Abstract
Background:
The use of sensor-augmented insulin pump (SAP) therapy is increasing. Currently, glucose sensors and insulin infusion cannulas are inserted separately. A new device, MiniMed Duo, combines sensing and infusion capabilities on the same platform and is intended to simplify device insertion and site management. We evaluated the device’s performance with respect to insulin delivery and glucose sensing, and its acceptability with patients.
Methods:
Forty-five patients (mean ± SD age, 45.5 ± 10.9 years, 48% female) with type 1 diabetes and previous use of SAP participated. Each subject was to wear 5 devices connected to insulin pumps over 15 days (3 days/device) and test capillary blood glucose (SMBG) 7 times/day. The primary endpoint was the percentage of sensor-SMBG paired values within 20% of one another. Subject experiences were assessed via questionnaires.
Results:
Overall, 74.8% of sensor-SMBG paired values were within 20%, meeting the primary accuracy endpoint, and the mean absolute relative difference was 15.5 ± 17.1%. Consensus error grid analysis showed that >95% of points were within the A+B zones, exceeding the threshold for adequate clinical accuracy. Insulin dosage and SMBG values did not change significantly compared to prestudy values. The functional survival of the device entering day 3 was 90.5%. There were no serious adverse events. Mean questionnaire results indicated overall satisfaction with the device.
Conclusion:
Duo provided insulin infusion and glucose sensing capabilities in a single device, which provided accurate glucose readings during routine use, was safe to wear, and was acceptable to most patients. It may improve satisfaction and convenience for patients using sensor-augmented insulin pumps.
Keywords: accuracy, combo-set, continuous glucose monitoring, duo, insulin infusion
For many patients who require insulin, continuous subcutaneous insulin infusion (CSII) offers significant advantages compared to multiple daily injections (MDI). In part because CSII can more closely match the endogenous insulin profile of normal pancreatic β cells, it offers reduced rates of severe hypoglycemia, better metabolic control, and improved quality of life compared to MDI.1
Pumps are often used in conjunction with continuous glucose monitoring (CGM) systems that provide real-time data on glucose concentrations in the interstitial fluid; the CGM data can be displayed on the pump itself, or on a separate device. As shown in the STAR 3 study2 and in the Eurythmics study,3 sensor-augmented pump (SAP) therapy offers rapid, significant, and durable improvements in metabolic control compared to MDI therapy. A significant “dose effect” of CGM use was noted in STAR 3, with increasing frequencies of sensor use being associated with greater reductions in A1C levels at 1 year. The incremental advantage of CGM was more recently demonstrated in the INTERPRET study, in which factors associated with A1C reductions included more frequent sensor use.4 Average sensor use over the 12 months of the study was only 30% (range, 0 to 94%), and decreased with time. Abandonment of CGM in the INTERPRET study may have been in response to physician recommendations, initial unfavorable experiences with routine use, perceived flexibility/lifestyle concerns, skin irritation, or the burden of 2 separate insertion procedures for an infusion set and a glucose sensor. In other contexts, cost aversion may contribute to CGM abandonment.5
A device that combines insulin delivery and glucose sensing in a single platform addresses some of these concerns and may encourage more patients to routinely use CGM. Feasibility of an early prototype device using Sof-sensor technology (Combo-set) was disclosed in 20126 and more fully described in 2013;7 this study showed that there was no interference between the glucose sensing and insulin delivery functions. A second study assessed sensor performance in patients using relatively large insulin boluses.8 A feasibility study of Duo™ included use of a companion Enlite sensor during inpatient and outpatient intervals.9 The aims of the current pivotal study were to evaluate the performance attributes of Duo in terms of insulin delivery and glucose sensing, to evaluate the safety of the device, and to evaluate its acceptability to patients during routine outpatient use, which included device changes at 3-day intervals. It included more patients, as well as more days and devices per patient, than previous studies.10
Methods
Devices
MiniMed Duo (Medtronic, Inc., Northridge, CA) includes a sensor and a steel insulin delivery catheter separated by a distance of 11 mm at the skin surface; the superficial side of the device has 2 ports to which the insulin delivery tubing and the glucose sensor transmitter are attached (Figure 1). A separate spring-loaded device is used to facilitate insertion. Veo insulin pumps and MiniLink glucose sensor transmitters were used throughout the study; CareLink Clinical Therapy Management Software for Diabetes was used for data collection (Medtronic). Self-monitoring of blood glucose was done with Contour XT meters (Bayer Diabetes Care, Tarrytown, NY). Sensors were to have been calibrated 3-4 times per day using the study meter. SG values in the 40-400 mg/dL range were only paired and included in accuracy calculations if taken within 5 minutes of a SMBG value.
Figure 1.

MiniMed Duo. The deep surface shows the steel insulin infusion cannula (left) and the glucose sensor (right), separated by 11 mm and surrounded by an adhesive patch. The superficial surface has been connected to the insulin delivery tubing (left) and to the glucose sensor transmitter (right).
Study Design
The study was nonrandomized, interventional, and conducted at 2 centers in Denmark (Hvidovre University Hospital and Fredericia Hospital, Sygehus Lillebælt). Approval from relevant institutional review boards was obtained. Written informed consent was provided by all subjects prior to entry into the study. Included patients were provided with and instructed to wear 5 separate Duo devices over the course of 15 days (1 device at a time, to be replaced at 3-day intervals). Data were labeled as “day 1,” “day 2,” or “day 3” based on the time from device insertion.
Patients
To be eligible for inclusion, subjects were 18 years of age or older at the time of screening, had a diagnosis of type 1 diabetes, and had been using a Paradigm sensor-augmented insulin pump (Medtronic) for at least 3 months at the time of enrollment. CGM usage and an average of 3 SMBG per day in the month prior to enrollment were also requirements. Baseline meter glucose values and insulin use information for the 30 days prior to the study were gathered from subjects’ pumps and used for comparison with data collected during the study phase.
Data Analysis
The primary accuracy endpoint was based on comparative readings of paired sensor and study glucose meter values, measured on days 1 through 3 in 5 separate periods of outpatient testing. The null hypothesis—that less than 60% of paired measurements were within 20% of one another, or within 20 mg/dL for any study meter measure ≤80 mg/dL—was to be rejected if the lower boundary of the 95% confidence interval for agreement rate was ≥60%. Analyses were performed using a repeated measures ANOVA design. Information regarding adverse events and device complaints that could have led to an adverse event was collected via questionnaires. Descriptive summaries of insulin usage in the prestudy and study periods were calculated.
Results
Patient Enrollment and Disposition
Forty-eight patients were enrolled. All were Caucasian, all had type 1 diabetes, and 47.9% were female. The enrolled subjects’ mean (±SD) age was 45.5 ± 10.9 years; diabetes duration, 23.3 ± 10.9 years; weight, 78.4 ± 13.0 kg; BMI, 25.4 ± 3.6 kg/m2; and A1C, 7.2 ± 0.8%. There were no screen failures. Three subjects were withdrawn during the course of the study—in 1 case, the subject requested withdrawal; in another case, the subject complained about itching and redness in the area of the MiniLink transmitter; and in the third case, withdrawal was related to a pump performance issue.
Insulin Delivery
As shown in Table 1, summary statistics regarding total daily insulin doses, SMBG values, and bolus insulin deliveries obtained during the baseline 30-day interval were not significantly different from values obtained during the study phase.
Table 1.
Mean ± SD Total Daily Insulin Doses, Self-Monitored Blood Glucose Values, and Daily Bolus Deliveries From Baseline (30 Days Prior to the Study) and Study Phases.
| Baseline (30-day use) |
Study Phase (15 days) |
|||
|---|---|---|---|---|
| Value | Subject-days | Value | Subject-days | |
| TDD (U), per subject-day | 44.3 ± 18.2 | 1382 | 43.2 ± 19.6 | 663 |
| SMBG (mg/dL) | 164.6 ± 43.8 | 1435 | 155.5 ± 36.1 | 727 |
| Bolus deliveries, per subject-day | 7 ± 2.8 | 1437 | 7 ± 2.7 | 725 |
SMBG, self-monitoried blood glucose; TDD, total daily insulin dose.
Sensor Performance
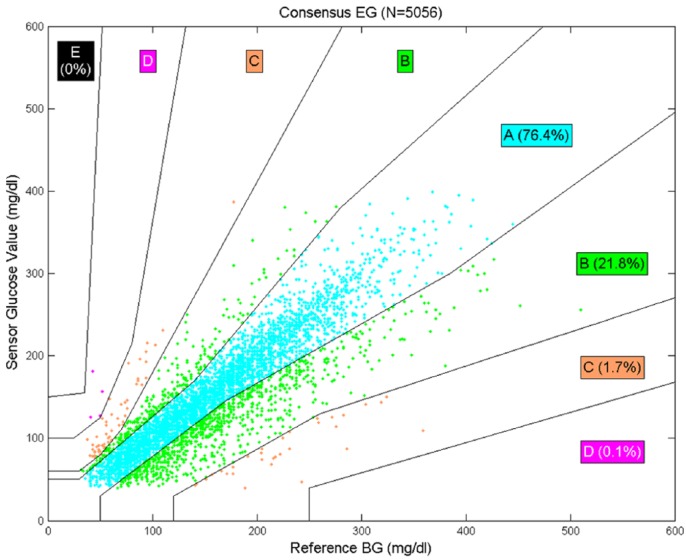
Data were analyzed with the Veo pump. The mean (±SD) number of sensor calibrations per day was 4.7 ± 1.6. Accuracy statistics (overall and for each of the 3 study days) are provided in Table 2. Of the 5874 SMBG values collected, 5056 were paired and evaluated. SMBG values were discarded if they were taken less than 5 min prior to another value, taken outside the sensor’s functional life, taken when no corresponding SG value was available for pairing, or taken when the SG value was <40 or >400 mg/dL. The overall mean (±SD) agreement rate within 20% (or within 20 mg/dL for values ≤80 mg/dL) was 74.8 ± 1.2% and the lower limit of the 95% confidence interval was 72.4%, meeting the prespecified success criterion. The overall mean (±SD) ARD was 15.5 ± 17.1%, the median ARD was 10.5%, the mean bias was -6.8 ± 32.8 mg/dL, and the median bias was -3 mg/dL. Sensor accuracy was also evaluated on a per-day basis, and the success criterion was met on each individual day of device wear. Sensor performance on day 2 and day 3 was significantly better than on day 1 (P < .001). All 5056 paired points were used in the consensus error grid analysis, which showed that 98.2% were within the A or B zones (Figure 2). Table 3 shows alert rates for hypoglycemia and hyperglycemia using various sensor glucose cutoff values. Kaplan-Meier analysis showed that the functional survival of the device at the start of day 3 was 90.5%.
Table 2.
Agreement Rates and Absolute Relative Differences (ARD) Between Sensor and SMBG Paired Points Using the Veo Pump.
| Day | No. of paired points | 20% agreement rate (mean ± SD) | 20% agreement rate (95% lower CL) | ARD (mean ± SD) | ARD (median) |
|---|---|---|---|---|---|
| 1 | 1918 | 65.8 ± 1.4% | 62.9% | 18.7 ± 19.9% | 13.3% |
| 2 | 1745 | 81.4 ± 1.3% | 78.8% | 13.1 ± 13.8% | 9.5% |
| 3 | 1393 | 78.4 ± 1.9% | 74.5% | 14.1 ± 15.9% | 9.3% |
| Overall | 5056 | 74.8 ± 1.2% | 72.4% | 15.5 ± 17.1% | 10.5% |
The 20% agreement rate was based on the number paired points within 20% of one another or within 20 mg/dL for SMBG values ≤80 mg/dL. CL, confidence limit.
Figure 2.
Consensus error grid using the Veo pump algorithm.
Table 3.
Hypoglycemia and Hyperglycemia Alerts.
| CGM alert setting (mg/dL) | Events correctly detected (%) ± 30 min | Events not detected (%) ± 30 min | Alerts verified by events (%) ± 30 min | False alerts (%) ± 30 min |
|---|---|---|---|---|
| Hypoglycemia | ||||
| 60 | 84.1 | 15.9 | 24.9 | 75.1 |
| 70 | 92.4 | 7.6 | 43.5 | 56.5 |
| 80 | 96 | 4 | 54.9 | 45.1 |
| 90 | 97.4 | 2.6 | 64 | 36 |
| 100 | 97.7 | 2.3 | 69.1 | 30.9 |
| Hyperglycemia | ||||
| 180 | 93.5 | 6.5 | 84.1 | 15.9 |
| 220 | 90.5 | 9.5 | 77 | 23 |
| 250 | 87.2 | 12.8 | 67.8 | 32.2 |
| 300 | 77.9 | 22.1 | 49.8 | 50.2 |
Safety
There were no serious adverse events or unanticipated adverse device effects during the study. Of the 44 adverse events, 43 (97.7%) were classified as mild in severity and were skin-related, such as minor bruising and bleeding. One adverse event was classified as moderate in severity; this was a furuncle/abscess that was treated with antibiotics and resolved without sequelae.
Questionnaire Results
The median response for each assertion in the “Insertion Questionnaire” was neutral or favorable toward the device, as was the median response for each assertion in the “Removal Questionnaire” with the exception of the assertion that “it was easier to remove the combo set than two sites.” We observed a wide range of scores, as indicated in the tables, in both the “Insertion Questionnaire” (Table 4) and from the “Removal Questionnaire” (Table 5). At the time of insertion, 75% of subjects indicated that they felt “no pain,” 89% agreed with a statement that the overall experience with the device was acceptable, and 74% of subjects preferred the device to insertion of 2 devices at 2 separate sites.
Table 4.
“Insertion Questionnaire” Results.
| Question | Assertion | Mean (SD) | Median | Min, max |
|---|---|---|---|---|
| 1 | Setting up the Combo seta for insertion was easy | 6.3 (0.9) | 6 | 3, 7 |
| 2 | It was easier to setup the Combo set than 2 separate sites | 4.9 (1.7) | 5 | 1, 7 |
| 3 | The amount of time it took to setup and insert the Combo set was acceptable | 6.2 (0.9) | 6 | 3, 7 |
| 4 | It took less time to setup and insert the Combo set compared to 2 sites | 4.9 (1.6) | 5 | 1, 7 |
| 5 | It was easy to find and rotate sites for the Combo set | 5.6 (1.7) | 6 | 1, 7 |
| 6 | It was easier to find and rotate sites for the Combo set compared to 2 sites | 4.8 (1.9) | 5 | 1, 7 |
| 7 | The 2 needle insertion did not increase my anxiety during insertion | 6.5 (1.1) | 7 | 2, 7 |
| 8 | The amount of pain at time of insertion was acceptable | 6.4 (1.2) | 7 | 2, 7 |
| 9 | The amount of bleeding at time of insertion was acceptable | 6.5 (0.9) | 7 | 3, 7 |
| 10 | The amount of pain at time of insertion was less compared to 2 sites | 4.8 (1.6) | 4 | 1, 7 |
| 11 | It was easy to apply the overtape to the Combo set | 5.6 (1.4) | 6 | 3, 7 |
The device was referred to as “Combo set” during the study. Integer responses 1-7 were allowed, with 1 indicating strong disagreement, 4 indicating neutrality, and 7 indicating strong agreement. N = 47 except for questions 8 and 9, where N = 46.
Table 5.
“Removal Questionnaire” Results.
| Question | Assertion | Mean (SD) | Median | Min, max |
|---|---|---|---|---|
| 1 | It was easy to remove the combo seta | 4.6 (1.9) | 5 | 1, 7 |
| 2 | It was easier to remove the combo set than 2 sites | 3.2 (1.7) | 3 | 1, 7 |
| 3 | Pain during removal was acceptable | 6.0 (1.4) | 6.5 | 2, 7 |
| 4 | Bleeding during removal was acceptable | 6.3 (1.0) | 7 | 3, 7 |
| 5 | There was less pain during removal compared to 2 sites | 4.0 (1.5) | 4 | 1, 7 |
| 6 | Combo Set was comfortable to wear during the day | 5.8 (1.4) | 6 | 2, 7 |
| 7 | Combo Set was comfortable to wear during the night | 5.8 (1.5) | 6 | 1, 7 |
| 8 | Combo set was more comfortable to wear compared to 2 sites | 4.7 (1.8) | 4 | 1, 7 |
| 9 | Combo Set stayed on the skin for the entire study | 6.3 (1.4) | 7 | 2, 7 |
| 10 | Overall the combo-set caused less irritation to my skin than 2 sites | 4.7 (1.7) | 4 | 1, 7 |
| 11 | Overall experience with the Combo Set was acceptable | 6.1 (1.3) | 7 | 2, 7 |
| 12 | I did not mind changing the combo set every 3 days | 5.7 (1.7) | 7 | 2, 7 |
| 13 | Overall I trusted the infusion and sensor of the Combo Set | 5.5 (1.9) | 6 | 1, 7 |
| 14 | I am not ashamed of people seeing my infusion site and sensor | 6.4 (1.3) | 7 | 1, 7 |
| 15 | Having a combined site made me feel better about my body than 2 sites | 5.1 (1.6) | 5 | 1, 7 |
| 16 | I prefer the Combo Set to my current 2 site setup | 5.4 (1.8) | 6 | 1, 7 |
| 17 | I would wear the Combo set more often than I wear my current sensor | 4.3 (2.1) | 4 | 1, 7 |
| 18 | Given the choice to wear the Combo set longer than the recommended 3 days I would try to push it longer | 5.4 (1.8) | 6 | 1, 7 |
The device was referred to as “Combo set” during the study. Integer responses 1-7 were allowed, with 1 indicating strong disagreement, 4 indicating neutrality, and 7 indicating strong agreement. N = 47 except for questions 3-5 and 17, where N = 46.
Discussion
For the first time, a common platform for insulin infusion and CGM sensing was tested with the Duo system in routine clinical care of adults with type 1 diabetes. The insulin need was comparable to observations before the study using single entry infusion, and acceptable accuracy of the CGM data was established with 74.8% of sensor glucose values falling within the 20% of corresponding SMBG values. No serious adverse events were observed, and 74% of patients preferred Duo over 2 separately inserted devices.
At hypoglycemic alert settings of 60 or 70 mg/dL, and at the hyperglycemic alert setting of 300 mg/dL, most of the predictive alerts were false. These high false alert rates may be attributable to appropriate preventive intervention(s) such as carbohydrate intake or additional insulin. Further analysis of the high false alert rates revealed that the differences between sensor and blood glucose were rather small (<10 mg/dL). In particular, the mean and median differences were −8.3 and −6 mg/dL at the alert setting of 60 mg/dL. As with other predictive systems, users are tasked with balancing sensitivity with burdensome “nuisance” alerts when choosing appropriate settings.
These data suggest that Duo will be an appropriate choice for some patients who are currently inserting infusion sets and glucose sensors separately. As with other sensors,11 the sensor used by Duo requires a warm-up phase, and its performance improves with time. Insulin delivery may also change over the course of an infusion set’s functional life, with faster absorption occurring later.12
Mean responses for most survey questions related to comfort, convenience, and ease of use were favorable; however, most questions elicited responses ranging from 1 (strong disagreement) to 7 (strong agreement). Narrative responses were not allowed, so the reasons underlying individual responses were not collected. However, all subjects had extensive good experience with the 2-site configuration, and none of the subjects had previously used a steel infusion cannula—both sources of a potential status quo bias. Clinicians and patients may wish to include these factors when considering Duo.
Additional studies are warranted to determine the factors contributing to patient preference—in particular, to the preference for 2 separate devices expressed by 26% of subjects—and to changes that might increase its ease of use and durability beyond the recommended 3-day lifespan. Subsequent versions of Duo may incorporate Teflon (rather than steel) cannulas to accommodate patient preferences. Further research may result in glucose sensors located on the actual insulin infusion cannula, which will simplify device management and facilitate interdevice communication.
The study has several limitations. Reference instruments were not used for blood glucose determinations, and the Contour XT blood glucose meters were used for calibrations as well as for accuracy determinations. In this study, insulin delivery was inferred by comparing insulin usage and sensor glucose patterns before and during use of the integrated device, in contrast to an earlier study7 that established the presence of exogenous insulin in patients’ blood by radioimmunoassay. The lack of a control arm in which Duo was only used for glucose sensing prevented assessment of the effect of nearby insulin delivery on Duo sensor accuracy. Two previous studies comparing Duo and Enlite sensor accuracy provided MARD values of 14.86% and 15.42%, respectively,8 and 18.46% and 15.78%, respectively.9 Duo sensor accuracy should be considered in the context of other recent studies that report MARD values for the Enlite sensor ranging from 18.9% to 19.9% in the home setting and from 16.4% to 16.6% in a clinical research center setting.13-15
Patients using SAP therapy to manage their diabetes may avoid having separate sites and procedures for an infusion cannula and a glucose sensor by using Duo. This new device, along with improved sensors and control algorithms, is expected to be a key component of future artificial pancreas systems.
Acknowledgments
We thank M. M. Andersen and B. Reino (Hvidovre Hospital, Hvidovre, Denmark), U. L. Jørgensen, C. C. Møller, and J. Pedersen (Fredericia Hospital, Fredericia, Denmark), and J. Theander (Medtronic, Hørsholm, Denmark) for assistance with the study. We also thank the patients who participated in the study. Presented in abstract and poster form at the Seventh International Congress on Advanced Technologies & Treatments for Diabetes, Vienna, Austria, February 5-8, 2014 (P-227). Published in abstract form as Nørgaard K, Andersen MM, Reino B, et al. Clinical evaluation of Duo, a combined insulin infusion/glucose sensing device. Diabetes Technol Ther. 2014;16(suppl 1):A88-A89.
Footnotes
Abbreviations: BMI, body mass index; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; SAP, sensor-augmented pump; SD, standard deviation; SMBG, self-monitored blood glucose.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JS and JBW are employees of Medtronic, Inc. KN and HG have received research support from Medtronic, Inc.
References
- 1. Linkeschova R, Raoul M, Bott U, Berger M, Spraul M. Less severe hypoglycaemia, better metabolic control, and improved quality of life in Type 1 diabetes mellitus with continuous subcutaneous insulin infusion (CSII) therapy; an observational study of 100 consecutive patients followed for a mean of 2 years. Diabet Med. 2002;19(9):746-751. [DOI] [PubMed] [Google Scholar]
- 2. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320. [DOI] [PubMed] [Google Scholar]
- 3. Hermanides J, Norgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med. 2011;28(10):1158-1167. [DOI] [PubMed] [Google Scholar]
- 4. Nørgaard K, Scaramuzza A, Bratina N, et al. Routine sensor-augmented pump therapy in type 1 diabetes: the INTERPRET study. Diabetes Technol Ther. 2013;15(4):273-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirsch IB. Diabetes care entering 2014: more ranting. Diabetes Technol Ther. 2014;16(2):iii-iv. [DOI] [PubMed] [Google Scholar]
- 6. O’Neal DN, Adhya S, Jenkins A, Voskanyan G, Ward G, Welsh JB. Feasibility of adjacent insulin infusion and glucose sensing via the Medtronic Combo-set. Diabetes. 2012;61(S1):A229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Neal DN, Adhya S, Jenkins A, Ward G, Welsh JB, Voskanyan G. Feasibility of adjacent insulin infusion and continuous glucose monitoring via the Medtronic Combo-Set. J Diabetes Sci Technol. 2013;7(2):381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frid A, Voskanyan G, Adhya S, et al. Adequacy of the integrated glucose sensor and infusion set’s sensor performance in the high insulin requiring population. Pediatr Diabetes. 2013;14(S18):143-144. [Google Scholar]
- 9. Nørgaard K, Voskanyan G, Adhya S, et al. Feasibility assessment of the MiniMed Duo Device: combined insulin delivery and glucose sensing. Diabetes. 2013;62(S1):A248. [Google Scholar]
- 10. Nørgaard K, Andersen MM, Reino B, et al. Clinical evaluation of Duo, a combined insulin infusion/glucose sensing device. Diabetes Technol Ther. 2014;16(S1):A88-A89. [Google Scholar]
- 11. Bailey T, Ahmann A, Brazg RL, et al. Accuracy and acceptability of the 6-day Enlite continuous subcutaneous glucose sensor. Diabetes Technol Ther. 2014;16(5):277-283. [DOI] [PubMed] [Google Scholar]
- 12. Clausen TS, Kaastrup P, Stallknecht B. Effect of insulin catheter wear-time on subcutaneous adipose tissue blood flow and insulin absorption in humans. Diabetes Technol Ther. 2009;11(9):575-580. [DOI] [PubMed] [Google Scholar]
- 13. Kropff J, Bruttomesso D, Doll W, et al. Accuracy of two continuous glucose monitoring systems: a head-to-head comparison under clinical research centre and daily life conditions [published online ahead of print August 11, 2014]. Diabetes Obes Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matuleviciene V, Joseph JI, Andelin M, et al. A clinical trial of the accuracy and treatment experience of the Dexcom G4 Sensor (Dexcom G4 System) and Enlite Sensor (Guardian REAL-Time System) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther. 2014;16(11):759-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luijf YM, Mader JK, Doll W, et al. Accuracy and reliability of continuous glucose monitoring systems: a head-to-head comparison. Diabetes Technol Ther. 2013;15(8):722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]