Abstract
The efficient diagnosis and accurate monitoring of diabetic patients are cornerstones for reducing the risk of diabetic complications. The current diagnostic and prognostic strategies in diabetes are mainly based on two tests, plasma (or capillary) glucose and glycated hemoglobin (HbA1c). Nevertheless, these measures are not foolproof, and their clinical usefulness is biased by a number of clinical and analytical factors. The introduction of other indices of glucose homeostasis in clinical practice such as fructosamine and glycated albumin (GA) may be regarded as an attractive alternative, especially in patients in whom the measurement of HbA1c may be biased or even unreliable. These include patients with rapid changes of glucose homeostasis and larger glycemic excursions, and patients with red blood cell disorders and renal disease. According to available evidence, the overall diagnostic efficiency of GA seems superior to that of fructosamine throughout a broad range of clinical settings. The current method for measuring GA is also better standardized and less vulnerable to preanalytical variables than those used for assessing fructosamine. Additional advantages of GA over HbA1c are represented by lower reagent cost and being able to automate the GA analysis on many conventional laboratory instruments. Although further studies are needed to definitely establish that GA can complement or even replace conventional measures of glycemic control such as HbA1c, GA may help the clinical management of patients with diabetes in whom HbA1c values might be unreliable.
Keywords: diabetes, glycated hemoglobin, fructosamine, glycated albumin
Diabetes is one of the most severe and frequent human disorders. According to recent statistics, this condition afflicts as many as 382 million persons around the globe, with an estimated prevalence of approximately 8.3% in 2013. At variance with other frequent pathologies such as cardiovascular disease and bacterial infections, the trend toward an increased prevalence is not expected to soon reverse. Worldwide, as many as 592 million individuals may be affected by diabetes in 2035, a remarkable 55% increase in prevalence over the next 2 decades.1 Due to its high global prevalence and severe, frequently life-threatening complications (eg, retinopathy, nephropathy, neuropathy, cardiovascular disease), diabetes must be regarded as a serious and increasing global health burden.
The most recent Standards of Medical Care in Diabetes published by the American Diabetes Association (ADA) emphasize that early diagnosis and monitoring are critical for preventing or delaying the onset of acute complications and lowering the risk of long-term complications of diabetes.2 The current diagnostic criteria for this condition are based on the presence of (1) glycated hemoglobin (HbA1c) value ≥6.5% (ie, ≥48 mmol/mol), (2) fasting plasma glucose (FPG) ≥126 mg/dL (ie, ≥7.0 mmol/L), (3) 2-hour plasma glucose ≥200 mg/dL (ie, ≥11.1 mmol/L) during an oral glucose tolerance test (OGTT) using a 75 g glucose load, or (4) random plasma glucose ≥200 mg/dL (ie, ≥11.1 mmol/L). An increased risk of diabetes (ie, prediabetes) is defined in the presence of (1) HbA1c value between 5.7-6.4% (ie, 39-46 mmol/mol), (2) FPG between 100-126 mg/dL (ie, 5.6-6.9 mmol/L), (3) 2-hour plasma glucose between 140-199 mg/dL (ie, 7.8-11.0 mmol/L) during an OGTT. With regard to diabetes monitoring, the glycemic targets for nonpregnant adults with diabetes include HbA1c value <7.0% (ie, <53 mmol/mol), preprandial capillary plasma glucose between 70-130 mg/dL (ie, 3.9-7.2 mmol/L), and peak postprandial capillary plasma glucose <180 mg/dL (ie, <10.0 mmol/L).
According to these widespread recommendations, the current diagnostic and prognostic strategies in diabetes are strongly based on two historical tests, plasma (or capillary) glucose and HbA1c. Both these measures are not foolproof.3 FPG is highly vulnerable to a number of preanalytical variables including recent food ingestion, sample storage, high within-subject biological variability, acute stress and diurnal variations, common drugs which influence glucose metabolism such as corticosteroids, fibrates, cyclosporine, beta-blockers, sulfamethoxazole, thiazide diuretics, and thyroid hormones, among others.4 With regard HbA1c, well-recognized drawbacks include a lower diagnostic performance in specific populations such as pregnant women, the elderly and non-Hispanic blacks, the risk of overdiagnosing diabetes in the presence of iron deficiency anemia (ie, hemoglobin level lower than 130 g/L in males and 120 g/L in females, respectively),4 and in subjects genetically predisposed to hyperglycation,5 the uncertain significance of this measure in subjects with increased red blood cell turnover (eg, hemolytic anemia, major blood loss, athletes), end-stage renal disease or heavy alcohol consumption, the interference from hemoglobin variants, potentially larger analytical imprecision when not using high pressure liquid chromatography (HPLC), and the higher costs compared to glucose measurement.6 In particular, genetic variants such as hemoglobin S and C traits or elevated fetal hemoglobin along with chemically modified derivatives of hemoglobin (eg, carbamylated hemoglobin in patients with impaired renal function) can substantially reduce the accuracy of HbA1c measurements. The bias is mainly dependent on the specific hemoglobin variant and method used for measuring HbA1c.6
Interestingly, the ADA has acknowledged that in patients in whom HbA1c and blood glucose are unreliable (especially those with hemoglobinopathies, altered red cell turnover or impaired renal function), the assessment of other indices of chronic glycemia may be advisable, although their relation with average glucose and prognosis remains uncertain.2 These alternative measures essentially include fructosamine and glycated albumin (GA). As such, the aim of this article is to provide an overview of the molecular and biological properties of these emerging biomarkers, along with a succinct description of the main studies that have investigated the role of fructosamine and GA in diabetes.
Biochemistry and Biology of Fructosamine and Glycated Albumin
Human serum albumin is the most abundant extracellular protein in plasma, accounting for 60-70% of total serum proteins. It is a globular protein with a molecular mass of 67 KDa and a serum half-life of approximately 20 days. The protein consists of 585 amino acids residues organized in a single polypeptide chain stabilized by 17 disulphide bridges and comprising 3 homologous domains (I, II, and III) assembled to form a heart-shaped molecule. Each domain is further organized into 2 subdomains (A and B), which share analogous structural motifs.7 The maintenance of osmotic pressure is the major function of albumin. Besides its role as a protein reservoir, a third function is attributable to the ability to bind, stabilize and transport metabolic products, regulatory mediators, nutrients, ions, and other proteins. In addition, human serum albumin interacts with lipid metabolism (ie, free fatty acids are transported in the blood bound to albumin), sequesters endogenous or exogenous toxins, and acts as a putative antioxidant compound.8
Because of its high sensitivity to glycation, the interest in this multifunctional protein has increased exponentially over the last decade, as a biomarker of hyperglycemia. Glycation is a nonenzymatic process, also known as Maillard reaction, in which glucose and other sugars react spontaneously with free amino terminal residues of serum proteins, specifically lysine and arginine.9 Initially, the condensation of the free aldehyde group of the carbohydrate in its open (acyclic) form with the N-terminal amino acid of the protein forms a reversible Schiff base product, the aldimine intermediate. This product may be reconverted to glucose and protein or undergo an Amadori rearrangement to form a fructosamine derivative by a stable, though slightly reversible, ketoamine linkage (Figure 1). The term “fructosamine,” therefore, typically refers to all ketoamine linkages that result from glycation of serum proteins.
Figure 1.
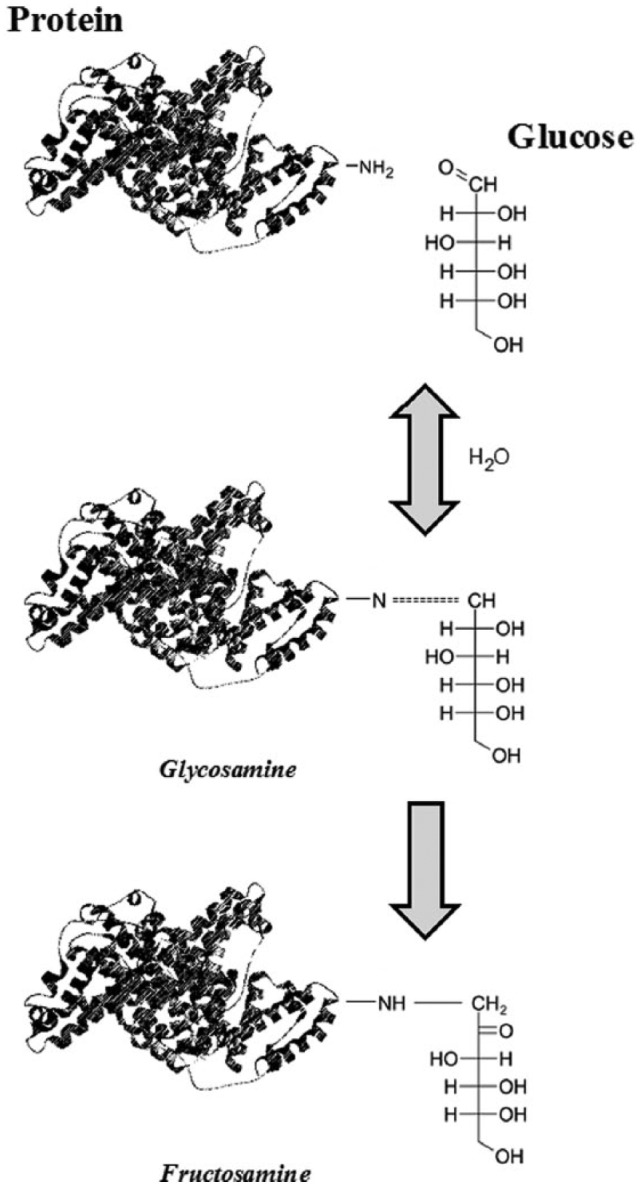
Mechanism of fructosamine (and glycated albumin) formation.
Because albumin is the most abundant of serum protein, fructosamine is predominantly a measure of GA, although other circulating proteins such as glycated lipoproteins and glycated globulins may contribute to determine the total concentration of fructosamine. Both fructosamine and GA levels increase in states of abnormally high glucose concentrations such as diabetes, and can hence be used for assessing glucose control over a short to intermediate time frame. With respect to hemoglobin, whose life span in red blood cells is of approximately 90-120 days, nonimmunoglobulin serum proteins have a much lower half-life, approximately 14-21 days.10 This implicitly means that while HbA1c provides a long-term record of glycemic control (ie, over a period of 2-3 months), the measurement of fructosamine or GA provides information on glucose control mostly limited to the previous 2 weeks.10 Another important difference with HbA1c is the rate of nonenzymatic glycation of albumin, which is approximately 9- to 10-fold higher than that of human hemoglobin.11,12
As a consequence of the greater susceptibility to glycation of albumin and other plasma proteins compared to intracellular proteins such as hemoglobin, the blood levels of GA exhibit a broader fluctuation than those of HbA1c, thus allowing an earlier detection of rapid changes of blood glucose.12 Accordingly, the measurement of fructosamine and GA seems useful not only as an alternative index of glycemic control in conditions in which HbA1c is unreliable, but also for identifying impaired control of blood glucose before any noticeable changes in HbA1c may occur,10,13 as well as for monitoring diabetics with fluctuating and/or poorly controlled diabetes.14
A number of methods have been developed for the assessment of fructosamine in serum and plasma. Colorimetric-based assays are indeed the most widely used and those better standardized, and typically exploit the unique property of fructosamine to be a reducing agent under alkaline conditions. The first technique, developed in 1983, was based on the reduction of the dye nitroblue tetrazolium (NBT) to formazane. The rate of formazane formation, which is directly proportional to the fructosamine concentration, can then be monitored with spectrophotometric technique.15 The test has been considerably improved in 1990, by addition of a nonionic detergent containing uricase which eliminated the interference from uric acid and polylysine, thus allowing a more accurate and sensitive measurement.16 The modified assay is currently available and broadly used in clinical laboratories. Although rapid, technically easy, inexpensive, and available for automation, the method is however affected by changes in ambient temperature and remains poorly standardized. Moreover, due to the technical nature of assay, all molecules with reducing activity such as bilirubin and vitamins may interfere in the measurement, thus biasing test results especially when present in large concentrations.
The concentration of GA can be directly measured by several methods, including boronate affinity chromatography, ion exchange chromatography, high performance liquid chromatography and immunoassays (eg, enzyme-linked immunosorbent assays or radioimmunoassays). A number of alternative methods have been developed, including Raman spectroscopy,17 refractive index measurements,18 capillary electrophoresis,19 and other electrophoretic techniques,20 but their usefulness in clinical practice has been challenged by requirements for dedicated instrumentation and poor analytical performance. Recently, a user-friendly, highly accurate and automated enzymatic assay (Lucica GA-L kit, Asahi Kasei Pharma, Tokyo, Japan) has been developed.21 The method is based on initial elimination of endogenous glycated amino acids and peroxide by a ketoamine oxidase, which is then followed by a peroxidase reaction.22 GA is then hydrolyzed by an albumin-specific proteinase and the products of this reaction are oxidized by ketoamine oxidase. The derived hydrogen peroxide is then measured quantitatively by a colorimetric method. The albumin concentration is concurrently measured with the bromocresol purple technique. The final result is expressed as ratio of glycated to total albumin. The assay can be implemented on a large number of automated clinical chemistry analyzers, and offers optimal analytical performances in term of linearity, recovery and precision.23,24 It is also noteworthy that the preliminary purification step enhances the specificity of GA assessment and makes it less vulnerable to interference from endogenous glycated amino acids.22 With respect to the NBT method used for fructosamine quantification, the GA enzymatic assay is better standardized and more precise,24 and is not influenced by the concentration of bilirubin in the specimen.
Some physiological and pathological conditions can significantly influence the metabolism of both fructosamine and GA. In brief, all those clinical conditions that affect protein metabolism potentially influence the concentrations of glycated proteins. In particular, the blood levels of fructosamine and GA may be modified in patients with protein losing states such as nephrotic syndrome, diminished protein production (ie, hepatic cirrhosis) and thyroid disease.25 However, GA levels can be presented as a ratio (ie, percentage) of total albumin, while fructosamine levels are not generally corrected for albumin or total protein concentration. Thus, physiologic or pathologic conditions linked to hypo-proteinemia (ie, pregnancy or malnutrition) are more likely to affect the concentration of fructosamine. Another disadvantage of fructosamine is that its concentration is considerably influenced by the levels of immunoglobulins), especially IgA, which are present in abnormal concentration in a broad range of clinical conditions.26
When tested with the above mentioned reference methods, fructosamine and GA were found to be highly correlated (ie, r = .86).27 Nevertheless, given the higher specificity and accuracy, GA testing is currently preferred over that of fructosamine.
Clinical Studies About Glycated Albumin and Fructosamine
Glycated Albumin and Fructosamine for Diabetes Screening and Diagnosis
Despite the unquestionable utility of HbA1c in diabetes mellitus, several studies have highlighted a number of limitations in patients affected by microvascular and macrovascular complications, as well as in special patient populations. In these conditions the use of alternative markers may overcome the drawbacks of HbA1c, by providing additional information about shorter-term glycemic control.28 In particular, the measurement of fructosamine and GA has been proposed to improve diagnosis and monitoring of diabetes, alone or in combination with HbA1c.29-31 Moreover, since both fructosamine and GA are associated with the future risk of diabetes independent of FPG and HbA1c,32,33 they have also been proposed in diabetes risk prediction, especially in subjects with prediabetes.34
Shima et al used HbA1c, fructosamine, and GA to screen for diabetes in 302 adults,35 and concluded that the plasma levels of GA and HbA1c, but not fructosamine, could efficiently identify subjects at risk of diabetes. In a community-based Japanese population study including 1575 subjects, Furusyo et al reported that GA was useful for screening diabetes in the general population. A GA cutoff of >15.5% showed acceptable diagnostic performance for identifying early-phase diabetes (0.91 area under the curve [AUC], 0.83 sensitivity, and 0.83 specificity).36 Li and colleagues obtained similar results in the screening of 1480 Chinese outpatients.37 Serum GA exhibited an overall acceptable diagnostic performance (AUC of 0.88), and a level ≥17.1% was identified as the most efficient threshold for performing confirmatory OGTTs. In the Atherosclerosis Risk in Communities (ARIC) Study including 1600 participants (227 with a history of diabetes and 1323 without), Selvin et al also showed that GA and fructosamine were strongly associated with the subsequent risk of diabetes.38 In particular, diabetic patients in the highest tertile of GA exhibited an odds ratio (OR) of 3.9 and 9.3 for developing albuminuria and retinopathy compared to those in the lowest tertile. Similarly, diabetic patients in the highest tertile of fructosamine exhibited an OR of 5.9 and 6.3 for developing albuminuria and retinopathy compared to those in the lowest tertile. In a following community-based population cross-sectional study including 1211 subjects, Yang et al investigated the role of GA for predicting undiagnosed diabetes,39 and found that the AUC of this biomarker was virtually identical to that of FPG (0.86 versus 0.88). A cutoff of 15.7% exhibited 0.73 sensitivity and 0.80 specificity for diagnosing diabetes. In a cross-sectional and longitudinal study including 10 987 subjects, Malmström and colleagues showed that fructosamine was effective in discriminating subjects with and without diabetes (AUC, 0.95), displaying 0.61 sensitivity and 0.97 specificity at a threshold level of 2.5 mmol/L (Table 1).40
Table 1.
Summary of Clinical Studies Investigating the Clinical Usefulness of Fructosamine and Glycated Albumin in Diabetes.
| Authors | Endpoint | n | Outcome | Reference |
|---|---|---|---|---|
| Shima et al 1989 | Screening of impaired glucose tolerance | 302 | GA: Significantly higher values in subjects with impaired glucose tolerance FA: Values nonsignificantly different between controls and subjects with impaired glucose tolerance |
35 |
| Furusyo et al 2011 | Screening of diabetes | 1575 | GA: AUC of 0.91 for screening diabetes | 36 |
| Li et al 2011 | Screening of diabetes | 1480 | GA: AUC of 0.88 for screening diabetes | 37 |
| Selvin et al 2011 | Prediction of diabetic complications | 1600 | GA: OR of 3.8-9.3 for diabetic complications FA: OR of 5.9-6.3 for diabetic complications |
38 |
| Yang et al 2012 | Screening of diabetes | 1211 | GA: AUC of 0.86 for screening diabetes | 39 |
| Malmström et al 2014 | Screening of diabetes | 10987 | FA: AUC of 0.95 for screening diabetes | 40 |
AUC, area under the curve; FA, fructosamine; GA, glycated albumin; OR, odds ratio.
Glycated Albumin and Fructosamine in Therapeutic Monitoring of Diabetes
As highlighted in a previous part of this article, the level of GA is strongly dependent on recent changes of blood glucose, but also reflects very rapid variations that cannot be accurately identified measuring blood glucose.41 The concentration of GA also decreases more rapidly than that of HbA1c during intensive insulin therapy, so that it can be of value for monitoring glycemic control during treatment with hypoglycemic agents and insulin.42,43 Moreover, continuous glucose measurements were more tightly correlated to GA compared to HbA1c.44,45 Since fructosamine reflects the average levels of blood glucose during the former 1 to 3 weeks, fructosamine would also expectedly mirror a poorly controlled glucose metabolism better than HbA1c.40,46,47
Lindsey et al investigated fructosamine and HbA1c in a prospective, randomized, multicenter, controlled trial including 72 diabetic patients,48 and showed that the combination of weekly fructosamine testing and daily blood glucose monitoring were no better than daily glucose monitoring alone. Subsequent studies demonstrated the utility of fructosamine and GA in diabetic patients who required tighter control, or in patients with conditions that rendered HbA1c testing unreliable such as gestational diabetes mellitus, postprandial hyperglycemia or gastric resection.49-51 Pu et al also studied 320 consecutive patients with type 2 diabetes,52 and showed that GA level was a significant predictor of coronary artery disease, exhibiting a diagnostic performance that exceeded that of HbA1c (AUC, 0.62 vs 0.53).
Glycated Albumin and Fructosamine in Diabetic Patients Affected by Chronic Kidney Disease
The anemias associated with chronic kidney disease (CKD) are usually accompanied by increased red cell turnover. Patients with CKD are frequently treated with iron and/or erythropoietin therapy or blood transfusion, so that the measurement of HbA1c might be unreliable.53-56
Since GA is not influenced by anemia and associated treatments, GA is now considered a superior index of glycemic control in patients on predialysis or dialysis.57 Peacock et al measured GA and HbA1c in 307 diabetic subjects (258 on hemodialysis and 49 without overt renal disease),58 and showed that the dialysis status had a substantial impact on HbA1c levels, but not on GA concentration. HbA1c levels significantly underestimated glycemic control in diabetic hemodialysis patients, whereas GA more accurately reflected glucose homeostasis. Interestingly, Chen et al reported that the estimated average glucose calculated from HbA1c and fructosamine substantially underestimated the mean blood glucose levels in patients with CKD stages 3-4.59 Accordingly, Freedman et al also showed that HbA1c was inversely associated with glomerular filtration rate (GFR) in patients with CKD disease stages 3 and 4, whereas GA was not significantly associated with GFR (r = –.08, P = .24).60
These findings were supported by Sany et al, who studied 50 hemodialyzed patients (25 with diabetes),61 and concluded that classification of glycemic control into quartiles of GA better reflected glycemic control than HbA1c. It was also shown that GA, but not HbA1c, is predictive of mortality and hospitalization in dialysis patients with diabetes,56 and that GA levels ≥29% are strongly predictive of cardiovascular death in diabetic patients undergoing hemodialysis (hazard ratio [HR], 2.97, P = .038).54 In a national prospective cohort study including 503 participants with a median follow-up of 3.5 years, Shafi et al demonstrated that an increased value of serum GA is significant a risk factor for all-cause mortality (HR, 1.40; 95% CI, 1.09-1.80), cardiovascular death (HR, 1.55; 95% CI, 1.09-2.21) and sepsis (HR, 1.39; 95% CI, 0.94-2.06).62
Unlike GA, a large number of clinical trials have reported poor correlations between fructosamine and glycemic control in patients with renal failure. Nunoi et al63 and Morgan and colleagues64 demonstrated that fructosamine is not a reliable marker of medium-term integrated blood glucose in diabetic patients with CKD. In a study of 23 diabetic hemodialysis patients, Joy et al65 showed that fructosamine was not significantly associated with long-term glycemic control in diabetic patients receiving hemodialysis (r = .345, P = .11).
Glycated Albumin and Fructosamine in Gestational Diabetes Mellitus
In pregnancy, HbA1c exhibits biphasic changes, decreasing between the first and second trimester and increasing in the third.66 This pattern has been attributed to decreased blood glucose in the first trimester, which is then followed by a relative iron deficiency. To reduce adverse maternal and fetal outcomes,67 the measurement of GA or fructosamine offers advantages over HbA1c in gestational diabetes mellitus.
Unlike HbA1c, GA is not influenced by the iron deficiency of pregnancy, so GA better reflects average glucose.68,69 Interestingly, Pan et al measured HbA1c and GA in a cross-sectional and hospital-based study of 713 pregnant Chinese women with an abnormal 50-g oral glucose-screening test,70 and reported that GA was independently associated with 0- and 120-min blood glucose.
As in other clinical settings, mixed evidence has been reported on the utility of fructosamine in screening and monitoring of gestational diabetes mellitus. Khan et al measured FPG and serum fructosamine in 165 pregnant women,71 and found that FPG and fructosamine could identify high-risk individuals to be screened with the OGTT avoiding unnecessary glucose challenges. At variance with these findings, Li et al measured fructosamine in 161 pregnant women,72 and reported that this biomarker may be useful for identifying patients at higher risk of abnormal glucose tolerance, but could not be used to predict gestational diabetes mellitus in early pregnancy due to the poor correlations with the outcome of the OGTT.
Serum fructosamine levels are correlated with maternal and gestational age and this complicates fructosamine utility for screening or diagnosing diabetic pregnancy,73,74 so that specific reference ranges should be established throughout pregnancy to increase its diagnostic efficiency.
Conclusions
An early diagnosis of diabetes and a strict glucose control are crucial for preventing or delaying the onset of serious, even life-threatening complications. Although HbA1c remains the standard for diagnosing diabetes and glycemic monitoring,2 emerging evidence attests that additional biomarkers such as fructosamine and GA are becoming HbA1c surrogates, especially in select patients, in whom the measurement of HbA1c may be biased or even unreliable. This especially include patients with rapid changes of glucose homeostasis and larger glycemic excursions (ie, temporarily high blood glucose spikes), red blood cell disorders and CKD. The diagnostic efficiency of GA seems superior to that of fructosamine over a broad range of clinical settings (Table 1), and is attributable to the fact that the fructosamine reflects a total concentration of glycated serum proteins, which can fluctuate in response to a variety of systemic disorders. Conversely, GA can be expressed as the ratio of GA to total albumin, thus minimizing the interference due to the concentrations of glycated and nonglycated albumin. The current method for measuring GA is also better standardized and less susceptible to preanalytical variables than fructosamine. Additional advantages of GA over HbA1c are represented by its lower cost and the portability of commercially available reagents to conventional laboratory instrumentation. Although further studies are needed to definitely establish whether GA may complement (or even replace) conventional measures of glycemic status such as HbA1c, it is undeniable that GA is already helping the clinical management of patients with diabetes in whom HbA1C values are unreliable.
Footnotes
Abbreviations: ADA, American Diabetes Association; AUC, area under the curve; CKD, chronic kidney disease; FA, fructosamine; FPG, fasting plasma glucose; GA, glycated albumin; GFR, glomerular filtration rate; HbA1c, glycated hemoglobin; HR, hazard ratio; Ig, immunoglobulin; NBT, nitroblue tetrazolium; OGTT, oral glucose tolerance test; OR, odds ratio.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80. [DOI] [PubMed] [Google Scholar]
- 3. Lippi G, Mattiuzzi C, Targher G. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:2030. [DOI] [PubMed] [Google Scholar]
- 4. Lippi G, Targher G. Glycated hemoglobin (HbA1c): old dogmas, a new perspective? Clin Chem Lab Med. 2010;48:609-614. [DOI] [PubMed] [Google Scholar]
- 5. Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care. 2002;25:275-8. [DOI] [PubMed] [Google Scholar]
- 6. Lippi G, Targher G. A laboratory standpoint on the role of hemoglobin A1c for the diagnosis of diabetes in childhood: more doubts than certainties? Pediatr Diabetes. 2011;12:183-186. [DOI] [PubMed] [Google Scholar]
- 7. He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209-215. [DOI] [PubMed] [Google Scholar]
- 8. Zilg H, Schneider H, Seiler FR. Molecular aspects of albumin functions: indications for its use in plasma substitution. Dev Biol Stand. 1980;48:31-42. [PubMed] [Google Scholar]
- 9. Anguizola J, Matsuda R, Barnaby OS, et al. Review: glycation of human serum albumin. Clin Chim Acta. 2013;425:64-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol. 2008;2:1114-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garlick RL, Mazer JS. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983;258:6142-6146. [PubMed] [Google Scholar]
- 12. Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011;93:645-658. [DOI] [PubMed] [Google Scholar]
- 13. Guerin-Dubourg A, Catan A, Bourdon E, Rondeau P. Structural modifications of human albumin in diabetes. Diabetes Metab. 2012;38:171-178. [DOI] [PubMed] [Google Scholar]
- 14. Lee EY, Lee BW, Kim D, et al. Glycated albumin is a useful glycation index for monitoring fluctuating and poorly controlled type 2 diabetes patients. Acta Diabetol. 2011;48:167-172. [DOI] [PubMed] [Google Scholar]
- 15. Johnson RN, Metcalf PA, Baker JR. Fructosamine: a new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin Chim Acta. 1983;127:87-95. [DOI] [PubMed] [Google Scholar]
- 16. Schleicher ED, Vogt BW. Standardization of serum fructosamine assays. Clin Chem. 1990;36:136-139. [PubMed] [Google Scholar]
- 17. Dingari NC, Horowitz GL, Kang JW, Dasari RR, Barman I. Raman spectroscopy provides a powerful diagnostic tool for accurate determination of albumin glycation. PLOS ONE. 2012;7:e32406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhernovaya OS, Tuchin VV, Meglinski IV. Monitoring of blood proteins glycation by refractive index and spectral measurements. Laser Phys Lett. 2008;5:460-464. [Google Scholar]
- 19. Hinton DJS, Ames JM. Analysis of glycated protein by capillary electrophoresis. Int Congress Ser. 2002;1245:471-474. [Google Scholar]
- 20. Pereira Morais MP, Mackay JD, Bhamra SK, et al. Analysis of protein glycation using phenylboronate acrylamide gel electrophoresis. Proteomics. 2010;10:48. [DOI] [PubMed] [Google Scholar]
- 21. Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324:61-71. [DOI] [PubMed] [Google Scholar]
- 22. Kouzuma T, Uemastu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 2004;346:135-143. [DOI] [PubMed] [Google Scholar]
- 23. Kohzuma T, Koga M. Lucica GA-L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther. 2010;14:49-51. [DOI] [PubMed] [Google Scholar]
- 24. Montagnana M, Paleari R, Danese E, et al. Evaluation of biological variation of glycated albumin (GA) and fructosamine in healthy subjects. Clin Chim Acta. 2013;423:1-4. [DOI] [PubMed] [Google Scholar]
- 25. Schleicher ED, Olgemöller B, Wiedenmann E, Gerbitz KD. Specific glycation of albumin depends on its half-life. Clin Chem. 1993;39:625-628. [PubMed] [Google Scholar]
- 26. Rodriguez-Segade S, Lojo S, Camiña MF, Paz JM, Del Río R. Effects of various serum proteins on quantification of fructosamine. Clin Chem. 1989;35:134-138. [PubMed] [Google Scholar]
- 27. Beck R, Steffes M, Xing D, et al. Diabetes Research in Children Network (DirecNet) Study Group. The interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitrol, and continuous glucose monitoring. Pediatr Diabetes. 2011;12:690-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010;57:751-762. [DOI] [PubMed] [Google Scholar]
- 29. Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58:1648-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furusyo N, Koga T, AiM, et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia. 2011;54:3028-3036. [DOI] [PubMed] [Google Scholar]
- 31. Ma X-J, Pan J-M, Bao Y-Q, et al. Combined assessment of glycated albumin and fasting plasma glucose improves the detection of diabetes in Chinese subjects. Clin Exp Pharmacol Physiol. 2010;37:974-979. [DOI] [PubMed] [Google Scholar]
- 32. Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective color analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2:279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Juraschek SP, Steffes MW, Miller ER, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35:2265-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14:933-946. [DOI] [PubMed] [Google Scholar]
- 35. Shima K, Abe F, Chikakiyo H, Ito N. The relative value of glycated albumin, hemoglobin A1c and fructosamine when screening for diabetes mellitus. Diabetes Res Clin Pract. 1989;7:243-250. [DOI] [PubMed] [Google Scholar]
- 36. Furusyo N, Koga T, Ai M, et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia. 2011;54:3028-3036. [DOI] [PubMed] [Google Scholar]
- 37. Li Q, Pan JM, Ma XJ, et al. Combined utility of hemoglobin A1c and glycated albumin in diabetic screening. Zhonghua Yi Xue Za Zhi. 2011;91:1813-1816. [PubMed] [Google Scholar]
- 38. Selvin E, Francis LM, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care. 2011;34:960-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang C, Li H, Wang Z, et al. Glycated albumin is a potential diagnostic tool for diabetes mellitus. Clin Med. 2012;12:568-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malmström H, Walldius G, Grill V, Jungner I, Gudbjörnsdottir S, Hammar N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies—cross-sectional and longitudinal experience from the AMORIS cohort. PLOS ONE. 2014;9:e111463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koga M, Murai J, Morita S, Saito H, Kasayama S. Comparison of annual variability in HbA1c and glycated albumin in patients with type 1 vs. type 2 diabetes mellitus. J Diabetes Complications. 2013;27:211-213. [DOI] [PubMed] [Google Scholar]
- 42. Takahashi S, Uchino H, Shimizu T, et al. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007;54:139-144. [DOI] [PubMed] [Google Scholar]
- 43. Won HK, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC. Reduction in glycated albumin can predict change in HbA1c: comparison of oral hypoglycaemic agent and insulin treatments. Diabet Med. 2012;29:74-79. [DOI] [PubMed] [Google Scholar]
- 44. Ogawa A1, Hayashi A, Kishihara E, Yoshino S, Takeuchi A, Shichiri M. New indices for predicting glycaemic variability. PLOS ONE. 2012;7:e46517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suwa T, Ohta A, Matsui T, et al. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J. 2010;57:135-140. [DOI] [PubMed] [Google Scholar]
- 46. Shima K, Komatsu M, Noma Y, Miya K. Glycated albumin (GA) is more advantageous than hemoglobin A1c for evaluating the efficacy of sitagliptin in achieving glycemic control in patients with type 2 diabetes. Intern Med. 2014;53:829-835. [DOI] [PubMed] [Google Scholar]
- 47. Moura BP, Amorim PR, Silva BP, Franceschini SC, Reis JS, Marins JC. Effect of a short-term exercise program on glycemic control measured by fructosamine test in type 2 diabetes patients. Diabetol Metab Syndr. 2014;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lindsey CC, Carter AW, Mangum S, et al. A prospective, randomized, multicentered controlled trial to compare the annual glycemic and quality outcomes of patients with diabetes mellitus monitored with weekly fructosamine testing versus usual care. Diabetes Technol Ther. 2004;6:370-377. [DOI] [PubMed] [Google Scholar]
- 49. Yoshiuchi K, Matsuhisa M, Katakami N, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J. 2008;55:503-507. [DOI] [PubMed] [Google Scholar]
- 50. Yogev Y, Hod M. Use of new technologies for monitoring and treating diabetes in pregnancy. Obstet Gynecol Clin North Am. 2007;34:241-253. [DOI] [PubMed] [Google Scholar]
- 51. Koga M, Murai J, Saito H, et al. Glycated albumin levels are higher relative to glycated haemoglobin levels in gastrectomized subjects. Ann Clin Biochem. 2009;47:39-43. [DOI] [PubMed] [Google Scholar]
- 52. Pu LJ, Lu L, Shen WF, et al. Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J. 2007;71:1067-1073. [DOI] [PubMed] [Google Scholar]
- 53. Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896-903. [DOI] [PubMed] [Google Scholar]
- 54. Fukuoka K, Nakao K, Morimoto H, et al. Glycated albumin levels predict long-term survival in diabetic patients undergoing haemodialysis. Nephrology (Carlton). 2008;13:278-283. [DOI] [PubMed] [Google Scholar]
- 55. Nagayama H, Inaba M, Okabe R, et al. Glycated albumin as an improved indicator of glycemic control in hemodialysis patients with type 2 diabetes based on fasting plasma glucose and oral glucose tolerance test. Biomed Pharmacother. 2009;63:236-240. [DOI] [PubMed] [Google Scholar]
- 56. Freedman BI, Andries L, Shihabi ZK, et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol. 2011;6:1635-1643. [DOI] [PubMed] [Google Scholar]
- 57. Zheng CM, Ma WY, Wu CC, Lu KC. Glycated albumin in diabetic patients with chronic kidney disease. Clin Chim Acta. 2012;413:1555-1561. [DOI] [PubMed] [Google Scholar]
- 58. Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73:1062-1068. [DOI] [PubMed] [Google Scholar]
- 59. Chen HS, Wu TE, Lin HD, et al. Hemoglobin A(1c) and fructosamine for assessing glycemic control in diabetic patients with CKD stages 3 and 4. Am J Kidney Dis. 2010;55:867-874. [DOI] [PubMed] [Google Scholar]
- 60. Freedman BI, Shihabi ZK, Andries L, et al. Relationship between assays of glycemia in diabetic subjects with advanced chronic kidney disease. Am J Nephrol. 2010;31:375-379. [DOI] [PubMed] [Google Scholar]
- 61. Sany D, Elshahawy Y, Anwar W. Glycated albumin versus glycated hemoglobin as glycemic indicator in hemodialysis patients with diabetes mellitus: variables that influence. Saudi J Kidney Dis Transpl. 2013;24:260-273. [PubMed] [Google Scholar]
- 62. Shafi T, Sozio SM, Plantinga LC, et al. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care. 2013;36:1522-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nunoi K, Kodama T, Sato Y, et al. Comparison of reliability of plasma fructosamine and glycosylated hemoglobin assays for assessing glycemic control in diabetic patients on hemodialysis. Metabolism. 1991;40:986-989. [DOI] [PubMed] [Google Scholar]
- 64. Morgan L, Marenah CB, Jeffcoate WJ, et al. Glycated proteins as indices of glycaemic control in diabetic patients with chronic renal failure. Diabet Med. 1996;13 514-519. [DOI] [PubMed] [Google Scholar]
- 65. Joy MS, Cefalu WT, Hogan SL, et al. Long-term glycemic control measurements in diabetic patients receiving hemodialysis. Am J Kidney Dis. 2002;39:297-307. [DOI] [PubMed] [Google Scholar]
- 66. Phelps RL, Honig GR, Green D, et al. Biphasic changes in haemoglobin A1c concentrations during normal human pregnancy. Am J Obstet Gynecol. 1983;147:651-653. [DOI] [PubMed] [Google Scholar]
- 67. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [DOI] [PubMed] [Google Scholar]
- 68. Hashimoto K, Noguchi S, Morimoto Y, et al. A1C but not serum glycated albumin is elevated in late pregnancy owing to iron deficiency. Diabetes Care. 2008;31:1945-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hashimoto K, Osugi T, Noguchi S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care. 2010;33:509-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pan J, Zhang F, Zhang L, Bao Y, Tao M, Jia W. Influence of insulin sensitivity and secretion on glycated albumin and hemoglobin A1c in pregnant women with gestational diabetes mellitus. Int J Gynaecol Obstet. 2013;121:252-256. [DOI] [PubMed] [Google Scholar]
- 71. Khan HA, Sobki SH, Alhomida AS, Khan SA. Paired values of serum fructosamine and blood glucose for the screening of gestational diabetes mellitus: a retrospective study of 165 Saudi pregnant women. Indian J Clin Biochem. 2007;22:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li K, Yang HX. Value of fructosamine measurement in pregnant women with abnormal glucose tolerance. Chin Med J (Engl). 2006;119:1861-1865. [PubMed] [Google Scholar]
- 73. Roberts AB, Baker JR. Serum fructosamine: a screening test for diabetes in pregnancy. Am J Obstet Gynecol. 1986;154:1027-1030. [DOI] [PubMed] [Google Scholar]
- 74. Frandsen EK, Sabagh T, Bacchus RA. Serum fructosamine in diabetic pregnancy. Clin Chem. 1988;34:316-319. [PubMed] [Google Scholar]


