 Thus, the aim of this study was an analysis of the current prevalence of lipoatrophy at insulin injection sites in a large cohort of young patients with type 1 diabetes.
Methods
In 2013, a total of all 678 patients (341 male, 337 female; median age 13.5 years) with type 1 diabetes were examined 4 to 8 times per year (mean 5.5 times) in the outpatient clinic. At diabetes onset or at the beginning of treatment in our clinic, all patients and their caregivers underwent a standardized education based on an age-adjusted, evaluated program including instructions on regular rotation of the injection sites, changing of pen needles immediately after single use as well as rotation and change of pump catheters every 2 to 3 days. Insulin preparations have to be taken out of refrigerator for at least 1.5 hours before injection or use in the pump.
At each outpatient visit, standardized inspection of the insulin injection sites was performed and abnormalities were documented. Patients with lipoatrophy and their parents underwent a standardized interview including items on kind of treatment, art and dose of insulin, as well as injection equipment at the time of first manifestation of lipoatrophy, as well as data on further comorbidities, that is, autoimmune diseases like thyroiditis, celiac disease, and vitiligo. Patient consent for photography was obtained.
Differences in the lipoatrophy prevalence and clinical or laboratory parameters between patient groups were tested by Pearson chi-square statistics and Mann–Whitney U test, respectively, with P < .05 indicating statistical significance.
Results
We identified a total of 16 patients (6 male, 10 female) with lipoatrophy (overall prevalence 2.4%). The current mean age (±SD) of the affected patients was 14.4 ± 3.9 years. Age and diabetes duration at onset of lipoatrophy were 11.5 ± 3.8 years and 5.4 ± 3.6 years, respectively. Lipoatrophic areas were found only at injection sites; no lipoatrophy was developed contralaterally to the injection site: unilateral thigh n = 6, both thighs n = 5, buttocks n = 3, abdomen n = 6. Figure 1 demonstrates a typical finding of bilateral lipoatrophy in a 9-year-old girl at both thighs.
Figure 1.
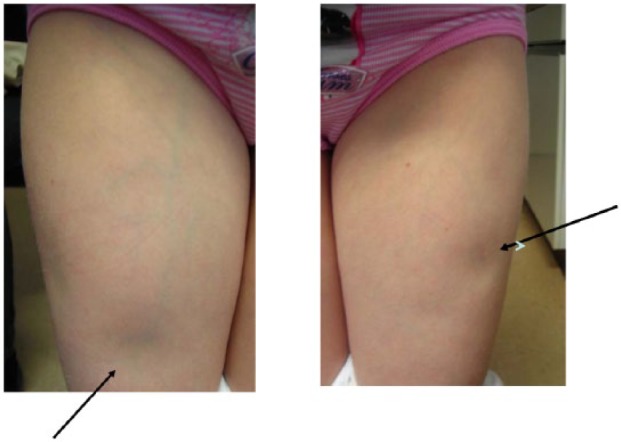
Lipoatrophic sites on both thighs in a young girl (9 years of age) with insulin pump therapy.
At the time of lipoatrophy onset, all patients were using analogs (insulin lispro: n = 3, insulin aspart: n = 13) compared with 76.2% in the whole cohort. In all, 14 of 16 patients (87.5%) were on insulin pump compared with 52% of patients without lipoatrophy (P = .0018). The number of patients using steel needles or Teflon catheters was equal in patients with pump therapy (n = 7 each).
Concomitant autoimmune diseases were present in 37.5% of the patients (thyroiditis: n = 3, thyroiditis and celiac disease: n = 2, celiac disease: n = 1) compared with 15.0% in those without lipoatrophy (P = .0128). In 2 patients lipoatrophy occurred prior to and in 4 patients after the diagnosis of the autoimmune disorder.
Patients with lipoatrophy had a longer diabetes duration than those without lipoatrophy (P < .001; Table 1). No statistically significant differences were found regarding age, gender distribution, glycemic control (A1c), frequency of hypoglycemic, or severe hypoglycemic episodes between both groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of Patients With Type 1 Diabetes With and Without Lipoatrophy.
| Patients with lipoatrophy (n = 16) | Patients without lipoatrophy (n = 662) | P value | |
|---|---|---|---|
| Gender (male/female) | 6/10 | 335/327 | .621 |
| Age (years) | 14.4 ± 3.9 | 13.7 ± 4.3 | .185 |
| Diabetes duration (years) | 9.4 ± 3.3 | 5.8 ± 3.8 | <.001 |
| A1c (%) | 8.2 ± 1.6 | 7.9 ± 1.3 | .997 |
| Episodes of severe hypoglycemia per year | 0.0 ± 0.0 | 0.01 ± 0.06 | .357 |
| Hypoglycemic episodes per month | 3.2 ± 1.2 | 3.9 ± 2.2 | .084 |
Values are mean ± standard deviation.
Affected patients were encouraged to change the injection area away from the lipoatrophic sites. As there is no established treatment of lipoatrophy so far, several actions have been taken: 6 patients changed the catheter type from Teflon to steel needle, 3 switched the insulin from aspart to lispro, and 7 adolescents started a local treatment with 4% sodium cromolyn cream twice a day. No local or systemic treatment with glucocorticoids was used. All these actions remained without visible success.
In 1 patient, lipoatrophy disappeared after heart-lung transplantation and immunosuppressive treatment due to idiopathic pulmonary hypertension at the age of 13.5 years. This was a girl with diabetes onset at the age of 30 months who started insulin pump treatment at the age of 10 years and developed lipoatrophy 10 months later (lipoatrophic areas 3 × 5 cm on both thighs).
Discussion
In general, the etiology of localized acquired lipoatrophy is unknown and thought to be heterogeneous. It may result from sequelae of abscess formation, repetitive trauma, constant or intermittent pressure, localized connective tissue diseases, systemic autoimmune disorders or iatrogenic causes.6 Insulin-induced localized lipoatrophy is thought to be an immune complex-mediated inflammatory lesion associated with all types of insulin,4 which has been confirmed in a recent review by Holstein et al.7 Furthermore, the authors stated that lipoatrophy is found more frequently in lean patients, females, and those with other autoimmune diseases. They postulated that autoimmune mechanisms could be triggered by the subcutaneous injection of any insulin and by further interactions with unknown local molecules. In a cross-sectional study of young 50 patients with type diabetes and pump therapy, Conwell et al found dermatological changes to be more frequent and severe in patients with lower adiposity.8
Although in the present study no biopsies were performed, the disappearing of lipoatrophy under immunosuppressive treatment in a single patient with heart-lung transplantation supports the theory of an immune-mediated pathogenesis. Furthermore, our data may support the hypothesis that the presence of a constant mechanical element such as a subcutaneous catheter may trigger the development of lipoatrophy, particularly in those patients with more than 1 autoimmune disease. However, this hypothesis remains unproven as long as these associations may be biased by unknown clinical parameters. In the past, continuous subcutaneous insulin infusion has been successfully used to treat patients with lipoatrophy.9 Therefore, large prospective follow-up studies are necessary to identify relevant factors and etiological relationships.
Currently there is no established therapy of lipoatrophy in patients with type 1 diabetes. Changing between different insulin preparations and changing the mode of insulin administration, for example switching from injection therapy to insulin pump infusion, local treatment with sodium cromolyn,10 low-dose oral corticosteroid,11 or subcutaneous coadministration of corticosteroid (eg, dexamethasone or betamethasone) are some therapeutic options used in case reports or small series of affected patients. Due to the low prevalence of this complication, a multicenter collaborative study is needed to improve our knowledge about the causes as well as the treatment options of lipoatrophy in patients with type 1 diabetes.
In conclusion, we found a relatively low prevalence of lipoatrophy of 2.4% indicating the need for continuous awareness on lipoatrophy in young patients with type 1 diabetes despite the use of modern insulins and treatment options like insulin pumps.
Footnotes
Authors’ Note: Parts of this study were presented in abstract form at the 39th International Society for Pediatric and Adolescent Diabetes Annual Conference, Gothenburg, Sweden, October 16-19, 2013.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kordonouri O, Lauterborn R, Deiss D. Lipohypertrophy in young patients with type 1 diabetes. Diabetes Care. 2002;25:634. [DOI] [PubMed] [Google Scholar]
- 2. Schernthaner G. Immunogenicity and allergenic potential of animal and human insulins. Diabetes Care. 1993;16(suppl 3):155-165. [DOI] [PubMed] [Google Scholar]
- 3. Renold AE, Winegrad AI, Martin DB. Diabète sucré et tissue adipeux. Helv Med Acta. 1957;24:322-327. [PubMed] [Google Scholar]
- 4. Radermecker RP, Pierard GE, Scheen AJ. Lipodystrophy reactions to insulin: effects of continuous insulin infusion and new insulin analogs. Am J Cin Dermatol. 2007;8:21-28. [DOI] [PubMed] [Google Scholar]
- 5. Rosenbloom AL. Insulin injection lipoatrophy recidivus. Pediatr Diabetes. 2014;15:73-74. [DOI] [PubMed] [Google Scholar]
- 6. Breznik V, Kokol R, Luzar B, Miljkovic J. Insulin-induced localized lipoatrophy. Acta Dermatovenerol. 2013;22:83-85. [PubMed] [Google Scholar]
- 7. Holstein A, Stege H, Kovacs P. Lipoatrophy associated with the use of insulin analogues: a new case associated with the use of insulin glargine and review of the literature. Expert Opin Drug Saf. 2010;9:225-231. [DOI] [PubMed] [Google Scholar]
- 8. Conwell LS, Pope E, Artiles AM, Mohanta A, Daneman A, Daneman D. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J Pediatr. 2008;152:622-628. [DOI] [PubMed] [Google Scholar]
- 9. Chantelau E, Reuter M, Schotes S, Starke AA. Severe lipoatrophy with human insulin: successfully treated by CSII. Diabet Med. 1993;10:580-581. [DOI] [PubMed] [Google Scholar]
- 10. Lopez X, Casteels M, Ricker A, Velazquez EF, Mun E, Goldfine AB. Human insulin analog-induced lipoatrophy. Diabetes Care. 2008;31:442-444. [DOI] [PubMed] [Google Scholar]
- 11. Chantelau EA, Praetor R, Praetor J, Poll LW. Relapsing insulin-induced lipoatrophy, cured by prolonged low-dose oral prednisone: a case report. Diabetol Metab Syndr. 2011;3:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]


