Abstract
Group A rotaviruses (RV-A) are the leading cause of viral gastroenteritis in children worldwide and genotype G9P[8] is one of the five most common genotypes detected in humans. In order to gain insight into the degree of genetic variability of G9P[8] strains circulating in Cameroon, stool samples were collected during the 1999–2000 rotavirus season in two different geographic regions in Cameroon (Southwest and Western Regions). By RT-PCR, 15 G9P[8] strains (15/89 = 16.8%) were identified whose genomic configurations was subsequently determined by complete or partial gene sequencing. In general, all Cameroonian G9 strains clustered into current globally-spread sublineages of the VP7 gene and displayed 86.6–100% nucleotide identity amongst themselves and 81.2–99.5% nucleotide identity with global G9 strains. The full genome classification of all Cameroonian strains was G9-P[8]-I1–R1–C1–M1–A1–N1–T1–E1–H1 but phylogenetic analysis of each gene revealed that the strains were spread across 4 or more distinct lineages. An unusual strain, RVA/Human-wt/CMR/6788/1999/G9P[8], which shared the genomic constellation of other Cameroonian G9P[8] strains, contained a novel G9 subtype which diverged significantly (18.8% nucleotide and 19% amino acid distance) from previously described G9 strains. Nucleotide and amino acid alignments revealed that the 3′ end of this gene is highly divergent from other G9 VP7 genes suggesting that it arose through extensive accumulation of point mutations. The results of this study demonstrate that diverse G9 strains circulated in Cameroon during 1999–2000.
Keywords: Rotavirus A, Genotype P[8]G9, Genomic phylogenetic analysis, Structural proteins, Non-structural proteins
1. Background
Childhood mortality has been declining worldwide as a result of socioeconomic development and implementation of prevention and survival interventions (Claeson et al., 2000). Group A rotaviruses (RV-A) are the main etiologic agent of acute gastroenteritis in infants and young children worldwide (Estes and Kapikian, 2007) and an estimated 453,000 children aged <5 years die from rotavirus diarrhea each year, with >85% of these deaths occurring in low-income countries of Africa and Asia (Parashar et al., 2009; Tate et al., 2011). Rotaviruses belong to the family Reoviridae, and the rotavirus genome consists of 11 double-stranded RNA gene segments that encode six structural (VP) and six non-structural proteins (NSP). Based on the two genes that encode the outer capsid proteins, VP4 (P-type) and VP7 (G-type), a widely used binary classification system was established for RV-A (Estes and Kapikian, 2007). This system has been recently standardized and extended to all 11 genes (Matthijnssens et al., 2008b). To date, at least 27 G, 35 P, 16 I, 9 R, 9 C, 8 M, 16 A, 9 N, 12 T, 14 E and 11 H genotypes have been identified based on the eleven rotavirus A genes (Esona et al., 2010b; Matthijnssens et al., 2011). In humans, at least five RV-A G types (G1–G4 and G9), and two common P types (P[8] and P[4]) circulate worldwide (Banyai et al., 2012; Gentsch et al., 2005; Santos and Hoshino, 2005). G9 strains emerged in 1990s, and there has been a global description of the appearance and dominance of this genotype (Gentsch et al., 2005; Laird et al., 2003; Matthijnssens et al., 2009; Santos and Hoshino, 2005). Genotype G9 strains with a Wa-like or a DS-1-like genomic configuration or a mixture thereof have been detected sporadically in localized outbreaks (Page et al., 2010). In Cameroon, the first molecular identification of genotype G9 in human samples was reported in a study conducted by Steele and colleagues in 2003 (Steele and Ivanoff, 2003).
At least seven major phylogenetic lineages and eleven minor lineages within G9 VP7 genes have been described (Phan et al., 2007; Wu et al., 2011). A molecular evolutionary analysis study utilizing Bayesian inference supported the idea that one single sub-lineage introduced in the 1980s was responsible for all the worldwide spread of G9 in the 1990s (Matthijnssens et al., 2010).
In order to gain insight into the degree of genetic variability of G9P[8] strains circulating in Cameroon, Central Africa, sequence determination and phylogenetic analysis of all eleven genome segments from G9P[8] RV-A strains detected in two different geographic regions of Cameroon (Southwest and Western Regions) was performed in order to infer the genetic relationship of Cameroonian strains with G9P[8] worldwide. The results of these studies revealed a new G9 genetic variant circulating in Cameroon during the 1999–2000 rotavirus seasons.
2. Material and methods
2.1. Fecal samples, strains and nomenclature
Fifteen diarrheic stool specimens collected from children <5 years of age, genotyped as G9P[8] (Esona et al., 2010a), were obtained during the 1999–2000 rotavirus season in two different geographic regions in Cameroon (Southwest and Western Regions). The strains and nomenclature are shown in Table 1.
Table 1.
Characteristics of the Cameroon G9P[8] strains.
| Genotypes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VP7 | Lineage | VP4 | Lineage | VP6 | VP1 | VP2 | VP3 | NSP1 | NSP2 | NSP3 | NSP4 | NSP5 | |
| Length of gene sequenced (nucleotide)a | 840 | 834 | 1191 | 1917 | 1367 | 1187 | 1043 | 948 | 930 | 525 | 591 | ||
| Presence of ORFb | P | P | C | P | P | P | P | C | C | C | C | ||
| Strain name | |||||||||||||
| RVA/Human-wt/CMR/6735/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6778/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6779/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6788/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6791/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6796/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6807/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6806/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6795/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6777/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6790/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6792/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6793/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6805/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
| RVA/Human-wt/CMR/6794/1999/G9P[8] | G9 | III | P[8] | P[8]-3 | I1 | R1 | C1 | M1 | A1 | N1 | T1 | E1 | H1 |
Length of gene sequenced in nucleotides.
P and C denotes partial or complete ORF, respectively.
2.2. Viral RNA extraction, amplification, and sequencing
Viral RNA from each of the 15 specimens was extracted from a 10% stool suspension made from 0.1 g or 100 μl stool in 2 ml of a 1:1 Vertrel/Water solution using either a commercial RNA extraction kit (NucliSens automated extractor, BIOMERIEUX, Durham, NC) according to the protocol specified by the manufacturer or a silica binding method described previously (Boom et al., 1990).
Previously published forward and reverse primers (Das et al., 1994; Gentsch et al., 1992; Iturriza-Gomara et al., 2001, 2002; Kerin et al., 2007; Matthijnssens et al., 2006; Mijatovic-Rustempasic et al., 2011) were used for the amplification of the different gene segments. The extracted dsRNA of each strain was denatured at 97 °C for 5 min and RT-PCR was carried out using a one step RT-PCR kit (Qiagen, Inc., Valencia, CA) according to manufacturer’s instructions. Reverse transcription (RT) of each gene from each sample was carried out for 30 min at 42 °C, followed by 15 min at 95 °C to inactivate the reverse transcriptase and activate the Taq polymerase. The cDNA was then subjected to 35 cycles of PCR in a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, Inc., Foster City, CA) using the following conditions: 30 s at 94 °C; 30 s at 42 °C; 45–90 s at 72 °C (depending upon the expected size of the amplified gene fragment), followed by a 7 min final extension at 72 °C. Amplicons were analyzed by gel electrophoresis in 1% SeaKem agarose gels (Thermo Fisher Scientific, Inc., Waltham, MA) then excised and purified with the QIAquick Gel Extraction kit (Qiagen, Inc., Valencia, CA) following the manufacturer’s instructions.
DNA cycle sequencing of each amplicon was performed with the same consensus primers used for RT-PCR, using a Big Dye Terminator cycle sequencing Ready kit v1.1 (Applied Biosystems, Inc., Foster City, CA). Previously published primers homologous to internal regions of each gene segment were also used (Mijatovic-Rustempasic et al., 2011). Cycle sequencing products were purified using Centri-sep spin columns (Princeton Separations, Inc., Adelphia, NJ), dried in a DNA speed VacR (Savant Instruments, Inc., Holbrook, NY) and reconstituted in 15 ml Hi-Di formamide. Automated separation and base-calling of cycle sequencing products was performed using an ABI 3130xl sequencer (Applied Biosystems, Foster City, CA). Overlapping sequence fragments were assembled and edited using Sequencher 4.8 (Gene Codes Corporation, Inc., Ann Arbor, MI).
2.3. Computational analysis
Sequences were aligned using the MUSCLE program within MEGA version 5 (Tamura et al., 2011). Once aligned, the JModel-Test 2 program (Posada, 2008) was used to identify the optimal evolutionary model that best fitted the sequence datasets. Using corrected Akaike Information Criterion (AICc) the following models; TPM3uf + I + G (NSP1), TIM2 + I + G (NSP2), GTR + I + G (NSP3, VP1, VP2, VP6), HKY + G (NSP4), TVM + G (NSP5), TIM3 + I + G (VP3), TPM1uf + G (VP4), and TPM3uf + G (VP7) were found to best fit the sequence data for the different genes. Using these models, maximum likelihood trees were constructed using PhyML 3.0 along with approximate likelihood-ratio test (aLRT) statistics for branch support (Guindon et al., 2010). Nucleotide and amino acid distance matrixes were prepared using the p-distance algorithm of MEGA version 5 software (Tamura et al., 2011).
Using the crystal structure of the RRV VP7 protein (PDB accession number 3fmg; (Aoki et al., 2009), amino acid substitutions found in Cameroonian strain RVA/Human-wt/CMR/6788/1999/G9P[8] were mapped spatially onto the 3D protein structure using the PyMOL Molecular Graphics System, version 1.5.0.1 (Schrodinger, 2010).
3. Results
3.1. The genotype configuration of Cameroonian G9P[8] strains
The names and characteristics of the Cameroonian strains analyzed in this study, and lengths of each gene are presented in Table 1. The accession numbers of each gene of the Cameroonian G9 strains and those from the GenBank are in the appendix. Accession numbers in bold face characters represent the gene sequences of these Cameroon G9 strains. Nucleotide sequences analysis based on VP7–VP4–VP6–VP1–VP2–VP3–NSP1–NSP2–NSP3–NSP4–NSP5 genes from all Cameroonian samples analyzed revealed a consensus genotype constellation of G9-P[8]-I1–R1–C1–M1–A1–N1–T1–E1–H1, respectively, according to the classification proposed by Matthijnssens and colleagues (Matthijnssens et al., 2008b).
3.2. Analysis of VP7 nucleotide sequences
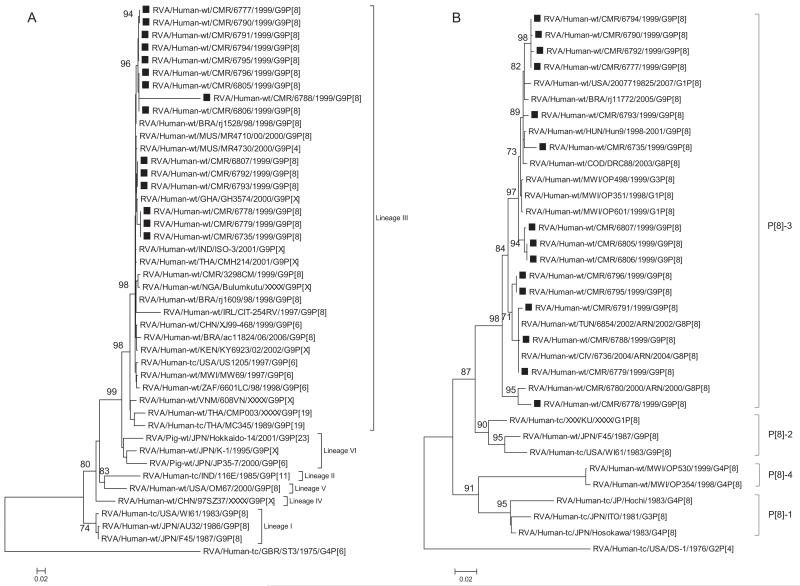
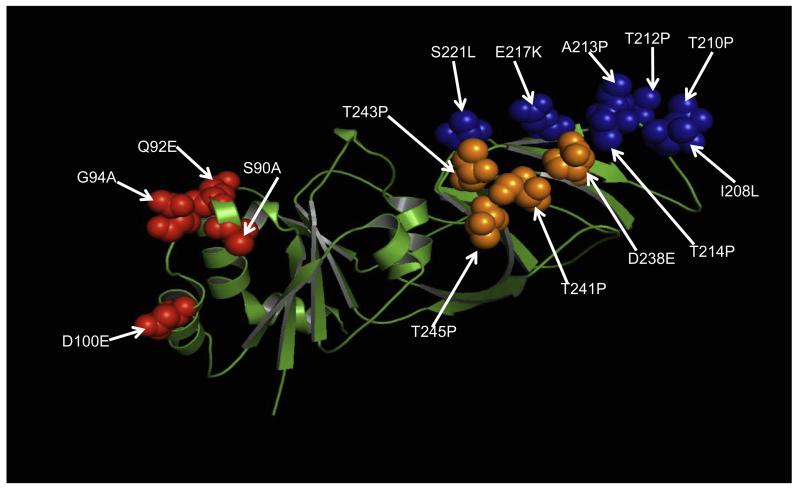
Phylogenetic analyses of the eleven genes determined the genetic relationships of the Cameroonian strains with a global collection of rotavirus genotypes. Phylogenetic analysis based on VP7 nucleotide sequences showed that all G9 strains detected in Cameroon during 1999–2000 clustered together in three distinct sub-clusters with lineage III G9 strains isolated worldwide (Fig. 1A). Nucleotide and amino acid identities among Cameroonian strains ranged between 88.6–100% and 84.6–100%, respectively. The strain RVA/Human-wt/CMR/6788/1999/G9P[8] showed a maximum nucleotide identity of 90% with Cameroonian strains RVA/Human-wt/CMR/6806/1999/G9P[8], RVA/Human-wt/CMR/6805/2000/G9P[8] and RVA/Human-wt/CMR/6796/2000/G9P[8], while its nucleotide identity with global G9 strains ranges from 81% to 89% (data not shown). Comparison of the amino acid sequence of strain RVA/Human-wt/CMR/6788/1999/G9P[8] to reference and contemporary human G9 genotypes from the GenBank revealed a low identity in the range of 81–85% as well as one to numerous substitutions in the nine major VP7 variable regions VR-1–VR-9 (Green et al., 1989) described for this protein (Fig. 2); VR-1 (I16L), VR-3 (A43 V), VR-4 (A68T), VR-5/antigenic epitope A (S90A, Q92E, G94A, and D100E), VR-8/antigenic epitope C (I208L, T210P, T212P, A213P, T214P, E217 K, and S221L), and VR-9/antigenic epitope F (D238E, T241P, T243P, and T245P) (Green et al., 1989; Kirkwood et al., 2003). The VR-2, VR-6 and VR-7 were highly conserved amongst both contemporary and older G9 strains. Substitutions in the three major variable regions; VR-5/antigenic epitope A, VR-8/antigenic epitope C and VR-9/antigenic epitope F were mapped to the VP7 crystal structure of the RRV strain (G3P[3]) available in the Protein Data Bank (Fig. 3). Out of the 15 substitutions identified in these three regions, 11 were radical in nature. Radical changes are associated with changes in size, charge and polarity (Zhang, 2000). Out of the 11 radical changes 9 were associated with changes in polarity with the strain 6788 being non-polar when compared to the consensus G9 strains. In VR-5/antigenic epitope A mutation in site 94 is associated with neutralization escape mutants (Aoki et al., 2009). Similar sites associated with neutralization escape mutants were observed in VR-8/antigenic epitope C (positions 213, 217, 221) and in VR-9/antigenic epitope F (position 238). Comparative analysis of this strain with representative strains belonging to the major G9 VP7 lineages revealed a high similarity in the 5′ end of the gene and a lower similarity downstream of the central part of the gene (data not shown).
Fig. 1.
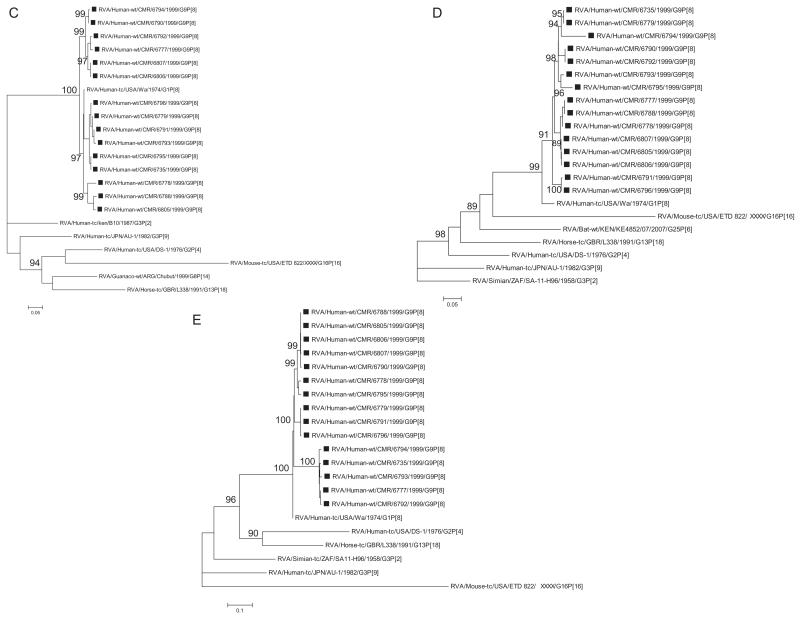
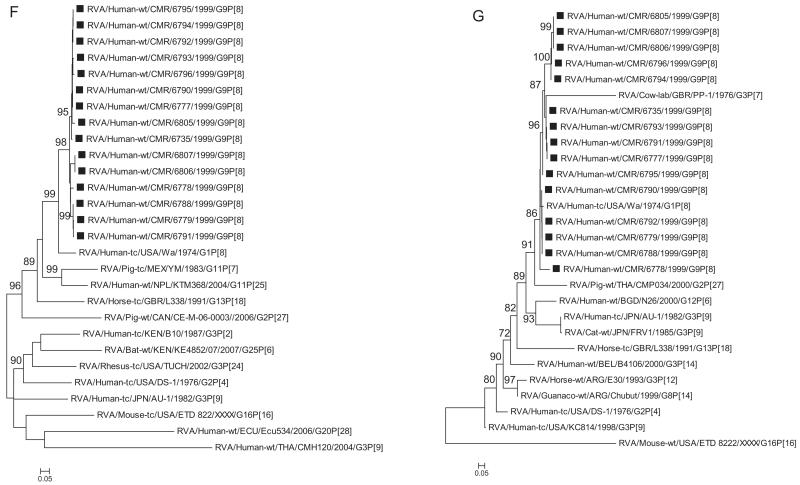
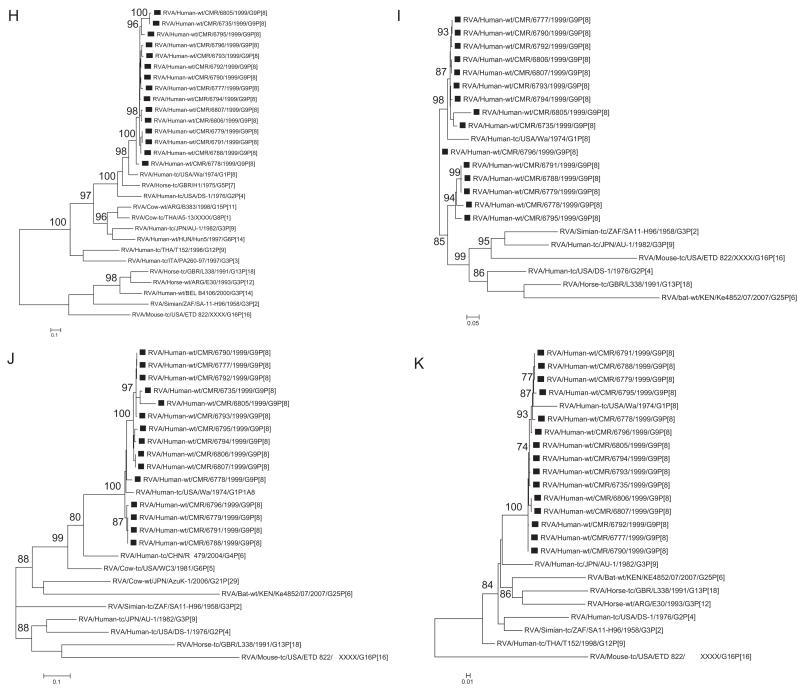
A–K Maximum likelihood phylogenetic trees built in PhyML with aLRT statistics as support show the genetic relationships of nucleotide sequences of VP7 (A), VP4 (B), VP1(C), VP2 (D), VP3 (E), VP6 (F), NSP4 (G), NSP1 (H), NSP2 (I), NSP3 (J) and NSP5 (K) of human G9P[8] rotaviruses from Cameroon with known human and animal rotavirus strains from GenBank database. The trees were drawn to scale. Only aLRT values of 70% and greater are shown. The strains labeled with filled squares indicate the Cameroon G9P[8] isolates sequenced in this study. The scale bar at the bottom of the trees indicates genetic distance.
Fig. 2.
Comparison of the deduced amino acid sequence of gene segment 9 of strain RVA/Human-wt/CMR/6788/1999/G9P[8] to a selection of older and contemporary G9 sequences from the GenBank. Only amino acids which differ are shown. Variable regions designated VR-1-VR-9 are shown.
Fig. 3.
Substitutions in strain RVA/Human-wt/CMR/6788/1999/G9P[8] highlighted on the crystal structure of RRV VP7 protein (3fmg). The molecule is colored in green. Residues corresponding to previously describe major antigenic sites A, C and F are indicated in red, blue and orange spheres, respectively. Arrows indicate substitutions at amino acid positions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Analysis of VP4 nucleotide sequences
Evolutionary analysis of VP4 nucleotide sequences from Cameroonian strains and representatives of genotype P[8] from the Gen-Bank database demonstrated that strains from both regions of Cameroon grouped in four different sub-clusters of lineage P[8]-III together with other rotaviruses from around the world (Fig. 1B). Nucleotide (amino acid) identities between Cameroonian strains ranged between 95.4% and 100% (95.7–100%). Strain RVA/Human-wt/CMR/6735/1999/G9P[8] showed a close genetic relationship with strains isolated in Acre-Brazil during 2005–2006 rotavirus season. Strain RVA/Human-wt/CMR/6778/1999/G9P[8] grouped in a separate cluster together with a previously detected African P[8] strain. Two Cameroonian strains, RVA/Human-wt/CMR/6779/1999/G9P[8] and RVA/Human-wt/CMR/6788/1999/G9P[8], showed complete identity with P[8] strains detected in Tunisia (North Africa) in 2002 and Cote D’Ivoire (West Africa) in 2004 (data not shown).
3.4. Analysis of VP1, VP2, VP3 and VP6 nucleotide sequences
Phylogenetic analysis based on VP1, VP2, VP3 and VP6 nucleotide sequences demonstrated that each gene of Cameroonian strains detected during the 1999–2000 rotavirus season grouped in several separate clusters together with strains isolated worldwide (Fig. 1C–F). The Cameroonian strains showed a close genetic relationship with cognate gene sequences of previously reported G1P[8], G3P[8], and G4P[8] strains detected in the USA, Bangladesh and Belgium (McDonald et al., 2009; Mijatovic-Rustempasic et al., 2011; Rahman et al., 2007). Nucleotide (amino acid) identity values among Cameroonian strains ranged from 91.8–100% (94–100%), 90.7–100% (86.8–100%), 89.6–100% (93.9–100%), and 96–100% (99.2–100%) for VP1, VP2, VP3 and VP6, respectively. Complete nucleotide and amino acid similarity (100%) was shared between strains RVA/Human-wt/CMR/6735/1999/G9P[8] and RVA/Human-wt/CMR/6795/1999/G9P[8] (VP1 gene); RVA/Human-wt/CMR/6735/1999/G9P[8] and RVA/Human-wt/CMR/6779/1999/G9P[8], RVA/Human-wt/CMR/6805/1999/G9P[8] and RVA/Human-wt/CMR/6806/1999/G9P[8], RVA/Human-wt/CMR/6790/1999/G9P[8] and RVA/Human-wt/CMR/6792/1999/G9P[8] (VP2 gene); RVA/Human-wt/CMR/6788/1999/G9P[8] and RVA/Human-wt/CMR/6805/1999/G9P[8] (VP3 gene); and RVA/Human-wt/CMR/6806/1999/G9P[8] and RVA/Human-wt/CMR/6807/1999/G9P[8] (VP6 gene). However, when the nucleotide and amino acid homologies of the VP1–VP3 and VP6 gene sequences of the Cameroonian strains were compared with cognate gene sequences of strains belonging to previously identified VP1–VP3 and VP6 genotypes, all of them were more closely related to strains in the R1, C1, M1 and I1 genotypes, respectively. Further comparison showed that within each genotype, the Cameroonian strains had maximum nucleotide (amino acid) identities of 84.8–98.6% (93.1–99.4%) for VP1, 89.8–98.8% (89.8–99.8%) for VP2, 88.3–99.2% (91.4–99.5%) for VP3 and 89.4–99.5% (97.5–100%) for VP6.
3.5. Analysis of NSP4 nucleotide sequences
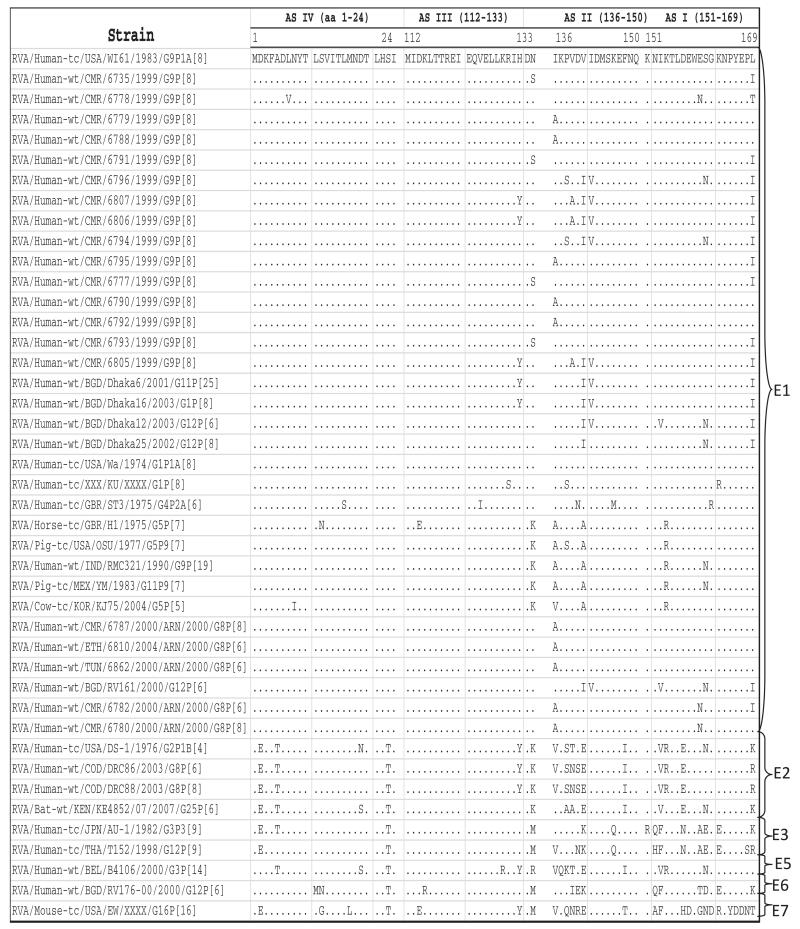
Phylogenetic analysis based on NSP4 nucleotide sequences showed that the Cameroonian strains grouped into four different sub-clusters together with strains belonging to genotype E1 (Fig. 1G). The nucleotide (amino acid) identity among Cameroonian strains ranged between 90.9%-100% (94.3%-100%). Analysis of deduced amino acid sequences of Cameroonian strains and rotaviruses detected worldwide demonstrated that seven of the fifteen strains detected in Cameroon showed an amino acid substitution within the enterotoxin domain (aa 114–135) (Ball et al., 2005). Three strains exhibited changes at position H131Y and the other four at position N133S. Amino acid substitutions were observed within previously described antigenic sites (Ball et al., 2005; Borgan et al., 2003): ASIV (aa 1–24) at position L7 V; ASIII (aa 112–133) at positions H131Y and N133S; ASII (aa136–150) at positions I136A, P138S, V139A, V141I and I142V and ASI (151–169 aa) at positions E160N, S161N, and L169I when aligned with other E1 genotype strains (Fig. 4).
Fig. 4.
Alignment showing amino acid substitutions inside the four NSP4 antigenic sites of the Cameroon G9 strains.
3.6. Analysis of NSP1- NSP3 and NSP5 nucleotide sequences
Phylogenetic analysis of NSP1- NSP3 and NSP5 nucleotide sequences, demonstrated that for each gene, the Cameroonian strains grouped in small separate sub-clusters of genotypes A1, N1, T1, and H1, respectively, together with other strains from around the world (Fig. 1H–K). Nucleotide (amino acid) identity values among Cameroonian strains ranged from 87.8–99.8% (82.9–99.7%), 86.8–100% (88–100%), 92.8–100% (93.1–100%) and 97.6–100% (98.5–100%) for NSP1, NSP2, NSP3 and NSP5, respectively. Complete nucleotide and amino acid identity was shared between strains RVA/Human-wt/CMR/6777/1999/G9P[8] and RVA/Human-wt/CMR/6790/1999/G9P[8] (NSP2, NSP3 and NSP5 genes) and RVA/Human-wt/CMR/6806/1999/G9P[8] and RVA/Human-wt/CMR/6807/1999/G9P[8] (NSP3 and NSP5 genes), while only strains RVA/Human-wt/CMR/6779/1999/G9P[8] and RVA/Human-wt/CMR/6791/1999/G9P[8] were completely identical in their NSP5 gene sequences. However, when the nucleotide and amino acid homologies of the NSP1–NSP3 and NSP5 gene sequences of the Cameroonian strains were compared with similar gene sequences of strains belonging to already identified NSP1–NSP3 and NSP5 genotypes, all of them were more closely related to strains in the A1, N1, T1, and H1 genotypes, respectively. Within each of these genotypes, the Cameroonian strains shared maximum nucleotide (amino acid) identity of 75.4–99.6% (68.7–98.8%), 81.2–99.5% (84.5–100%), 85.9–99.6% (87.9–100%), and 94.6–99.8% (93.9–100%), respectively.
4. Discussion
The genetic variability of RV-A strains is the result of accumulation of single nucleotide mutations (genetic drift) and sudden changes in the RV-A genome (genetic shift), primarily by reassortment and recombination events (Estes and Kapikian, 2007; Matthijnssens et al., 2008c; McDonald et al., 2009; Ramig, 1997). Since the proposal that RV-A classification should be based on all 11 RV-A gene segments (Matthijnssens et al., 2008b), the number of studies reporting RV-A full genome sequences has increased (Banyai et al., 2011; Esona et al., 2010b, 2011; Matthijnssens et al., 2008a; McDonald et al., 2009, 2011; Mijatovic-Rustempasic et al., 2011). Previous studies have shown that the predominance of a specific G type is related to the emergence of atypical VP7 lineages (Banyai et al., 2009; Parra, 2009; Parra et al., 2005). The results obtained in this study revealed multiple amino acids changes in 6 of the 9 variable regions (Green et al., 1989; Kirkwood et al., 1993) when comparing Cameroon G9 strain RVA/Human-wt/CMR/6788/1999/G9P[8] to both contemporary and older G9 strains from the GenBank database. This suggests that this Cameroon G9 strain might represent a new genetic variant of VP7 gene G9 genotype. The relatively low overall amino acid homology with other G9 strains together with numerous changes in important antigenic regions raises questions on whether this strain may be antigenically distinct from typicalG9 strains. Also, the previously described conserved N-glycosylation site found within VR-4 at amino acid residues 69–71 (Green et al., 1989) was found to be conserved in all G9 strains used in this analyses. Definitive conclusions on the possible origin of this variant could not be made by bioinformatics analysis of the VP7 gene sequence. However, in this case it remains unclear what selective pressures on this gene fragment could have driven this strong diversification, given that other gene segments of this strain retained their identity with related G9P[8] strains identified in the same region and time period. In this context, it is difficult to determine if a single sub-lineage of G9 can be responsible for the worldwide spread of G9 rotavirus as proposed recently (Matthijnssens et al., 2010).
At least six different neutralization epitopes (A through F) have been identified in the RVA VP7 protein, with A–C and F described as the most important (Kirkwood et al., 2003). The strain RVA/Human-wt/CMR/6788/1999/G9P[8] shows distinct changes in its antigenic regions when compared to G9 strains circulating in the same region and also globally (Fig. 2). A strong shift in polarity, with strain RVA/Human-wt/CMR/6788/1999/G9P[8] being strongly non-polar as compared to other G9 strains suggest possible inaccessibility of epitopes on the VP7 protein of strain RVA/Human-wt/CMR/6788/1999/G9P[8] as the region becomes more hydrophobic in nature. Multiple sites previously identified as important in producing neutralization escape mutants show substitutions in strain RVA/Human-wt/CMR/6788/1999/G9P[8] when compared with global G9 strains. If strain RVA/Human-wt/CMR/6788/1999/G9P[8] is also a neutralization escape mutant it could be due in part to changes in polarity at the antigenic epitopes. The amino acid sequences at positions 87–101 and 208–211 (epitope region A and C) is said to be conserved within serotypes (Green et al., 1988). However, we observe substitutions within these regions in an alignment of a global collection of G9 strains.
Seven of the fifteen strains detected in Cameroon showed an amino acid substitution in the enterotoxin domain (114–135 aa) of NSP4. These changes occur at amino acid positions 131 and 133, which are in the region (amino acid 131–140) reported to be responsible for altered pathogenesis mediated by the NSP4 protein (Zhang et al., 1998). Also observed are amino acid substitutions in the four previously described NSP4 antigenic sites (Ball et al., 2005). Antigenic sites AS IV, AS III, AS II and AS I had 1, 1, 5, 3 amino acids substitutions, respectively, when compared with strains in the E1 genotype. NSP4 is a trans-membrane glycoprotein known to be involved in virus assembly and is capable of inducing diarrhea in infant mice (Ball et al., 1996; Tian et al., 1996). It is possible these changes may affect the conformation or activity of NSP4 and also alter ability of host responses to neutralize enterotoxic function of the NSP4 gene segment (Ball et al., 1996; Tian et al., 1995).
Recent studies on origin and spread of the G9 rotavirus have revealed a high degree of genetic diversity within this genotype worldwide (Martinez-Laso et al., 2009; Phan et al., 2007; Wu et al., 2011). These studies have also shown close similarity between human and porcine G9 strains and zoonotic transmission and convergent evolution were proposed as the possible evolutionary mechanisms. Studies in Brazil have implicated porcine G9 strains in many outbreaks (Leite et al., 2008). Also, reassortment events among animal and human strains have continued to be an important mechanism for rotavirus evolution and emergence in developing countries (Banyai et al., 2010; Esona et al., 2010b). This present report confirms the circulation of a diverse G9 genotype among children in two regions of Cameroon. However, all of the G9P[8] strains reported here showed close similarity in all gene segments except VP7 with human rotavirus strains of different G genotypes including G1P[8], G3P[8] and G4P[8]. Nonetheless, due to the increased close contact between human and animals in most developing countries, the full genome sequence data obtained from any future G9 study from Cameroon maybe entirely different.
Two live oral vaccines from Merck (RotaTeq®) and Glaxo-SmithKline (Rotarix®) have been licensed in more than 100 countries and are being introduced into routine immunization programs in several countries worldwide (Glass et al., 2006; Vesikari et al., 2007), but has not been introduced in Cameroon. Therefore, it will be important to monitor the circulation of G9s in Cameroon before the introduction of these vaccines and, once introduced, to monitor the abilities of the vaccines to provide heterotypic protection against divergent G9 strains.
Furthermore, monitoring temporal changes in all 11 gene segments may help us to comprehend the nature and pattern of rotavirus evolution. This study revealed the presence of a novel VP7 genetic variant and diverse G9P[8] strains in Cameroon. Surveillance to monitor the strain diversity of circulating RV-A to detect possible strain replacement following the introduction of universal RV-A vaccine is a priority of the World Health Organization. Such studies are important to estimate potential impact of vaccination programs on circulating strains including whether escape mutants of known serotypes or novel strains that evade vaccine immunity will emerge.
Supplementary Material
Acknowledgements
We thank the staff of the Gastroenteritis and Respiratory Viruses Laboratory Branch at the Centers for Disease Control and Prevention for invaluable assistance and the anonymous reviewers for constructive comments. This study was supported by the Centers for Disease Control and Prevention. K.B. was supported by the Hungarian Scientific Research Fund (PD76364).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.meegid.2013.06.005.
References
- Aoki ST, Settembre EC, Trask SD, Greenberg HB, Harrison SC, Dormitzer PR. Structure of rotavirus outer-layer protein VP7 bound with a neutralizing Fab. Science. 2009;324:1444–1447. doi: 10.1126/science.1170481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- Ball JM, Mitchell DM, Gibbons TF, Parr RD. Rotavirus NSP4: a multifunctional viral enterotoxin. Viral Immunol. 2005;18:27–40. doi: 10.1089/vim.2005.18.27. [DOI] [PubMed] [Google Scholar]
- Banyai K, Gentsch JR, Martella V, Bogdan A, Havasi V, Kisfali P, Szabo A, Mihaly I, Molnar P, Melegh B, Szucs G. Trends in the epidemiology of human G1P[8] rotaviruses: a hungarian study. J. Infect. Dis. 2009;200(Suppl. 1):S222–S227. doi: 10.1086/605052. [DOI] [PubMed] [Google Scholar]
- Banyai K, Papp H, Dandar E, Molnar P, Mihaly I, Van Ranst M, Martella V, Matthijnssens J. Whole genome sequencing and phylogenetic analysis of a zoonotic human G8P[14] rotavirus strain. Infect. Genet. Evol. 2010;10:1140–1144. doi: 10.1016/j.meegid.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Banyai K, Mijatovic-Rustempasic S, Hull JJ, Esona MD, Freeman MM, Frace AM, Bowen MD, Gentsch JR. Sequencing and phylogenetic analysis of the coding region of six common rotavirus strains: evidence for intragenogroup reassortment among co-circulating G1P[8] and G2P[4] strains from the United States. J. Med. Virol. 2011;83:532–539. doi: 10.1002/jmv.21977. [DOI] [PubMed] [Google Scholar]
- Banyai K, Laszlo B, Duque J, Steele AD, Nelson EA, Gentsch JR, Parashar UD. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine. 2012;30(Suppl. 1):A122–A130. doi: 10.1016/j.vaccine.2011.09.111. [DOI] [PubMed] [Google Scholar]
- Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgan MA, Mori Y, Ito N, Sugiyama M, Minamoto N. Antigenic analysis of nonstructural protein (NSP) 4 of group A avian rotavirus strain PO-13. Microbiol. Immunol. 2003;47:661–668. doi: 10.1111/j.1348-0421.2003.tb03429.x. [DOI] [PubMed] [Google Scholar]
- Claeson M, Bos ER, Mawji T, Pathmanathan I. Reducing child mortality in India in the new millennium. Bull. World Health Organ. 2000;78:1192–1199. [PMC free article] [PubMed] [Google Scholar]
- Das BK, Gentsch JR, Cicirello HG, Woods PA, Gupta A, Ramachandran M, Kumar R, Bhan MK, Glass RI. Characterization of rotavirus strains from newborns in New Delhi, India. J. Clin. Microbiol. 1994;32:1820–1822. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esona MD, Armah GE, Steele AD. Rotavirus VP4 and VP7 genotypes circulating in Cameroon: identification of unusual types. J. Infect. Dis. 2010a;202(Suppl.):S205–S211. doi: 10.1086/653575. [DOI] [PubMed] [Google Scholar]
- Esona MD, Mijatovic-Rustempasic S, Conrardy C, Tong S, Kuzmin IV, Agwanda B, Breiman RF, Banyai K, Niezgoda M, Rupprecht CE, Gentsch JR, Bowen MD. Reassortant group A rotavirus from straw-colored fruit bat (Eidolon helvum) Emerg. Infect. Dis. 2010b;16:1844–1852. doi: 10.3201/eid1612.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esona MD, Banyai K, Foytich K, Freeman M, Mijatovic-Rustempasic S, Hull J, Kerin T, Steele AD, Armah GE, Geyer A, Page N, Agbaya VA, Forbi JC, Aminu M, Gautam R, Seheri LM, Nyangao J, Glass R, Bowen MD, Gentsch JR. Genomic characterization of human rotavirus G10 strains from the African rotavirus network: relationship to animal rotaviruses. Infect. Genet. Evol. 2011;11:237–241. doi: 10.1016/j.meegid.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Estes MK, Kapikian A. Rotaviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5th ed. Kluwer/Lippincott, Williams and Wilkins; Philadelphia, PA: 2007. pp. 1917–1974. [Google Scholar]
- Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, Das BK, Bhan MK. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, Jain V, Cunliffe NA, Nakagomi O, Kirkwood CD, Fischer TK, Parashar UD, Bresee JS, Jiang B, Glass RI. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J. Infect. Dis. 2005;192:S146–S159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–332. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- Green KY, Sears JF, Taniguchi K, Midthun K, Hoshino Y, Gorziglia M, Nishikawa K, Urasawa S, Kapikian AZ, Chanock RM, et al. Prediction of human rotavirus serotype by nucleotide sequence analysis of the VP7 protein gene. J. Virol. 1988;62:1819–1823. doi: 10.1128/jvi.62.5.1819-1823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KY, Hoshino Y, Ikegami N. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology. 1989;168:429–433. doi: 10.1016/0042-6822(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Iturriza Gomara M, Wong C, Blome S, Desselberger U, Gray J. Molecular characterization of VP6 genes of human rotavirus isolates: correlation of genogroups with subgroups and evidence of independent segregation. J. Virol. 2002;76:6596–6601. doi: 10.1128/JVI.76.13.6596-6601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gomara M, Isherwood B, Desselberger U, Gray J. Reassortment in vivo: driving force for diversity of human rotavirus strains isolated in the United Kingdom between 1995 and 1999. J. Virol. 2001;75:3696–3705. doi: 10.1128/JVI.75.8.3696-3705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerin TK, Kane EM, Glass RI, Gentsch JR. Characterization of VP6 genes from rotavirus strains collected in the United States from 1996 to 2002. Virus Genes. 2007;35:489–495. doi: 10.1007/s11262-007-0119-7. [DOI] [PubMed] [Google Scholar]
- Kirkwood C, Masendycz PJ, Coulson BS. Characteristics and location of cross-reactive and serotype-specific neutralization sites on VP7 of human G type 9 rotaviruses. Virology. 1993;196:79–88. doi: 10.1006/viro.1993.1456. [DOI] [PubMed] [Google Scholar]
- Kirkwood C, Bogdanovic-Sakran N, Palombo E, Masendycz P, Bugg H, Barnes G, Bishop R. Genetic and antigenic characterization of rotavirus serotype G9 strains isolated in Australia between 1997 and 2001. J. Clin. Microbiol. 2003;41:3649–3654. doi: 10.1128/JCM.41.8.3649-3654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Gentsch JR, Nakagomi T, Nakagomi O, Glass RI. Characterization of serotype G9 rotavirus strains isolated in the United States and India from 1993 to 2001. J. Clin. Microbiol. 2003;41:3100–3111. doi: 10.1128/JCM.41.7.3100-3111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite JP, Carvalho-Costa FA, Linhares AC. Group A rotavirus genotypes and the ongoing Brazilian experience: a review. Mem. Inst. Oswaldo Cruz. 2008;103:745–753. doi: 10.1590/s0074-02762008000800001. [DOI] [PubMed] [Google Scholar]
- Martinez-Laso J, Roman A, Head J, Cervera I, Rodriguez M, Rodriguez-Avial I, Picazo JJ. Phylogeny of G9 rotavirus genotype: a possible explanation of its origin and evolution. J. Clin. Virol. 2009;44:52–57. doi: 10.1016/j.jcv.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Rahman M, Martella V, Xuelei Y, De Vos S, De Leener K, Ciarlet M, Buonavoglia C, Van Ranst M. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J. Virol. 2006;80:3801–3810. doi: 10.1128/JVI.80.8.3801-3810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, Patton JT, Rahman M, Van Ranst M. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008a;82:3204–3219. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Banyai K, Estes MK, Gentsch JR, Iturriza-Gomara M, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Patton JT, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Desselberger U, Van Ranst M. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008b;153:1621–1629. doi: 10.1007/s00705-008-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Rahman M, Van Ranst M. Two out of the 11 genes of an unusual human G6P[6] rotavirus isolate are of bovine origin. J. Gen. Virol. 2008c;89:2630–2635. doi: 10.1099/vir.0.2008/003780-0. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Bilcke J, Ciarlet M, Martella V, Banyai K, Rahman M, Zeller M, Beutels P, Van Damme P, Van Ranst M. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 2009;4:1303–1316. doi: 10.2217/fmb.09.96. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Heylen E, Zeller M, Rahman M, Lemey P, Van Ranst M. Phylodynamic analyses of rotavirus genotypes G9 and G12 underscore their potential for swift global spread. Mol. Biol. Evol. 2010;27:2431–2436. doi: 10.1093/molbev/msq137. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, Buesa J, Esona MD, Estes MK, Gentsch JR, Iturriza-Gomara M, Johne R, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Parreno V, Rahman M, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Patton JT, Desselberger U, Van Ranst M. Uniformity of rotavirus strain nomenclature proposed by the rotavirus classification working group (RCWG) Arch. Virol. 2011;156:1397–1413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Aguayo D, Gonzalez-Nilo FD, Patton JT. Shared and group-specific features of the rotavirus RNA polymerase reveal potential determinants of gene reassortment restriction. J. Virol. 2009;83:6135–6148. doi: 10.1128/JVI.00409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Davis K, McAllen JK, Spiro DJ, Patton JT. Intra-genotypic diversity of archival G4P[8] human rotaviruses from Washington, DC. Infect. Genet. Evol. 2011;11:1586–1594. doi: 10.1016/j.meegid.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic-Rustempasic S, Banyai K, Esona MD, Foytich K, Bowen MD, Gentsch JR. Genome sequence based molecular epidemiology of unusual US Rotavirus A G9 strains isolated from Omaha, USA between 1997 and 2000. Infect. Genet. Evol. 2011;11:522–527. doi: 10.1016/j.meegid.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Page N, Esona M, Armah G, Nyangao J, Mwenda J, Sebunya T, Basu G, Pyndiah N, Potgieter N, Geyer A, Steele AD. Emergence and characterization of serotype G9 rotavirus strains from Africa. J. Infect. Dis. 2010;202(Suppl.):S55–63. doi: 10.1086/653551. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J. Infect. Dis. 2009;200(Suppl. 1):S9–S15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- Parra GI. Seasonal shifts of group A rotavirus strains as a possible mechanism of persistence in the human population. J. Med. Virol. 2009;81:568–571. doi: 10.1002/jmv.21423. [DOI] [PubMed] [Google Scholar]
- Parra GI, Bok K, Martinez V, Russomando G, Gomez J. Molecular characterization and genetic variation of the VP7 gene of human rotaviruses isolated in Paraguay. J. Med. Virol. 2005;77:579–586. doi: 10.1002/jmv.20495. [DOI] [PubMed] [Google Scholar]
- Phan TG, Okitsu S, Maneekarn N, Ushijima H. Genetic heterogeneity, evolution and recombination in emerging G9 rotaviruses. Infect. Genet. Evol. 2007;7:656–663. doi: 10.1016/j.meegid.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Posada D. JModelTest: phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Rahman M, Sultana R, Ahmed G, Nahar S, Hassan ZM, Saiada F, Podder G, Faruque AS, Siddique AK, Sack DA, Matthijnssens J, Van Ranst M, Azim T. Prevalence of G2P[4] and G12P[6] rotavirus, Bangladesh. Emerg. Infect. Dis. 2007;13:18–24. doi: 10.3201/eid1301.060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig RF. Genetics of the rotaviruses. Annu Rev Microbiol. 1997;51:225–255. doi: 10.1146/annurev.micro.51.1.225. [DOI] [PubMed] [Google Scholar]
- Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- Schrodinger LLC. The PyMOL Molecular Graphics System, Version 1.3r1. 2010. [Google Scholar]
- Steele AD, Ivanoff B. Rotavirus strains circulating in Africa during 1996-1999: emergence of G9 strains and P[6] strains. Vaccine. 2003;21:361–367. doi: 10.1016/s0264-410x(02)00616-3. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J, Burton A, Boschi-Pinto C, Steele A, Duque J, Parashar U, Global a.t.W.-c., Network/, R.S. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet. 2011;11 doi: 10.1016/S1473-3099(11)70253-5. DOI:10.1016/S1473-3099(1011)70253-70255. [DOI] [PubMed] [Google Scholar]
- Tian P, Estes MK, Hu Y, Ball JM, Zeng CQ, Schilling WP. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J. Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Ball JM, Zeng CQ, Estes MK. The rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activity. J. Virol. 1996;70:6973–6981. doi: 10.1128/jvi.70.10.6973-6981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- Wu FT, Banyai K, Huang JC, Wu HS, Chang FY, Yang JY, Hsiung CA, Huang YC, Lin JS, Hwang KP, Jiang B, Gentsch JR. Diverse origin of P[19] rotaviruses in children with acute diarrhea in Taiwan: detection of novel lineages of the G3, G5, and G9 VP7 genes. J. Med. Virol. 2011;83:1279–1287. doi: 10.1002/jmv.22052. [DOI] [PubMed] [Google Scholar]
- Zhang J. Rates of conservative and radical nonsynonymous nucleotide substitutions in mammalian nuclear genes. J. Mol. Evol. 2000;50:56–68. doi: 10.1007/s002399910007. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zeng CQ, Dong Y, Ball JM, Saif LJ, Morris AP, Estes MK. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virus virulence. J. Virol. 1998;72:3666–3672. doi: 10.1128/jvi.72.5.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.