Abstract
Background:
The effects of transition by individuals with type 1 diabetes (T1D) to more recently available continuous glucose monitoring (CGM)-enabled insulin pumps from either multiple daily insulin injections (MDI) or older insulin pumps on treatment satisfaction have not been well studied. We conducted a survey to assess treatment satisfaction among users of the Animas® Vibe™ insulin pump, a latest generation insulin pump (LGIP) system (CGM-enabled), after switching from MDI or earlier generation insulin pumps.
Methods:
Individuals with T1D from 141 centers in 5 countries and 4 language areas participated in the survey. Treatment satisfaction was assessed by the Insulin Treatment Satisfaction Questionnaire (ITSQ), which was included in a 50-item online questionnaire that also assessed preference for using the LGIP compared with previous treatment and satisfaction with key LGIP features.
Results:
A total of 356 individuals, ages 12-79 years, responded to the survey: mean (SD) age 38.4 (16.1) years; diabetes duration 19.1 (13.3) years; female 59%; previously treated with MDI 58%. Overall mean (SD) ITSQ scores were high among all respondents regardless of prior treatment: 95.1 (23.2) (scale: 0-132). No differences between previous-treatment groups were seen. Most (83%) of respondents rated the LGIP to be better than their previous insulin delivery system: “much better” (65%), “a bit better” (18%) regardless of age, and 95% would recommend using the LGIP to others.
Conclusions:
Use of the Animas Vibe was associated with high treatment satisfaction and perceived as a better method of insulin delivery regardless of previous insulin therapy or age.
Keywords: CSII, insulin pump, treatment satisfaction, ITSQ, CGM, continuous glucose monitoring
Treatment with insulin pumps has been shown to be an effective method for improving glycemic control and preventing/delaying long-term diabetic complications,1-3 and is the recommended therapy for most individuals with type 1 diabetes (T1D).4 Use of insulin pumps in combination with continuous glucose monitoring (CGM) technologies has been shown to further improve clinical outcomes.5-7
Despite these benefits, many individuals with T1D who are treated with multiple daily insulin injections (MDI) may be reluctant to make the transition to an insulin pump. In focus group interviews with 30 adults with T1D who were currently using insulin pumps, Ritholz and colleagues identified 4 major themes regarding respondents’ reluctance to transition to insulin pump therapy.8 These included the potential impact on diabetes self-care, emotional reactions to the insulin pump, body image, and social acceptance. Perceived complexity of insulin pumps, particularly among older patients, has also been suggested as a possible deterrent.9
The effect of transition from MDI to an insulin pump on perception and treatment satisfaction has not been well studied. Nor is it known whether and/or to what degree treatment satisfaction among experienced insulin pump users is impacted by transition to more recent insulin pump systems, which offer advanced features such as smaller incremental basal and bolus administration, programmable basal rates and CGM capability. However, an early study by Hanestad and colleagues showed a strong association between satisfaction with diabetes care and glycemic control.10
To address this knowledge deficit, we conducted the Comparing Perception of Insulin Therapies for T1D Patients with the Aim to Improve Quality of Care (CHOICE) study, a survey of individuals with T1D who recently transitioned to a latest generation (CGM-enabled) insulin pump (LGIP) from MDI or previous insulin pump use.
Methods
Design
The CHOICE study was a multicenter, noninterventional, cross-sectional survey to assess treatment satisfaction with insulin pump therapy among individuals with T1D who had recently transitioned to use of the Animas® Vibe™ (LifeScan, Wayne, USA), an LGIP system (CGM-enabled), from either MDI therapy or an earlier generation insulin pump. Thus, we established 2 study arms: insulin pump experienced and pump naïve T1D patients, including previous treatment with insulin injection pens, vial, and syringe or no prior insulin treatment.
Data were collected by online survey which included individuals with T1D who were recruited from 141 centers in 4 language areas and 5 countries (France [n = 42], Germany [n = 47], Netherlands [n = 16], and UK/Ireland [n = 36]). The study was conducted in strict compliance with EU-regulations, which are delineated in the Directives of the European Parliament No. 93/42/EWG (last modified: 2007/47/EG) and ICH-GCP (CPMP/ICH/135/95). Written, informed consent was obtained from all respondents prior to completing the survey.
Respondents
Sites that were known to use the LGIP were recruited randomly from a list of clinics provided by the study sponsor. Clinicians (physicians and diabetes nurses) were asked to randomly collect email addresses from up to 30 patients who met eligibility criteria, which included T1D, age ≥ 12 years, and use of the LGIP for at least 3 months. Clinicians identified a total of 368 potential respondents.
Study Device
The study LGIP is the Animas Vibe, CGM-enabled system. The insulin pump functions as a receiver for the Dexcom G4® PLATINUM CGM sensor (CGM system, Dexcom, La Jolla, CA, USA). The LGIP system presents real-time glucose information, alerts for high and low readings and glucose trend information on a high-contrast color screen. In addition, the system offers incremental basal dosages (0.025 U/hr to 25.0 U/hr), programmable basal rates, and automatic calculation of correction bolus dosages based on the latest blood glucose reading.
Measurements
The primary endpoint for the survey was overall treatment satisfaction with the LGIP, as assessed by the Insulin Treatment Satisfaction Questionnaire (ITSQ) (scale: 0-132, in the higher the better format), a validated measure of treatment satisfaction that is applicable to a wide range of insulin therapies.11 The 22-item ITSQ instrument provides an overall score and scores for 5 subscales: (1) insulin delivery device satisfaction, (2) glycemic control, (3) hypoglycemic control, (4) inconvenience of regimen, and (5) lifestyle flexibility. The ITSQ was included in a 50-item questionnaire, which also included questions to assess respondents’ perceptions regarding stable and safe glucose control, satisfaction with key LGIP features and preference for using the LGIP compared with their previous treatment.
Procedures
Clinicians at the selected study sites were provided with preformatted email invitations and patient-specific passwords. The emails were sent to 368 prospective respondents, who were redirected to the study server to complete the online questionnaire after providing written, informed consent.
Statistical Methods
Pearson chi-square tests were applied to tests for independency between 2 categorical variables: pump naïve versus pump experienced. T tests for independent samples were used to test for mean differences between 2 distinct groups. The analysis of variance (ANOVA) procedure tested for overall mean differences between multiple categories under the assumption of approximately normal distributed data. Given a significant overall test, several post hoc tests between distinct categories were conducted: least significant difference (LSD) in case of homogenous variances; Tamhane’s T2 if heterogeneous variances were assumed. Multivariate regression analysis (general linear model, GLM) modeled the association between an approximately normal distributed dependent variable and several independent factors and covariates. All statistical calculations had been performed with the software package SPSS version 22.
Results
Demographic Characteristics
A total of 356 individuals responded to the survey; 12 of the 368 individuals initially identified reported having type 2 diabetes and 2 respondents of the 356 T1D respondents failed to complete the questionnaire. Demographic characteristics of respondents are presented in Table 1. Glycemia in respondents was well controlled using the LGIP as assessed by self-reported fasting glucose data: 47% of respondents reported fasting glucose <7.0 mmol/L (126 mg/dL) and 30% reported fasting glucose of 7.0-9.0 mmol/L (126-162 mg/dL).
Table 1.
Demographic Characteristics.
| Respondents by country, n (%) | n = 354 |
|---|---|
| France | 156 (44) |
| UK/Ireland | 105 (30) |
| Germany | 42 (12) |
| Netherlands | 51 (14) |
| Gender, n (%) | |
| Male | 146 (41) |
| Female | 209 (59) |
| Mean age, years (SD) | 38.4 (16.1) |
| Age by category, n (%) | |
| 12-17 years | 61 (17) |
| 18-29 years | 45 (13) |
| 30-49 years | 157 (44) |
| 50-64 years | 74 (21) |
| 65+ years | 17 (5) |
| Mean diabetes duration, years (SD) | 19.1 (13.3) |
| Previous insulin delivery system, n (%) | |
| Insulin pump | 135 (38) |
| Insulin pen (premixed) | 14 (4) |
| Insulin pen (long-/fast-acting) | 190 (54) |
| Syringe/vial | 11 (3) |
| None | 4 (1) |
| Frequency of mild/moderate hypoglycemia, n (%) | |
| More than once per week | 164 (46.1) |
| Once per week | 111 (31.2) |
| Once per month | 33 (9.3) |
| Less than once per month | 17 (4.8) |
| Never | 5 (1.4) |
| Fasting glucose value (day of survey completion), n (%) | |
| Do not know | 12 (3.4) |
| <7.0 mmol/L (126 mg/dL) | 168 (47.2) |
| 7.0-9.0 mmol/L (126-162 mg/dL) | 107 (30.1) |
| >9.0 mmol/L (162 mg/dL) | 62 (17.4) |
| Mean glucose measurements/day, n (SD) | n = 348 |
| 6.6 (6.8) | |
| Mean correction boluses/week, n (SD) | n = 336 |
| 10.3 (9.1) | |
Respondents in France were slightly younger (34 [17.4] years) compared to the other countries in the study; however, the between-country difference in overall mean age was statistically significant only for the comparison between France and the UK/Ireland (42.7 [14.4] years, P < .001). By age category, there was a statistically significant overall country difference with a higher percentage of French respondents in the 12-17-year (29%) category, compared with the Netherlands (12%), UK/Ireland (8%), and Germany (5%) cohorts (P < .001). The gender distribution was slightly skewed toward more females (59%) but with no between-country difference or gender differences by age category.
As shown in Table 1, 61% of respondents were using MDI prior to transitioning to the LGIP. Remarkable within-country differences were seen in the distribution of prior insulin treatment with higher percentages of prior insulin pump users in Germany (62%) and the Netherlands (59%) compared with UK/Ireland (28%) and France (32%) (P = .001). No significant differences in prior insulin delivery methods were seen by age category or between countries.
Respondents from all countries reported similar behaviors in the number of glucose measurements per day. However, the number of correction boluses per week reported by French respondents was slightly lower than UK/Ireland and Netherlands respondents and significantly lower than reported by German respondents: 8.8 versus 16.0 (P = .006).
Current use of the CGM feature was reported by 28% of respondents; however, frequency (eg, days per week) of use was not queried. Note that only respondents > 18 years received this question in the survey due to regulatory restrictions regarding CGM use in younger patients. Significant between-country differences were seen in reported CGM use (P = .033), with Germany (45%) and the Netherlands (38%) showing the highest percentages of CGM use, and France (27%) and UK/Ireland (19%) showing the lowest percentages. CGM use varied with age: 18-29 years, 13%; 30 to 49 years, 68%; 50-64 years, 18%; and 65+ years, 1%.
Treatment Satisfaction: Total and Subscale ITSQ Score and Multivariate Regression Analysis
Overall ITSQ scores (range: 0-132) in a higher-the-better format showed that treatment satisfaction with the LGIP was high (95.1 [23.2]) among all respondents regardless of prior insulin delivery system use. No significant mean differences between previous pump users and MDI patients could be detected (Figure 1). Treatment satisfaction scores were also high in the 5 subscales: (1) insulin delivery device satisfaction, 29.5 (6.3) (scale 0-36); (2) glycemic control, 12.0 (4.4) (scale 0-18); (3) hypoglycemic control, 21.1 (6.5) (scale 0-30); (4) inconvenience of regimen, 20.4 (7.4) (scale 0-30); and (5) lifestyle flexibility, 12.3 (4.6) (scale 0-18).
Figure 1.
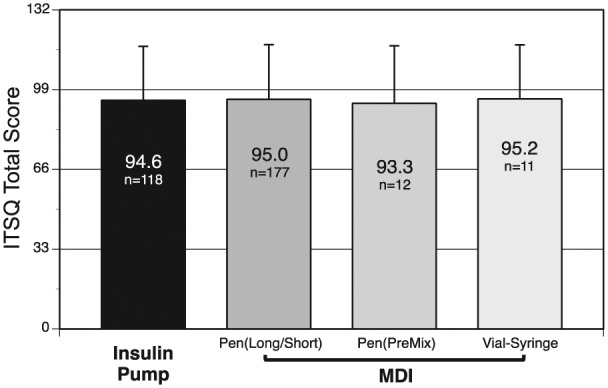
Total ITSQ scores showed no between-group difference in treatment satisfaction related to prior insulin delivery system use. Thirty-two respondents did not complete the ITSQ questions; data from the 4 respondents who indicated “no previous treatment” were excluded from this analysis.
Multivariate regression analysis showed that self-reported fasting blood glucose was the most significant contributing factor to treatment satisfaction; values < 7.0 mmol/L (126 mg/dL) were associated with a 21-unit increase in the overall diabetes treatment satisfaction. Fasting blood glucose was also strongly correlated with respondents’ satisfaction regarding glucose control as assessed by the ITSQ glycemic control subscale (P < .001); lower fasting glucose correlated with greater satisfaction with glucose control. High satisfaction with glucose control was strongly correlated with high overall treatment satisfaction (P < .001). No correlation between fasting blood glucose and CGM use was seen. Reduced insulin delivery device satisfaction was overall associated with lower educational status (lower secondary) (P = .046).
ITSQ hypoglycemic control subscale scores showed that patients experiencing no or infrequent hypoglycemic events reported higher satisfaction with the hypoglycemic control of the LGIP than patients with frequent hypoglycemic events (eg, at least once a month) (21.5 vs 18.7, P = .008). However, both of these groups of patients scored high on this subscale.
Comparison to Previous Treatment
Most respondents (83%) rated the LGIP to be better than their previous insulin delivery system: 65% as “much better,” 18% “a bit better.” A significantly greater percentage of respondents who previously used MDI rated the LGIP “much better” or “a bit better” compared with prior insulin pump users (93% vs 60%, P < .001) regardless of age (Figure 2).
Figure 2.
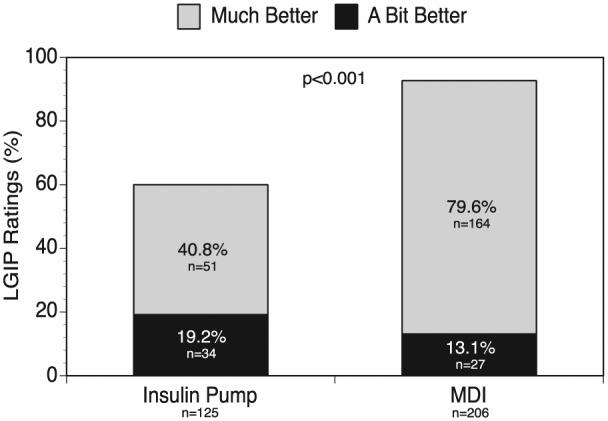
Respondent ratings of LGIP use (much better/a bit better) by prior treatment group. Nineteen respondents did not complete this portion of the questionnaire; data from the 4 respondents who indicated “no previous treatment” were excluded from this analysis.
Satisfaction With LGIP Features
The majority (95%) of respondents reported they would “definitely” (66%) or “probably” (28%) recommend the LGIP to others. Device ease of use, giving a bolus at meal or snack time, device warnings and alarm safety system and device size were identified as “important” features by greater than 90% of respondents, and most respondents were satisfied with these features (Table 2). Of all respondents, 81% rated the CGM feature as “important.” Among these respondents, 68% reported satisfaction with this feature.
Table 2.
Respondent Satisfaction With Features Considered to Be Important.
| Feature | Respondents who rated the feature “important” (%) | Respondents who were satisfied with the feature (%) |
|---|---|---|
| Ease of use (eg, menu, navigation) | 96.6 | 83.8 |
| Giving a bolus at meal or snack time | 95.3 | 94.2 |
| Warnings and alarm safety system | 93.5 | 72.0 |
| Size | 90.6 | 60.1 |
Discussion
In this multicenter, noninterventional, online survey, treatment satisfaction scores were high in all respondents using the LGIP regardless of previous insulin therapy or age. This is important because treatment satisfaction is associated with better glycemic control.10
It is noteworthy that the cohort that was previously treated with MDI therapy had the highest percentage of respondents who rated the LGIP as “much better” than their previous therapy, regardless of patient age. Perception of complexity of insulin pump therapy, especially among older patients, has been cited as a common obstacle to transitioning from MDI to insulin pump therapy.9
This survey also noted that less than one-third of respondents used the integrated CGM function within the LGIP. Although the survey questionnaire was not designed to identify the reason(s) for low CGM use, it is possible that this was due to limited reimbursement for CGM in the countries surveyed. Across Europe, remuneration models are variable with some countries providing no CGM reimbursement and others limiting its use to narrow populations such as children, pregnant women and those unable to achieve target glycemic control.
One limitation of the survey was the use of self-reported data, which may not accurately reflect respondents’ current level of glucose control (eg, reported fasting blood glucose values), which could not be verified. Another potential limitation is that the survey findings were limited to only 1 LGIP; it was felt necessary to do this to avoid confounding results due to differences in indication and features among CGM-enabled insulin pump systems. In addition, because the survey did not obtain information about frequency of CGM use, we were unable to determine how frequently respondents were actually using CGM. Although numerous studies have shown that use of CGM improves glycemic control,5,12-23 clinical benefits were seen mainly in those individuals who regularly wore their CGM devices at least 6 days per week.5,6,13,14,24-26 Another limitation was that our study design did not allow us to assess changes in treatment satisfaction. Use of a pre-post study design would have allowed us to determine the impact of transition to the LGIP system. Nevertheless, given the high treatment satisfaction scores seen in respondents, combined with the large percentage of respondents who rated the LGIP better than their previous therapy, we are confident that treatment satisfaction was preserved and may have improved.
Conclusion
In conclusion, our findings show that use of the Animas Vibe was associated with high treatment satisfaction and was perceived as a better method of insulin delivery regardless of previous insulin therapy or age. Our findings may provide guidance to clinicians when discussing transition to insulin pumps with their MDI-treated patients who express concerns about insulin pump complexity.
Acknowledgments
The authors wish to thank Christopher G. Parkin, MS (CGParkin Communications, Inc, Boulder City, NV, USA) for editorial assistance in preparing this article. Interim results from the study were presented at the 45th annual meeting of the European Association for the Study of Diabetes (EASD), September 29 to October 2, 2014, Vienna, Austria.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KB, ML, MH, ML, BL-G, KW, and RZ are members of an advisory board of Animas, a Johnson and Johnson company, division of Cilag GmbH International, receiving honoraria for participation and advice. MB and CGP have received consulting fees from Animas. BL is an employee of LifeScan, a Johnson and Johnson company.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Animas, a Johnson and Johnson company, division of Cilag GmbH International, 6300 Zug, Switzerland.
List of Abbreviations: ANOVA, analysis of variance; CGM, continuous glucose monitoring; CHOICE, Comparing Perception of Insulin Therapies for T1D Patients with the Aim to Improve Quality of Care; GLM, general linear model; ITSQ, Insulin Treatment Satisfaction Questionnaire; LGIP, latest generation insulin pump; LSD, least significant difference; MDI, multiple daily injections; SD, standard deviation; T1D, type 1 diabetes; UK, United Kingdom; USA, United States of America.
References
- 1. Diabetes C, Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765-774. [DOI] [PubMed] [Google Scholar]
- 3. Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51:941-951. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80. [DOI] [PubMed] [Google Scholar]
- 5. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52:1250-1257. [DOI] [PubMed] [Google Scholar]
- 6. Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32:2245-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choudhary P, Ramasamy S, Green L, et al. Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care. 2013;36:4160-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care. 2007;30:549-554. [DOI] [PubMed] [Google Scholar]
- 9. Bode BW. Insulin pump use in type 2 diabetes. Diabetes Technol Ther. 2010;12(suppl 1):S17-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanestad BR, Albrektsen G. Quality of life, perceived difficulties in adherence to a diabetes regimen, and blood glucose control. Diabet Med. 1991;8:759-764. [DOI] [PubMed] [Google Scholar]
- 11. Anderson RT, Skovlund SE, Marrero D, et al. Development and validation of the Insulin Treatment Satisfaction Questionnaire. Clin Ther. 2004;26:565-578. [DOI] [PubMed] [Google Scholar]
- 12. Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 13. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730-2732. [DOI] [PubMed] [Google Scholar]
- 15. Battelino T, Bode BW. Continuous glucose monitoring in. 2010. Int J Clin Pract. 2011;65(S170):10-15. [DOI] [PubMed] [Google Scholar]
- 16. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hermanides J, Phillip M, DeVries JH. Current application of continuous glucose monitoring in the treatment of diabetes. Diabetes Care. 2011;34:S197-S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riveline JP, Schaepelynck P, Chaillous L, et al. Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens: a 1-year multicenter study. Diabetes Care. 2012;35:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care. 2011;34:2403-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44-50. [DOI] [PubMed] [Google Scholar]
- 21. Garg S, Jovanovic L. Relationship of fasting and hourly blood glucose levels to HbA1c values: safety, accuracy, and improvements in glucose profiles obtained using a 7-day continuous glucose sensor. Diabetes Care. 2006;29:2644-2649. [DOI] [PubMed] [Google Scholar]
- 22. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. [DOI] [PubMed] [Google Scholar]
- 23. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9:203-210. [DOI] [PubMed] [Google Scholar]
- 26. Frontino G, Bonfanti R, Scaramuzza A, et al. Sensor-augmented pump therapy in very young children with type 1 diabetes: an efficacy and feasibility observational study. Diabetes Technol Ther. 2012;14:762-764. [DOI] [PubMed] [Google Scholar]


