Abstract
Background:
Hypoglycemia mitigation is critical for appropriately managing patients with diabetes. Advanced technologies are becoming more prevalent in diabetes management, but their benefits have been primarily judged on the basis of hemoglobin A1c. A critical appraisal of the effectiveness and limitations of advanced technologies in reducing both A1c and hypoglycemia rates has not been previously performed.
Methods:
The cost of hypoglycemia was estimated using literature rates of hypoglycemia events resulting in hospitalizations. A literature search was conducted on the effect on A1c and hypoglycemia of advanced technologies. The cost-effectiveness of continuous subcutaneous insulin infusion (CSII) and real-time continuous glucose monitors (RT-CGM) was reviewed.
Results:
Severe hypoglycemia in insulin-using patients with diabetes costs $4.9-$12.7 billion. CSII reduces A1c in some but not all studies. CSII improves hypoglycemia in patients with high baseline rates. Bolus calculators improve A1c and improve the fear of hypoglycemia but not hypoglycemia rates. RT-CGM alone and when combined with CSII improve A1c with a neutral effect on hypoglycemia rates. Low-glucose threshold suspend systems reduce hypoglycemia with a neutral effect on A1c, and low-glucose predictive suspend systems reduce hypoglycemia with a small increase in plasma glucose levels. In short-term studies, artificial pancreas systems reduce both hypoglycemia rates and plasma glucose levels. CSII and RT-CGM are cost-effective technologies, but their wide adoption is limited by cost, psychosocial, and educational factors.
Conclusions:
Most currently available technologies improve A1c with a neutral or improved rate of hypoglycemia. Advanced technologies appear to be cost-effective in diabetes management, especially when including the underlying cost of hypoglycemia.
Keywords: hypoglycemia, glycemic control, technology, cost-effectiveness
The New York Times published an article on April 5, 2014, titled “Even Small Medical Advances Can Mean Big Jumps in Bills” by Elizabeth Rosenthal,1 claiming that diabetes technologies and therapies are overpriced, offer little value, and place an unjust burden on the US health care system. “That captive audience of Type 1 diabetics has spawned lines of high-priced gadgets and disposable accouterments, borrowing business models from technology companies like Apple.” This controversial article failed to capture the benefits of technology not only in improving glycemic control as determined by hemoglobin A1c (A1c) but also in mitigating the frequency, severity, and cost of hypoglycemia. The article also did not address the increasing problem and resultant costs of hypoglycemia in patients with type 2 diabetes and how technology might be used to mitigate this as well.2 In light of Rosenthal’s article, it is important to objectively review the literature to answer the following questions:
What is the cost of hypoglycemia?
What is the evidence that technology can improve A1c and/or reduce the risk of hypoglycemia?
What are the limitations in using technology to accomplish this?
What is the cost-effectiveness of technology?
The Problem
Since the publication of the Diabetes Control and Complications Trial (DCCT) results in 1993 and the DCCT/EDIC study in 2005 an improvement in the hemoglobin A1c level has been the benchmark for demonstrating the benefit of any new therapy because these studies showed that there was a direct relationship between the degree of glycemic control and the development of micro- and macrovascular complications.3,4 Somewhat overlooked is the high rate of hypoglycemia that was the “price” for improved A1c in the intensively treated arm of the DCCT study. There was a greater than 3-fold increase in the rate of severe hypoglycemia as defined as requiring assistance, coma and/or seizure in the intensive arm of the study (77.5 events per year compared with 24.1 events/year).5 The prevalence of hypoglycemia remained about the same during the entirety of the trial. Indeed, it is the fear of hypoglycemia that often precludes more aggressive glycemic management since even a single episode results in patients and their providers becoming reluctant to adhere to the recommendations that led to that episode. Despite improvements in monitoring technology and the introduction of analog insulins, hypoglycemia continues to be a problem for patients with type 1 diabetes. Indeed, Weinstock and colleagues in the T1D Exchange Network, a database that is a reflection of real-world glycemic control, recently reported that the overall prevalence of severe hypoglycemia episodes (1 or more severe [seizure or coma] episodes per year) was 11.8% (range 5%-19%) per year depending on the duration of diabetes in the almost 5000 patients surveyed.6 Interestingly, they found a U-shaped curve of the prevalence of hypoglycemia whose nadir was an A1c of 7%-7.4%, suggesting that mean hyperglycemia does not protect against hypoglycemia. They also found that the prevalence was independently associated with a lower socioeconomic status, lower educational achievement, lower income, and not having private insurance. Over the last few years, it has become apparent that hypoglycemia is also a major problem in patients with type 2 diabetes particularly those on insulin only, insulin in combination with oral agents, and insulin secretagogues. The risk of severe hypoglycemia in patients with type 2 with respect to A1c can also be described by a U-shaped curve. The highest incidence was in those with the highest A1cs and was not related to whether or not the patient was on insulin, insulin plus an oral agent, or oral agents alone.7
These observations and others have led the Endocrine Society and the American Diabetes Association to recommend that for “healthy adults with diabetes, a reasonable glycemic goal might be the lowest A1c that does not cause severe hypoglycemia, preserves awareness of hypoglycemia, and results in an acceptable number of documented episodes of symptomatic hypoglycemia.”8 The recent reduction of A1c goals in children and young adults with type 1 diabetes to <7.5% exert more pressure on finding a way to achieve those goals.9
Methods
The cost of severe hypoglycemia in the United States was estimated in type 1 and 2 diabetes based on their current prevalence, the prevalence of hypoglycemia unawareness in those populations, the frequency of severe hypoglycemic events, and the number and cost of hospitalizations for hypoglycemia.10-15 A range of costs was estimated based on high and low rates of severe hypoglycemic events in insulin-using patients. A selective literature search was conducted on the effect and cost-effectiveness (where available) on A1c and hypoglycemia of insulin pumps (CSII), bolus calculators (BC), real-time continuous glucose monitors (RT-CGM), sensor-augmented pumps (SAP), low-glucose threshold suspend (LGTS) systems, low-glucose predictive suspend (LGPS) systems, and artificial pancreas (AP) systems.
Cost of Hypoglycemia
The published estimate for the total (direct and indirect) costs of diabetes is $245 billion a year as of 2012.16 This cost is 41% higher than 5 years earlier and now represents 20% of the health care expenditures in the United States. The cost of hypoglycemia is an important part of overall costs of diabetes care because it often results in hospital admissions. I have estimated that the cost of severe hypoglycemic episodes in the United States is $4.9-$14.7 billion in the 5 million insulin-using patients with type 1 and type 2 diabetes who have hypoglycemic unawareness (Table 1). This is based on the assumption that the prevalence of hypoglycemia unawareness in insulin-using patients with type 1 and type 2 diabetes is 20% and 9.8%, respectively;10,11 the annual number of severe hypoglycemic events in those with hypoglycemia unawareness that are likely to result in hospitalization is 2.4-8.1/year in type 1 diabetes10,12 and 2.1-5.9/year in type 2 diabetes;13,14 and the cost per hospitalization for severe hypoglycemia is $17 564.15 The actual cost of hypoglycemia in the United States is undoubtedly much higher since non-insulin-using patients, particularly those using insulin secretagogues either alone or in combination with another noninsulin therapy, also develop hypoglycemia resulting in hospitalizations. In addition, nonsevere hypoglycemia results in a significant impact on work productivity and additional resource utilization through additional clinic visits and blood glucose testing.16
Table 1.
Estimate Cost of Hypoglycemia Admissions in Patients With Type 1 and Type 2 Diabetes.
| Lowest rate of hypoglycemia/year for type 1 diabetes | Lowest rate of hypoglycemia/year for type 2 diabetes | Highest rate of hypoglycemia/year for type 1 diabetes | Highest rate of hypoglycemia/year for type 2 diabetes | |
|---|---|---|---|---|
| Patients | 1.0 million | 18.2 million | 1.0 million | 18.2 million |
| Insulin-requiring patientsa | 1.0 million | 4.0 million | 1.0 million | 4.0 million |
| Hypoglycemia unaware patientsb | 200 000 | 400 000 | 200 000 | 400 000 |
| Severe hypoglycemia events | 480 000c | 840 000c | 1 600 000d | 2 400 000d |
| Hospitalizationse | 100 800 | 176 000 | 336 000 | 504 000 |
| Cost of hospitalization ($)f | 1.8 billion | 3.1 billion | 5.9 billion | 8.8 billion |
22% of patients use insulin.
20% hypoglycemia unawareness in type 1 diabetes and 9.8% in type 2 diabetes.
Low annual rate of severe hypoglycemia events.
High annual rate of severe hypoglycemia events.
21% hospitalization rate for severe hypoglycemia.
Cost of hospitalization for severe hypoglycemia = $17 564.
Technologies
The evidence for the benefits and limitations of several currently available technologies in managing patients with both type 1 and type 2 diabetes is discussed below. A summary of these is shown in Table 2.
Table 2.
Effects of Currently Approved Technologies on Key Aspects of Management in Patients With Diabetes.
| A1c | Hypoglycemia | Limitations | |
|---|---|---|---|
| CSII |  |
 |
1. Cost2. Socioeconomic barriers |
| BC |  |
 |
1. Most are not FDA-approved2. Meal content not included in calculation |
| RT-CGM |  |
 |
1. Cost2. Low adherence rates |
| SAP |  |
 |
1. Cost2. 2 insertion sites |
| LGTS |  |
 |
1. Cost2. 2 insertion sites3. Limited number of studies |
| LGPS |  |
 |
1. Cost2. 2 insertion sites3. Limited number of studies |
LGPS systems are approved in the European Union but not yet by the US Food and Drug Administration.
Bolus Calculators (BC)
Benefits
Bolus calculators are widely available in a variety of formats—applications for smart phones, incorporation into blood glucose meters, and incorporation into insulin pumps. Bolus calculators estimate the “correction” insulin dose using a patient’s current glucose level, insulin sensitivity, target glucose level and insulin-on-board and the prandial insulin dose based on the carbohydrate content of the meal and the preestablished insulin:carbohydrate ratio. Cavanaugh and colleagues demonstrated that patients with diabetes have poor diabetes-related numeracy.17 BC’s can overcome this problem. Sussman and colleagues documented the numeracy problem in a study that found that patients made errors 63% of the time in calculation of their correction bolus dose and/or their prandial carbohydrate-based dose when provided with the standard formulae.18 In that study, the use of a BC improved the accuracy of the insulin dose to over 90%. At this time, there are 84 BCs that are downloadable from iTunes for use on smart phones.19 It should be noted that none of the smart phone BCs have been cleared by the Food and Drug Administration (FDA) for accuracy. Thus, patients and their diabetes providers should be cautious about relying on the dose recommendation until a particular application has been thoroughly tested. At the present time, there is only 1 blood glucose meter (ACCU-CHEK Aviva Expert) that contains an FDA-approved BC. It is available by prescription only. BCs that are included in insulin pumps have all been cleared by the FDA.
Most studies of BCs have demonstrated that they reduce A1c in both stand-alone and pump-based applications by 0.2%-0.7% as summarized by Schmidt and Norgaard.20 Except in 1 instance where the insulin-on-board calculation may have been inaccurate,21 there was no increase in the rate of severe hypoglycemia. A recent systematic review showed that the number of correction doses and the frequency of mild hypoglycemia was reduced in those using a BC that was imbedded in their insulin pump although only 2 studies were ultimately included in that meta-analysis.22 BCs have been shown to reduce the fear of hypoglycemia as well as other factors that engender patients to improve their glycemic control including providing more flexibility and an improved quality of life.23
Limitations
It is helpful to remember what BCs cannot do whether they are phone application, meter-based, or pump-based. They do not take into consideration several factors that could affect the final dose recommendation. These include the glycemic index of the carbohydrates in the meal, the relative mix of carbohydrate, fat, and protein which may affect gastric emptying, and the rate of insulin absorption which may vary from injection to injection. The effect of exercise is also not included in BCs because the intensity and duration of the exercise, which have important and prolonged effects on insulin requirements, are difficult to quantitate without the input of external devices, for example, accelerometers.
Continuous Subcutaneous Insulin Infusion (CSII) Pumps
Benefits
Insulin pumps have been used by patients with type 1 diabetes for over 30 years. Their effectiveness in improving A1c has been demonstrated in a number of studies. Recent systematic reviews and meta-analyses of those studies24-26 come to different conclusions about the effectiveness of CSII to improve A1c perhaps because of the differences in the selection of the studies used in the meta-analyses. Indeed, Pickup and Sutton used studies in which the duration of treatment was sufficiently long and reported outcomes in sufficient detail to draw conclusions about effects on both hyper- and severe hypoglycemia. Importantly, they excluded studies in which the baseline hypoglycemia rates were already low.24 They found that CSII can reduce A1c by about 0.6% when compared to multiple daily injection (MDI) therapy (with either isophane/lente plus regular insulin or insulin glargine plus insulin lispro or aspart) in both adults and children. In a separate meta-analysis, Pickup found that in those patients whose severe hypoglycemia rate was over 18 episodes per year that those subjects on MDI had twice the risk of hypoglycemia compared to CSII in all patients and 4 times the risk in children.27 Thus, patient selection is critical in deciding whether or not it is appropriate to prescribe CSII to an individual.
There have been only a few randomized clinical trials investigating the effect of CSII versus MDI on severe hypoglycemia in patients with type 2 diabetes as summarized by Golden and colleagues.25 Their meta-analysis showed that there was a non–clinically significant decrease in A1c of -0.16%, a moderate reduction in mild hypoglycemia, and no reduction of severe hypoglycemia. In an observational trial, Frias and colleagues found that CSII reduced A1c by 1.2% without engendering any episodes of severe hypoglycemia.28 However, no pre-CSII hypoglycemia rates were reported in that study.
Limitations
While the improvement in A1c and the rate of severe hypoglycemia are important, there are numerous other factors that predict who will be a successful “pumper” as described by Walsh and Roberts.29 A useful questionnaire which can help identify patients who are most likely to be successful is contained in that book. Many of the factors that predict successful management of diabetes in general are, in fact, the same ones indicative of success in using an insulin pump. In addition, a variety of other factors—many of which are socioeconomic—determine who is prescribed an insulin pump. In a study of factors that influence the prescription of CSII in the United States, the Pediatric Diabetes Consortium consisting of 7 independent clinics found that race, income, private insurance status, and family structure were all independent factors in whether or not a patient was started on an insulin pump within 1 year of diagnosis.30 The T1D Exchange found that while CSII was more effective in reducing A1c in African American compared with whites, after controlling for socioeconomic status, CSII was prescribed at less than half the frequency in African American children and young adults compared with whites.31 Similar factors have been found in other countries. In Germany, for example, pediatric patients of Turkish origin are half as likely as native Germans to have an insulin pump prescribed which is independent of other socioeconomic factors.32
Real-Time Continuous Glucose Monitors (RT-CGM)
Benefits
The accuracy and usability of continuous glucose monitors (CGM) have gradually improved over the past decade. As opposed to retrospective CGM, real-time CGM (RT-CGM) devices offer opportunities to improve both hyper- and severe hypoglycemia because they provide actionable information to patients in “real-time.” In actuality, there is a delay of 5-15 minutes between the blood and interstitial glucose depending on the specific technology but such a lag time does not appear to result in a diminution of the benefits of these devices.33 Two recent meta-analyses of randomized controlled trials showed that RT-CGM was superior to self-monitoring of blood glucose (SMBG) in reducing A1c by almost 0.4% in both children and adults.25,34 It should be noted that these meta-analyses include some studies in which the patient’s insulin delivery was CSII in some cases effectively making them studies of sensor-augmented pumping (see below). RT-CGM appears to benefit patients over a range of A1cs, with the most benefit in those with the highest A1cs. The benefit of RT-CGM has also been demonstrated in an observational trial which showed an overall improvement in A1c of 0.4% over a wide range of baseline A1cs without an increase in the rate of mild or severe hypoglycemia.35
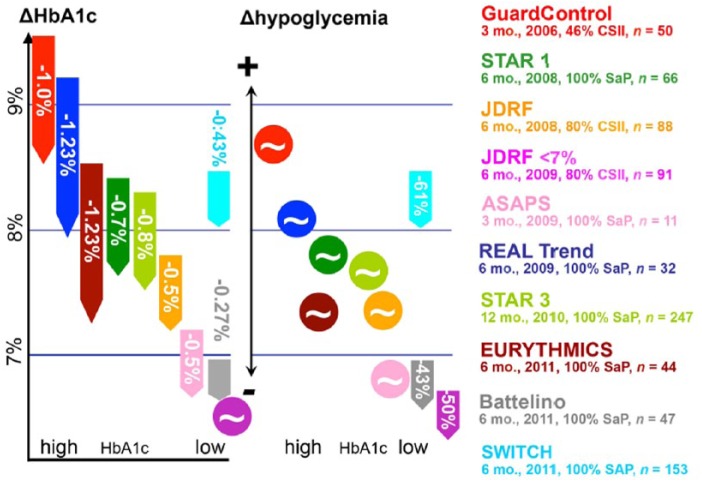
Whether or not RT-CGM improves the rate, severity and duration of hypoglycemia in patients with type 1 diabetes is not clear at this time. Neither the meta-analyses cited above found a significant change in the rate of mild or severe hypoglycemia in either adults or children perhaps because the primary endpoints of those studies was change in A1c. On the other hand, Floyd et al found a significant decrease in the duration of both mild and severe hypoglycemia and an increase in the time “in range” (70-180 mg/dl) in those using RT-CGM.34 Two studies in which reduction of hypoglycemia was the primary end-point did, in fact, demonstrate an improvement in hypoglycemia rates although this was in mild hypoglycemia.36,37 The severe hypoglycemia rate was low36 or zero37 in these studies. The relationship of A1c and hypoglycemia is beautifully depicted by Liebl and colleagues (Figure 1).38 The effect of RT-CGM on A1c reduction was, in general, proportional the baseline A1c. The reduction in the rate of hypoglycemia occurred in 3 studies—2 of which were those in which the A1c was already at or near goal. With higher A1cs there was a neutral effect on hypoglycemia. Finally, Choudhary et al found that RT-CGM almost eliminated episodes of severe hypoglycemia in 35 highly selected patients who had hypoglycemia unawareness.39 The majority of these patients were using an LGTS system, but there was no difference in the severe hypoglycemia rates between those who used the LGTS system (n = 23) compared to those who did not (n = 12).
Figure 1.
Combined effect of real-time continuous glucose monitoring on changes in A1c and hypoglycemia rates. Source: Liebl et al.38
Like studies using CSII, identifying the patients who are most likely to benefit from the technology is critical to demonstrating its effectiveness.
RT-CGM has also been used in patients with type 2 diabetes. Chico et al were among the first investigators to demonstrate that hypoglycemia was often unrecognized in patients with type 2 diabetes whether on MDI or oral agents.40 Approximately, 47% of their patients had hypoglycemia detected only with RT-CGM with 75% of those occurring during the night. RT-CGM improves A1c and/or reduces the frequency of hypoglycemic episodes in patients with type 2 diabetes taking prandial insulin.41-44 Yoo also showed that the reduction in A1c was accompanied by a reduction in glycemic variability without a significant increase in the rate of hypoglycemia.42 We showed that patients with type 2 diabetes not on prandial insulin (ie, they were either on oral agents, oral agents and insulin glargine, or insulin glargine alone), a 3-month “dose” of RT-CGM improved A1c at 3 months compared to SMBG and maintained its comparative efficacy over the next 9 months without an increase in hypoglycemia.44
Limitations
It is important to remember that the published results of the effects of RT-CGM most likely represent the best case estimates for use of the device since highly motivated patients are usually the ones who participate in clinical research trials. Even so, patients in those published studies did not always use their device as instructed. Several studies have documented a direct relationship between the reduction in A1c and hypoglycemia with the days per week of use in children.45-47 Adults 25 years of age and older benefit when they wear the device 60-70% of the time.48
While RT-CGM has been shown to be beneficial to many patients with type 1 diabetes, it has not been widely adopted to date. The penetration of device use in the United States is relatively small, ranging from 4% in adolescents to 21% in adults and it is discontinued by 41% of patients within 1 year.49 There are many possible reasons for this not the least of which is cost since even if the patient has insurance the out-of-pocket costs from copays can be a significant addition to the other substantial costs of managing diabetes. In fact, poor and low income persons have higher out-of-pocket costs (34%-35%) compared with high and middle income persons (10%-24%).50 The fact that CMS does not currently pay for RT-CGM precludes many patients with type 1 diabetes who are Medicare beneficiaries from adopting it and propels those already using the technology to abandon it because of the high costs associated with its use. In addition, there are other factors that may account for lack of adoption and/or abandonment of use. Ramchandani and associates investigated the limitations of RT-CGM in their urban, multiethnic pediatric patient population and found that fewer than half of their patients who were prescribed the device actually used it.51 The main reasons for discontinuation were problems with the equipment as well as concerns about its accuracy. In addition, many found it intrusive (both physically and psychosocially) and the insertion was painful. In those using CSII, some did not want an additional site.
Sensor-Augmented Pumps (SAP)
Benefits
The combination of RT-CGM and CSII—sensor-augmented pumping or SAP—would be predicted to be the best combination of advanced technologies for improving A1c and the rates and severity of hypoglycemia. Bergenstal and colleagues demonstrated that SAP produced an overall sustained reduction in A1c of 0.6% over 12 months compared to SMBG plus MDI in both adults and children.52 Most of the benefit was in adults whose A1c decreased by 1%, while it decreased 0.5% in children. There was a direct correlation of benefit with the time of sensor use with a 1.2% decrease in A1c in those using the RT-CGM 81-100% of the time. There was no improvement in the rate of hypoglycemia in this study. Among the reasons for this may be that the patients who had 2 or more severe hypoglycemic events in the previous year were excluded the trial and the overall hypoglycemia rate in the study group was 10% of that seen in the DCCT trial thus limiting the benefit that might have been seen in a higher risk population. This observation was confirmed in the meta-analysis by Golden et al,25 who found an A1c reduction of 0.68% in 4 studies using SAP but no improvement in mild or severe hypoglycemic events.
Limitations
The limitations described for CSII and RT-CGM individually also apply to SAP. In addition, the use of 2 devices requires 2 insertion sites which may be limited in some patients and, depending on the brands of pump and RT-CGM, the necessity of carrying using 2 independent display devices. The recent European approval of the Medtronic Duo—a single insertion platform which has both the RT-CGM sensor and the insulin infusion catheter—is a solution to the “real-estate” problem, but at the same time introduces the need to change the sensor at the same time as the infusion catheter thus limiting the time the sensor may be used.
Low-Glucose Threshold Suspend (LGTS) Systems
Benefits
LGTS systems are an extension of SAP and represents the first step toward an artificial pancreas. Bergenstal and colleagues reported a significant reduction in rate and severity of hypoglycemia during a 3 months trial in adults using an LGTS system compared to those using SAP without an increase in A1c in either group.53 There were important inclusion criteria for this study that inform us about who may benefit most from this technology. The inclusion criteria mandated that patients had to have been a pump user for at least the previous 6 months and had at least 2 nocturnal hypoglycemic events (≤65 mg/dl) lasting at least 20 minutes during the 2-week run-in period prior to randomization. These results were confirmed in a study by Ly and colleagues who found a reduction in the adjusted incidence rate of hypoglycemia after 6 months of use from 34.2 per 100 patient-months in the CSII group to 9.5 per 100 patient-months in the LGTS system.54 The severe hypoglycemia rate decreased from 2.2 per 100 patient-months to 0 per 100 patient-months and there was no change in the A1c.
Limitations
The limitations listed above for CGM and CSII are also operative here. There was no improvement in the quality of life in the ASPIRE study subjects but this may have been due to the relatively short duration (3 months) of the trial.
Low-Glucose Predictive Suspend (LGPS) Systems
Benefits
LGPS systems represent the next step in the advance toward the artificial pancreas. The LGPS system differs from the LGTS system in using a predictive algorithm to suspend insulin prior to reaching a predetermined threshold and thus would be expected to be more effective in preventing hypoglycemia but might result in hyperglycemia. There have been a limited number of outpatient studies to date using LGPS systems. Maahs and colleagues conducted a study of an LGPS system in 45 individuals with type 1 diabetes over 42 days.55 The study had a novel design in that the LGTS feature was randomly turned on or off on each of the 42 nights of the study and the patients were blinded to that action. It also included only patients who were at high risk to have hypoglycemia based on a run-in trial. They found that there was a 50% reduction in the rate of hypoglycemia and the duration of time with glucose levels under 60 mg/dl and 50 mg/dl were reduced from 23 to 7 minutes and 10 to 2 minutes, respectively. However, the mean overnight glucose was higher on the nights when the LGTS was activated (125 to 132 mg/dl) and the fasting blood glucose was also higher (144 mg/dl compared with 129 mg/dl). Given the relationship of blood glucose to A1c found in the ADAG study,56 it would be predicted that the A1c would increase by approximately 0.25%-0.5%. Concerns for the possibility that there would be large increases in blood glucose with ketosis were allayed by Beck and colleagues, who did not find a significant rate of ketonemia in a trial of an LGPS system overnight.57 The clinical significance particularly on long-term complication rates of the likely increase in A1c remains to be determined but might be mitigated by the fact that the study cohort already had a median A1c of 6.8%.
Limitations
Given the limited number of studies and their short duration, it is difficult to estimate at this time whether or not there will be any additional limitations of LGPS systems other than those of SAP and LGTS systems.
Artificial Pancreas (AP) Systems
Benefits
Several groups around the world are currently studying the benefits and risks of artificial pancreas (AP) systems. Studies have progressed from hospital settings to the outpatient world over the last 2 years. Most of these use only insulin as the therapeutic agent but at least 2 groups are using bi-hormonal systems of insulin and glucagon. The disadvantage of insulin-only systems is related to the pharmacokinetics of presently available insulin preparations. Nevertheless, Phillip and colleagues showed that in subjects who were randomly assigned to use either SAP or AP on consecutive nights, the AP system reduced both mean glucose (140 mg/dl to 126 mg/dl) and hypoglycemia rates (22 to 7 per night) compared to SAP.58 Leelarathna and colleagues using an insulin-only AP system found that subjects using an AP system spent significantly greater time in the range of 70-180 mg/dl compared with those on SAP (75% vs 62%) but there was no reduction in hypoglycemia over 7 days at home.59 Russell and colleagues used an insulin-glucagon AP system which they call the “bionic” pancreas” in adult and adolescent outpatients.60 The adults were under close observation but permitted to work, eat, exercise and sleep in the downtown Boston area. The adolescents were at a diabetes camp and closely supervised by the camp staff. Both groups had a reduction in their mean blood glucose (adults decreased by 26 mg/dl; adolescents decreased by 19 mg/dl) during the study. Extrapolated to a longer time horizon, this would correspond to a decrease in A1c of 0.8% and 0.6%, respectively. The adults but not the adolescents had a reduction in the hypoglycemia rate, perhaps due to the closer supervision and more rapid response to hypoglycemia that was provided to the adolescents by the camp staff. The number of carbohydrate interventions was significantly reduced (by 50%) in the adolescents.
Limitations
AP systems are rapidly progressing, but to date the number of subjects and duration of the trials are too limited to know what their limitations are. It will be critical to assess the robustness of the hardware and software in larger studies. This includes the efficacy of the alarms in these robotic systems since it is well-known that many patients disable alarms in their RT-CGM and CSII devices because of alarm fatigue.
Cost-Effectiveness
As noted above there are a number of barriers to using technology related to physical, socioeconomic, and educational factors. However, one of the most important at this time is cost since most of the currently available technologies that can improve hypoglycemia and A1c simultaneously are expensive although not dissimilar to the costs of newer pharmaceutical agents that have favorable hypoglycemia profiles such as glucagon-like peptide-1 (GLP-1) receptor antagonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose transporter-2 (SGLT-2) inhibitors. Ideally, there should be head-to-head studies comparing the efficacy and cost-effectiveness of an advanced technology to one of the newer pharmacologic agents.
In cost-effectiveness studies, a good value is considered to be an incremental cost-effectiveness ratio (ICER) of $50 000 per quality-adjusted life-years (QALY), although it may extend to as high as $300 000/QALY in the United States.61 Around the world, the threshold for QALY may differ from that in the United States. The World Health Organization suggests that the cost per QALY should be equal to 3 times the per-capita gross domestic product of a country.62
There have been a limited number of cost-effectiveness studies of CSII. A recent summary of the studies comparing CSII to MDI in adults and children with type 1 diabetes found that most of the technology interventions were cost-effective.63 SAP did not demonstrate cost-effectiveness,64 but that study modeled the results of the STAR 3 trial in adults which compared SAP to multiple dose insulin with self-monitoring of blood glucose not RT-CGM.65 Thus, the results did not really reflect the cost-effectiveness of the pump therapy since there were differences in both insulin delivery and glucose monitoring between the intervention and control groups. Studies analyzing cost-effectiveness of CSII in patients with type 2 have not been done, although David and colleagues found that the cost of the pump and supplies would be offset in 3 years because of lower insulin utilization.66
There have been 3 cost-effectiveness studies of RT-CGM in patients with type 1 diabetes (Table 3). Huang and colleagues modeled the Juvenile Diabetes Research Foundation (JDRF) RT-CGM trials but their calculations did not include any cost savings resulting from mitigation of severe hypoglycemia.67 They found that the technology was cost-effective with an ICER of $78 943/QALY for the A1c <7% cohort, that is, those patients who had a significant reduction in severe hypoglycemia. McQueen and colleagues found a somewhat lower ICER of $45 033/QALY but this also did not include cost-reduction from severe hypoglycemia avoidance.68 Ly and colleagues estimated the incremental cost of RT-CGM as part of a LGTS system and did include the costs associated with severe hypoglycemic events such as hospital admission, accidents, emergency visits, and ambulance calls. They found that the cost per QALY was AUS$40 803 in patients ≥12 years.69 Finally, Graham and colleagues, modeling the cost saving of RT-CGM using the same assumptions noted above,10-16 found that RT-CGM reduced the yearly cost of hypoglycemia by 13% in a population of 10 million insured patients.70 The cost-effectiveness of using RT-CGM in patients with type 2 diabetes not on prandial insulin was evaluated by Fonda and colleagues.71 They found that the ICER was $8896/QALY gained.
Table 3.
Cost-Effectiveness of Real-Time Continuous Glucose Monitoring in Patients With Type 1 Diabetes.
| Source | Setting/population | Cost per QALY gained |
|---|---|---|
| Huang et al67 | T1DM, Juvenile Diabetes Research Foundation-CGM trials, CGM vs SMBG, 2 cohorts: (1) A1c < 7%, all ages; and (2) A1c ≥ 7.0% and ≥ 25 years of age | When considering immediate quality of life benefit: |
| $98 679 for A1c ≥ 7.0% cohort and $78 943 for A1c < 7% cohort. | ||
| McQueen et al68 | T1DM, intensive insulin therapy with CGM (+SMBG) vs intensive insulin therapy with SMBG only, US | Using their individualized model: $45 033 |
| Ly et al68 | T1DM, sensor-augmented pump with low glucose suspend in hypoglycemic unaware patients | Over 6 months, cost per QALY gained is Australian $40 908 |
Conclusions
The advanced technologies reviewed herein have, in general, produced meaningfully improvements in A1c with either an improved/neutral effect on hypoglycemia, or they have reduced hypoglycemia rates with an improved/neutral effect on A1c or plasma glucose. The existing literature provides evidence to counter the assertions in Rosenthal’s New York Times article, which overlooked many important advantages of diabetes technologies, not the least of which is their effect on hypoglycemia since fear of hypoglycemia on the part of both patients and physicians is one of the major barriers that preclude more aggressive glycemic management In addition, it is clear that hypoglycemia is costly. It not only represents a significant component of the overall costs of diabetes treatment, but by preventing the A1c lowering in many patients it may also limit the ability to reduce the costly micro- and macrovascular complications. Finally, our A1c-centric world misses the boat on how to best assess an intervention whether it be one using technology or pharmacotherapy. It behooves us to find a single metric that incorporates the effect on both A1c and hypoglycemia to assess an intervention so that patients, providers, payers, regulators, and the lay press can properly understand the full impact of a new therapy.
Footnotes
Abbreviations: A1c, hemoglobin A1c; AP, artificial pancreas; BC, bolus calculator; CMS, Center for Medicare and Medicaid Services; CSII, continuous subcutaneous insulin infusion; DCCT, Diabetes Control and Complications Trial; DPP-4, dipeptidyl peptidase-4; FDA, Food and Drug Administration; GLP-1, glucagon-like peptide-1; ICER, incremental cost-effectiveness ratio; JDRF, Juvenile Diabetes Research Foundation; LGPS, low-glucose predictive suspend; LGTS, low-glucose threshold suspend; MDI, multiple daily injection; QALY, quality-adjusted life-years; RT-CGM, real-time continuous glucose monitor; SAP, sensor-augmented pump; SGLT-2, sodium-glucose transporter-2; SMBG, self-monitoring of blood glucose.
Author’s Note: The opinions expressed in this article reflect the personal views of the authors and not the official views of the United States Army or the Department of Defense.
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Rosenthal E. Even small medical advances can mean big jumps in bills. New York Times. April 5, 2014. Available at: http://www.nytimes.com/2014/04/06/health/even-small-medical-advances-can-mean-big-jumps-in-bills.html?_r=0. Accessed September 23, 2014.
- 2. Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin-related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med. 174:678-86, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Eng J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 4. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Eng J Med. 2005;353:2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271-276. [PubMed] [Google Scholar]
- 6. Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D exchange clinic registry. J Clin Endo Metab. 2013;98:3411-3419. [DOI] [PubMed] [Google Scholar]
- 7. Lipska KJ, Warton EM, Huang ES, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes. Diabetes Care. 2013;36:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endo Metab. 2013;98:1845-1859. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;36(suppl 1):S14-S80. [DOI] [PubMed] [Google Scholar]
- 10. Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med. 2008:25:501-504. [DOI] [PubMed] [Google Scholar]
- 11. Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2010;87:64-68. [DOI] [PubMed] [Google Scholar]
- 12. Choudhary P, Geddes J, Freeman JV, Emery CJ, Heller SR, Frier BM. Frequency of biochemical hypoglycaemia in adults with type 1 diabetes with and without impaired awareness of hypoglycaemia: no identifiable differences using continuous glucose monitoring. Diabet Med. 2010;27:666-672. [DOI] [PubMed] [Google Scholar]
- 13. Henderson JN, Allen KV, Deary IJ, Frier BM. Hypoglycaemia in insulin-treated type 2 diabetes: frequency, symptoms and impaired awareness. Diabet Med. 2003;20:1016-1021. [DOI] [PubMed] [Google Scholar]
- 14. Leiter Al, Yale FJ, Chiasson JL, Harris S, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes in glycemic and hypoglycemia management. Can J Diabetes. 2005; 29:186-192. [Google Scholar]
- 15. Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care. 2011;17:673-680. [PubMed] [Google Scholar]
- 16. Brod M, Wolden M, Christensen T, Bushnell DM. Understanding the economic burden of nonsevere nocturnal hypoglycemic events: impact on work productivity, disease management, and resource utilization. Value Health. 2013;16:1140-1140. [DOI] [PubMed] [Google Scholar]
- 17. Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Int Med. 2008;148:737-746. [DOI] [PubMed] [Google Scholar]
- 18. Sussman A, Taylor EJ, Patel M, et al. Performance of a glucose meter with a built-in automated bolus calculator versus manual bolus calculation in insulin-using subjects. J Diabetes Sci Technol. 2012;6:330-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diabetes In Control. Updated bolus calculators for diabetes management. Issue 721, March 20, 2014. Available at: http://www.diabetesincontrol.com/articles/diabetes-news/16063-updated-bolus-calculators-for-diabetes-management-. Accessed October 2014.
- 20. Schmidt S, Norgaard K. Bolus calculators. J Diabetes Sci Technol. 2014;8:1035-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garg SK, Bookout TR, McFann KK, et al. Improved glycemic control in intensively treated adult subjects with type 1 diabetes using insulin guidance software. Diabetes Technol Ther. 2008;10:369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramotowska A, Golicki D, Dzygalo K, Szypowska A. The effect of using the insulin pump bolus calculator compared to standard insulin dosage calculations in patients with type 1 diabetes mellitus—a systematic review. Exp Clin Endocrinol Diabetes. 2013;121:248-254. [DOI] [PubMed] [Google Scholar]
- 23. Barnard K, Parkin C, Young A, Ashraf M. Use of an automated bolus calculator reduces fear of hypoglycemia and improves confidence in dosage accuracy in patients with type 1 diabetes mellitus treated with multiple daily insulin injections. J Diabetes Sci Technol. 2012;6:144-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25:765-774. [DOI] [PubMed] [Google Scholar]
- 25. Golden SH, Brown T, Yeh HC, et al. Methods for insulin delivery and glucose monitoring: comparative effectiveness. Comparative Effectiveness Review 57, AHRQ Publication 12-EHC036-EF. Rockville, MD: Agency for Healthcare Research and Quality; July 2012. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 26. Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab. 2009;94(3):729-740. [DOI] [PubMed] [Google Scholar]
- 27. Pickup J. The evidence base for diabetes technology: appropriate and inappropriate meta-analysis. J Diabetes Sci Technol. 2013;7:1567-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frias JP, Bode BW, Bailey TS, Kipnes, Brunelle R, Edelman SV. A 16-week open-label, multicenter pilot study assessing insulin pump therapy in patients with type 2 diabetes suboptimally controlled with multiple daily injections. J Diabetes Sci Technol. 2011;5:887-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walsh J, Roberts R. Pumping Insulin. 5th ed. San Diego, CA: Torrey Pines Press; 2012. [Google Scholar]
- 30. Lin MH, Connor CG, Ruedy KJ, et al. Race, socioeconomic status, and treatment center are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes. Diabetes Technol Ther. 2013;15:929-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klingensmith GJ, Miller KM, Beck RW, et al. Racial disparities in insulin pump therapy and hemoglobin A1c among T1D exchange participants. Diabetes. 2012;61(suppl 1):A358. [Google Scholar]
- 32. Icks A, Razum O, Rosenbauer J, et al. Lower frequency of insulin pump treatment in children and adolescents of Turkish background with type 1 diabetes: analysis of 21,497 patients in Germany. Diabetes Technol Ther. 2012;14:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scuffi C, Lucarelli F, Valgimigli F. Minimizing the impact of time lag variability on accuracy evaluation of continuous glucose monitoring systems. J Diabetes Sci Technol. 2012;6:1383-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Floyd B, Chandra P, Hall S, et al. Comparative analysis of the efficacy of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes mellitus J Diabetes Sci Technol. 2012;6:1094-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1c with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9:203-210. [DOI] [PubMed] [Google Scholar]
- 36. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Hirsch IB, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liebl A, Henrichs HR, Heinemann L, et al. Continuous glucose monitoring: evidence and consensus statement for clinical use. J Diabetes Sci Technol. 2013;7:500-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choudhary P, Ramasamy S, Green L, et al. Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care. 2013;36:4160-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chico A, Vidal-Rios P, Subira M, Novials The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurement for improving metabolic control. Diabetes Care. 2003;26:1153-1157. [DOI] [PubMed] [Google Scholar]
- 41. Zick R, Petersen B, Richter M, Haug C, SAFIR Study Group. Comparison of continuous blood glucose measurement with conventional documentation of hypoglycemia in patients with type 2 diabetes on multiple daily insulin injection therapy. Diabetes Technol Ther. 2007;9:483-492. [DOI] [PubMed] [Google Scholar]
- 42. Yoo HJ. RT-CGM as a motivational tool for poorly controlled type 2. Diabetes Res Clin Pract. 2008;82:73-79. [DOI] [PubMed] [Google Scholar]
- 43. Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44-50. [DOI] [PubMed] [Google Scholar]
- 44. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Diabetes Research in Children Network (DirecNet) Study Group, Buckingham B, Beck RW, et al. Continuous glucose monitoring in children with type 1 diabetes. J Pediatr. 2007;15:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Danne T, de Valk HW, Kracht T, et al. Reducing glycaemic variability in type 1 diabetes self-management with a continuous glucose monitoring system based on wired enzyme technology. Diabetologia. 2009;52:1496-1503. [DOI] [PubMed] [Google Scholar]
- 47. Diabetes Research in Children Network Study Group, Weinzimer S, Xing D, et al. Prolonged use of continuous glucose monitors in children with type 1 diabetes on continuous subcutaneous insulin infusion or intensive multiple-daily injection therapy. Pediatr Diabetes. 2009;10:91-96. [DOI] [PubMed] [Google Scholar]
- 48. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 49. Wong JC, Foster NC, Maahs DM, et al. Real-time continuous glucose monitoring among participants in the T1D Exchange Clinic Registry. Diabetes Care. 2014;37:2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li R, Barker LE, Shrestha S, et al. Changes over time in high out-of-pocket health care burden in U.S. adults with diabetes 2001-2011. Diabetes Care. 2014;37:1620-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramchandani N, Arya S, Ten S, Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Technol. 2011;5:860-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bergenstal R, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Eng J Med. 2010;363:311-320. [DOI] [PubMed] [Google Scholar]
- 53. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia N Eng J Med. 2013;369:224-232. [DOI] [PubMed] [Google Scholar]
- 54. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes. JAMA. 2013;310:1240-1247. [DOI] [PubMed] [Google Scholar]
- 55. Maahs DM, Calhoun P, Buckingham BA, et al. A randomized trial of a home system to reduce nocturnal hypoglycemia in type 1 diabetes. Diabetes Care. 2014;37:1885-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beck RW, Raghinaru D, Wadwa RP, et al. Frequency of morning ketosis after overnight insulin suspension using an automated nocturnal predictive low glucose suspend system. Diabetes Care. 2014;37:1224-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Eng J Med. 2013;368:824-833. [DOI] [PubMed] [Google Scholar]
- 59. Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931-1937. [DOI] [PubMed] [Google Scholar]
- 60. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Eng J Med. 2014;371:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349-356. [DOI] [PubMed] [Google Scholar]
- 62. Baltussen RM, Adam T, Tan Torres T, et al. Generalized Cost-Effectiveness Analysis: A Guide. Global Programme on Evidence for Health Policy. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 63. Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists/American College of Endocrinology insulin pump management task force. Endo Pract. 2014;20:463-489. [DOI] [PubMed] [Google Scholar]
- 64. Kamble S, Schulman KA, Reed SD. Cost-effectiveness of sensor-augmented pump therapy in adults with type 1 diabetes in the United States. Value Health. 2012;15:632-638. [DOI] [PubMed] [Google Scholar]
- 65. Davis SN, Horton ES, Battelino T, Rubin RR, Schulman KA, Tamborlane WV. STAR 3 randomized controlled trial to compare sensor-augmented insulin pump therapy with multiple daily injections in the treatment of type 1 diabetes: research design, methods, and baseline characteristics of enrolled subjects. Diabetes Techno Ther. 2010;12:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. David G, Shafiroff J, Saulnier A, Gunnarsson C. Multiple daily injection therapy (MDI) versus durable insulin pump therapy in type II diabetics: a breakeven analysis. Value Health. 2012;15:A65. [Google Scholar]
- 67. Huang ES, O’Grady M, Basu A, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2010;33:1269-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McQueen RB, Ellis SL, Campbell JD, Nair KV, Sullivan PW. Cost-effectiveness of continuous glucose monitoring and intensive insulin therapy for type 1 diabetes. Cost Eff Resour Alloc. 2011;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ly TT, Brnabic AJM, Eggleston A, et al. Cost-effectiveness of sensor-augmented insulin pump and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17:561-569. [DOI] [PubMed] [Google Scholar]
- 70. Graham C, Bowman L, Lewis J, Murphy B. Decision analytic model: cost implications of RT-CGM use in insulin requiring patients with hypoglycemic unawareness. Paper presented at: 7th International Conference on Advanced Technologies and Treatments for Diabetes; February 2014; Vienna, Austria. [Google Scholar]
- 71. Fonda SJ, Graham C, Samyshkin Y, Munakata J, Powers J, Price D, Vigersky RA. Cost-effectiveness of real-time continuous glucose monitoring (RT-CGM) in type 2 diabetes (T2DM). Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]