Abstract
Aim
Many diseases of the heart are characterised by changes in substrate utilisation, which is in part regulated by the activity of the enzyme pyruvate dehydrogenase (PDH). Consequently, there is much interest in the in vivo evaluation of PDH activity in a range of physiological and pathological states to obtain information regarding the metabolic mechanisms of cardiac diseases. Hyperpolarized [1-13C]pyruvate, detected using MRS, is a novel technique for evaluating PDH flux non-invasively. PDH flux has been assumed to directly reflect in vivo PDH activity, although to date this assumption remains unproven.
Methods
Control animals and animals undergoing interventions known to modulate PDH activity, namely high fat feeding and dichloroacetate infusion, were used to investigate the relationship between in vivo hyperpolarized MRS measurements of PDH flux and ex vivo measurements of PDH enzyme activity (PDHa). Further, the plasma concentrations of pyruvate and other important metabolites were evaluated following pyruvate infusion to assess the metabolic consequences of the pyruvate infusion during hyperpolarized MRS experiments.
Results
Hyperpolarized MRS measurements of PDH flux significantly correlated with ex vivo measurements of PDHa, confirming that PDH activity directly influences the in vivo flux of hyperpolarized pyruvate through cardiac PDH. The maximum plasma concentration of pyruvate reached during hyperpolarized MRS experiments was ~250 μM, equivalent to physiological pyruvate concentrations reached during exercise or with dietary interventions. Concentrations of other metabolites, including lactate, glucose and β-hydroxybutyrate (BHB), did not vary during the 60 s following pyruvate infusion. Hence, during the 60 s data acquisition period, metabolism was minimally affected by pyruvate infusion.
Keywords: Pyruvate Dehydrogenase, PDH, Dichloroacetate, DCA, High Fat Feeding, Hyperpolarization, Magnetic Resonance Spectroscopy
Introduction
Myocardial metabolism is a highly regulated process that enables the heart to adapt to alterations in substrate supply, circulating hormone levels, coronary blood flow and myocardial oxygen supply. In order to maintain a constant high level of ATP synthesis regardless of the physiological environment, the heart can utilise many different substrates for energy generation, including fatty acids, glucose, lactate and ketone bodies. In the healthy adult myocardium, fatty acids are oxidized preferentially to other substrates, contributing 60-70% of total ATP production [1]. In contrast, many diseases of the heart can be characterised by alterations in substrate preference. For example the failing heart demonstrates an increased reliance upon glucose for energy generation, whilst fatty acid oxidation is decreased [2, 3]. Cardiac substrate utilisation is, in part, regulated by the activity of pyruvate dehydrogenase (PDH) [4-6]. PDH irreversibly converts the end product of glycolysis, pyruvate, to acetyl CoA, which can subsequently be used to generate ATP via the Krebs cycle. The PDH catalysed reaction is primarily regulated by a reversible phosphorylation cycle. PDH is covalently inactivated by phosphorylation via the enzyme PDH kinase (PDK). PDH is dephosphorylated and reactivated by PDH phosphatase (PDP). The relative activities of PDK and PDP determine the proportion of the PDH enzyme complex present in the active form, thus determining PDH activity. [4-6]. Therefore, by evaluating PDH activity in a range of physiological and pathological states, key information regarding the metabolic mechanisms of heart disease can be obtained.
Current methods of measuring PDH activity use ex vivo biochemical assays [7]. However, such invasive methods cannot account for the transient effects of hormones and substrate supply and are highly sensitive to tissue corruption from preparation and enzyme extraction processes. Further, biochemical assays cannot be used to monitor PDH activity repeatedly in the same organism. Therefore, the ability to assess PDH activity in vivo could provide a new understanding of the role of PDH in the development of cardiac diseases, such as heart failure, as well as providing a tool for the evaluation of metabolic interventions.
The recent development of hyperpolarized magnetic resonance spectroscopy (MRS) has enabled the non-invasive assessment of cardiac PDH flux in vivo [8, 9]. Hyperpolarized MRS improves MR signal-to-noise ratio (SNR) more than 10,000-fold [8] making it possible to non-invasively visualise the uptake of 13C-labelled metabolites and their metabolic conversion through specific enzymes in real time [9-11]. Using hyperpolarized [1-13C]pyruvate, PDH flux can be assessed by monitoring the rate of production of [13C]bicarbonate, a by-product of the PDH mediated reaction which exists in pH dependent equilibrium with 13CO2 [11]. However, the relationship between the in vivo assessment of PDH flux, obtained using hyperpolarized MRS, and ex vivo measurements of PDH activity remains to be established.
Previous studies designed to assess in vivo cardiac metabolism have primarily used positron emission tomography (PET) in conjunction with radiolabelled 2-fluoro-2-deoxy-D-glucose [12]. Although PET can be used to provide information about individual reactions, the detected signal represents the sum of both the tracer and the biochemical products produced by the heart and is therefore limited to the study of single specific reactions [10]. The other major disadvantage of this approach is that it exposes patients to radiation. In contrast to this, hyperpolarized MRS evaluates flux through multiple enzymes simultaneously as it can differentiate starting substrates from biochemical products. Furthermore, this approach only requires infusion of metabolites naturally occurring within the body, albeit at greater than physiological concentrations [11]. In this study, control animals and animals undergoing interventions known to modulate PDH activity, namely high fat feeding and dichloroacetate (DCA) infusion, were used to investigate the relationship between in vivo cardiac PDH flux, measured using hyperpolarized MRS, and the activity of the PDH enzyme measured using a conventional ex vivo assay. In a final experiment, the plasma concentrations of pyruvate and other important metabolites were evaluated following pyruvate infusion to assess the metabolic effects of infusing pyruvate during hyperpolarized MRS experiments.
Experimental
The [1-13C]pyruvic acid and OX063 trityl radical were obtained from GE Healthcare (Amersham, UK) and the gadolinium compound 1,3,5-tris-(N-(DO3A-acetamido)-N-methyl-4-amino-2-methylphenyl)-(1,3,5)-triazinane-2,4,6-trione, referred to here as 3-Gd, was obtained from Imagnia AB (Malmo, Sweden). Rats were housed on a 12:12-h light/dark cycle in animal facilities at the University of Oxford (lights on 7AM and off at 7PM). All animal studies were performed between 7AM and 1PM with the animals in the fed state. All investigations conformed to Home Office Guidance on the Operations of the Animals (Scientific Procedures) Act 1986 and to institutional guidelines.
Control Studies
Eleven male Wistar rats (~ 300 g, to match the end weight of the high fat fed animals) were examined using the hyperpolarized MRS protocol described below to act as controls.
High Fat Feeding
Seven male Wistar rats (~ 200 g) were placed on a high fat diet, with 55% of calories derived from saturated fat, 29% from protein and 16% from carbohydrates. Rats were examined using the hyperpolarized MRS protocol 4 weeks after implementation of the high fat diet.
Dichloroacetic Acid Infusion
Six male Wistar rats (~ 300 g, to match the end weight of the high fat fed animals) received a 1 ml bolus of DCA (30 mg/ml), followed by a 0.5 ml infusion over 15 min immediately prior to assessment of PDH flux using the hyperpolarized MRS protocol described below [13].
Hyperpolarized MRS Protocol
As previously described [14], animals were anaesthetised with isoflurane (2% in O2), and a catheter was inserted into the tail vein for intravenous administration of the hyperpolarized solution. ECG, respiration and body temperature were monitored throughout the experiment. A 1H/13C butterfly coil was placed over the rat chest and rats were positioned in a 7T horizontal bore MR scanner interfaced to a Varian Inova console (Varian Medical Systems, Palo Alto, USA). Correct positioning was confirmed by the acquisition of an axial proton FLASH image (TE/TR = 1.17/2.33 ms, matrix size = 64 × 64, FOV = 60 × 60 mm, slice thickness = 2.5 mm, excitation flip angle = 15°). An ECG-gated shim was used to reduce the proton linewidth to approximately 120 Hz. Anaesthesia was maintained by means of isoflurane (~1.7%) delivered to, and scavenged from, a nose cone during the experiment.
Approximately 40 mg [1-13C]pyruvate doped with 15 mM trityl radical and a trace amount of 3-Gd, was hyperpolarized in a prototype polarizer system with 45 min of microwave irradiation (GE Healthcare, Amersham, UK). [8]. The sample was subsequently dissolved in a pressurized and heated alkaline solution, containing 40 mM NaOH, 40 mM TRIS base buffer, and 0.27 mM EDTA. The resultant liquid contained 80 mM hyperpolarized sodium [1-13C]pyruvate with a polarization of ~ 25%, at physiological temperature and pH. One milliliter of the hyperpolarized solution was infused into each rat over 10 s via the tail vein catheter. Immediately prior to infusion, an ECG-gated 13C-MR pulse-acquire sequence was initiated and 60 individual cardiac spectra were acquired over 1 minute following infusion. Animals infused with DCA, and relevant control animals (n=5) were sacrificed immediately post hyperpolarized MRS analysis to preserve the effect of the DCA infusion on cardiac metabolism. In contrast, high fat fed animals and relevant controls (n=6) were sacrificed ~1 hour post MRS analysis. By varying the time the two control groups were sacrificed, it was possible to investigate the effect of pyruvate infusion on PDH activity. All animals were euthanized by exsanguination following anaesthesia overdose and loss of corneal reflexes. Hearts were removed, snap frozen in liquid N2 and stored at −80 °C for subsequent biochemical assessment.
MRS Data Analysis and Kinetic Modelling
Cardiac 13C MR spectra were analysed using the AMARES algorithm as implemented in the jMRUI software package [11, 15]. The resulting data were analysed using three different methods in order to determine the best approach for assessing in vivo PDH flux. Firstly, The peak areas of [1-13C]pyruvate and 13C-bicarbonate at each time point were quantified and used as input data for the kinetic model developed by Zierhut et al. specifically for the analysis of hyperpolarised MRS data [16]. Firstly the change in [1-13C]pyruvate signal over the 60 s acquisition time was fit to the integrated [1-13C]pyruvate peak area data using the equation:
| (1) |
In this equation, Mpyr(t) represents the [1-13C]pyruvate peak area as a function of time. This equation fits the parameters kpyr, the rate constant for pyruvate signal decay (s−1), rateinj, the pyruvate arrival rate (a.u. s−1), and tarrival, the pyruvate arrival time (s). The parameter tend is the sum of tarrival and the known injection duration (in this case 10 s). These parameters were then used to fit the following equation which uses the dynamic 13C-bicarbonate data to calculate kpyr→bic, the rate constant for pyruvate to bicarbonate exchange (s−1), and kbic, the rate constant for bicarbonate signal decay (s−1) which was assumed to consist of metabolite T1 decay and signal loss from the 5° RF flip angle pulses.
| (2) |
Two alternative assessments of PDH flux were obtained by dividing the maximum [1-13C]bicarbonate peak area by both the maximum [1-13C]pyruvate peak area and the maximum [1-13C]alanine peak area.
PDH Activity
The activity of the active and total fractions of PDH (PDHa and PDHt) were determined spectrophotometrically by the method of Seymour and Chatham [7]. Briefly, the assay required the preparation of cardiac tissue with one of two homogenisation buffers for either PDHa or PDHt measurement. PDHa was assessed when PDH was extracted under conditions where both PDP and PDK were inhibited (25 mM HEPES, 25 mM KH2PO4, 25 mM KF, 1 mM DCA, 3 mM EDTA, 1 mM ADP, 1 mM dithiothreitol, 0.05 mM leupeptin, 1 % Triton X-100; pH = 7.2). PDHt was assessed under conditions were PDP was stimulated by Mg2+, and PDK inhibited by DCA and ADP (75 mM HEPES, 5 mM DCA, 5 mM MgCl2, 1 mM ADP, 1 mM dithiothreitol, 0.05 mM leupeptin, 1 % Triton X-100; pH = 7.2).
Frozen cardiac tissue was powdered and 0.2 g was homogenized in 1 ml of the appropriate homogenization buffer using a polytron (30 s). The sample was snap frozen in liquid N2, thawed and re-homogenized 3 times. The sample was centrifuged (3,400 rpm, 7 min) and the supernatant removed for analysis. Assay buffer (950 μl; 50 mM HEPES, 1 mM MgCl2, 0.08 mM EGTA, 1 mM dithiothreitol, 4 μM rotenone, 1.7 mM NAD, 0.1 mM coenzymeA, 0.2 mM thiamine pyrophosphate HCl, and 16.7 mM lactate; pH = 7.2) was incubated with 2 μl LDH at 30 °C for 5 min. PDH activity was determined by adding and mixing a 25 μl aliquot of either PDHa or PDHt extract to the assay buffer and immediately following the reaction at 340 nm using the kinetic program on a spectrophotometer (2 min for both PDHa and PDHt samples). The rate of NADH production over the first 30 s was used to determine activity in units of μmol/min/g wet weight.
Plasma metabolites
Male Wistar rats (~250 g, n=14) were anaesthetised (2% isoflurane in O2) and the left femoral artery cannulated for blood sampling. A 2 cm ventral skin incision was made along the crease formed by the abdomen and the left thigh and the adductor muscles were blunt dissected to visualise the left femoral artery. A small incision was made into the artery and a polyethylene cannula was inserted into the vessel and secured in place with a suture. A second cannula was inserted into the tail vein for pyruvate infusion. Approximately 0.4 ml blood was sampled from the femoral artery for baseline metabolite measurements. One millilitre of 80 mM sodium pyruvate was infused via the tail vein and further 0.4 ml blood samples were collected at 30 s, 1 min, 2 min, 5 min, 10 min and 30 min post infusion. A 50 μl whole blood sample was separated from each larger sample and extracted using 7% ice cold perchloric acid. All samples were then immediately centrifuged (3,400 rpm for 10 min at 4 °C), and plasma was removed. A 50 μl aliquot of plasma (not treated with perchloric acid) was separated and 1 μl tetrahydrolipstatin (THL, 30 μg/ml) was added for non-esterified fatty acid (NEFA) analysis. Pyruvate concentrations were determined spectrophotometrically from the perchloric acid treated plasma using a pyruvate assay kit (Biovision). An ABX Pentra 400 (Horiba ABX Diagnostics) was used to perform plasma assays for glucose, lactate, β-hydroxybutyrate, NEFA and triacylglyceride (TAG). Insulin levels were measured using a rat insulin ELISA (Mercodia).
Statistical Analysis
Values reported are mean ± SEM. For the comparison between control, DCA treated and high fat fed rats, statistical significance was assessed using a two sample t-test assuming unequal variance and considered at the P < 0.05 level. Differences in metabolite concentrations in serially collected plasma samples were assessed using repeated measures ANOVA with post-hoc multiple paired t-tests employing a Bonferroni correction.
Results
In Vivo PDH Flux Assessment using Hyperpolarized MRS
In this study, PDH activity and PDH flux were assessed in four groups of rats; one group fed a high fat diet for 4 weeks (known to result in end product inhibition of PDH through excess acetyl CoA from enhanced fatty acid oxidation), one group infused with DCA (known to activate PDH through PDK inhibition) and the two respective control groups. PDH activity was measured using a well characterised ex vivo biochemical approach and PDH flux was determined in vivo using hyperpolarized MRS (Figure 1 shows example annotated 13C-MRS spectra for the different groups). Three different methods of assessing in vivo PDH flux from the hyperpolarized MRS data were tested in this study. In the first method the dynamic in vivo data was fitted to the kinetic model developed by Zierhut and colleagues for the analysis of hyperpolarized MRS data [16]. In the second method, used in a number of previously published hyperpolarized MRS studies [11, 17], the maximum [13C]bicarbonate peak area was divided by the maximum [1-13C]pyruvate peak area to normalise out any differences in polarization levels between experiments. In the third method, the maximum [13C]bicarbonate peak area was normalised to the maximum [1-13C]alanine peak area. A number of studies utilising radiolabelled pyruvate have suggested that labelled alanine represents a robust internal reference of intracellular pyruvate [18-20], therefore the maximum [1-13C]alanine peak area may be a good value to normalise against in hyperpolarized MRS studies. By using and comparing all three analyses, the best method of interpreting hyperpolarized MRS data could be found. Figure 1 summarises the results obtained using the various analysis techniques. Interestingly, the three approaches gave very similar results. The rate constants for the conversion of pyruvate to bicarbonate (kpyr→bic), obtained by modelling the in vivo data, correlated significantly with both the maximum [13C]bicarbonate/ maximum [1-13C]pyruvate values (P < 0.001; Figure 2A) and the maximum [13C]bicarbonate/ maximum [1-13C]alanine values (P < 0.001; Figure 2B). However, although this suggests that any of the three analysis techniques could be used to interpret the in vivo MRS data, when correlated against the ex vivo measurements of active PDH activity, the modelled data produced the most robust correlation (R2 = 0.86, P < 0.001; Figures 2D-F). This is likely due to the fact that the kinetic model, unlike other analysis approaches, considered the effect of variables including T1 relaxation, injection speed and rate of removal of metabolites, all of which can influence the hyperpolarized 13C-MRS signal. Therefore in all subsequent comparisons, only the modelled data is considered.
Figure 1.
Example annotated spectra from each of the 3 experimental groups used in this study (t=10 s).
Figure 2.
Comparison of the different techniques used to analyse the in vivo hyperpolarized 13C-MRS data. A – C shows the similarity between the results of the three different analyses. D – F shows the correlation between the results of the three analysis techniques and the activity of the active PDH fraction, determined ex vivo.
Comparing In Vivo PDH Flux and Ex Vivo PDH Activity Measurements
The ex vivo activity of the total PDH and active PDH fractions was not significantly different between the two control groups (total: 5.28 ± 0.7 and 4.91 ± 0.5 μmol.min.gww; active: 0.96 ± 0.3 and 1.23 ± 0.4 μmol.min.gww in the DCA controls and the HFF controls respectively). The DCA control hearts were harvested immediately post infusion of pyruvate, whereas the HFF control hearts were harvested ~1 hr post pyruvate infusion. This result confirms that the pyruvate infusion has no significant effect on PDH expression or phosphorylation in control animals up to 1 hr post infusion. Therefore, in subsequent analyses, all control animals are combined into a single control group. The activity of the total PDH in control animals and high fat fed animals was not significantly different (Table 1; P = 0.06). In contrast, DCA treated animals had significantly higher levels of total PDH activity than control animals (Table 1; P < 0.001).
Table 1.
Ex vivo measurements of PDHa and PDHt determined spectrophotometrically, and the in vivo rate constants for the conversion of pyruvate to bicarbonate (Kpyr→bic), determined using hyperpolarized MRS.
| Group | PDHa (μmol.min.gww) | PDHt (μmol.min.gww) | Kpyr→bic (s−1) |
|---|---|---|---|
| Control | 1.11 ± 0.2 | 5.08 ± 0.4 | 0.0039 ± 0.0004 |
| High Fat Fed | 0.77 ± 0.2 | 6.53 ± 0.6 | 0.0027 ± 0.0004* |
| DCA Infused | 6.75 ±0.7* | 7.86 ± 0.3* | 0.0103 ± 0.0008* |
P < 0.05 vs. controls
Infusion of DCA caused a significant 6.1-fold increase in PDHa relative to control values (Table 1; P = 0.02). Kinetic modelling of the in vivo hyperpolarized MRS data provided a quantitative rate constant for the conversion of pyruvate to bicarbonate in each animal (kpyr→bic). DCA infusion resulted in a 2.6-fold increase in kpyr→bic relative to control animals (Table 1; P < 0.001).
The provision of a high fat diet for 4 weeks significantly reduced the kpyr→bic rate constant to 69% of the control value (Table 1; P = 0.04). In contrast, the ex vivo biochemical approach failed to identify a significant difference in PDHa following 4 weeks of high fat feeding (Table 1; P = 0.3).
Using data from all animals it was observed that the kpyr→bic rate constant, determined in vivo using hyperpolarized MRS with subsequent kinetic modelling of the data, correlated significantly with the measurements of active PDH enzyme activity determined spectrophotometrically (P < 0.001; Figure 2F).
Effect of Pyruvate Infusion on Systemic Metabolism
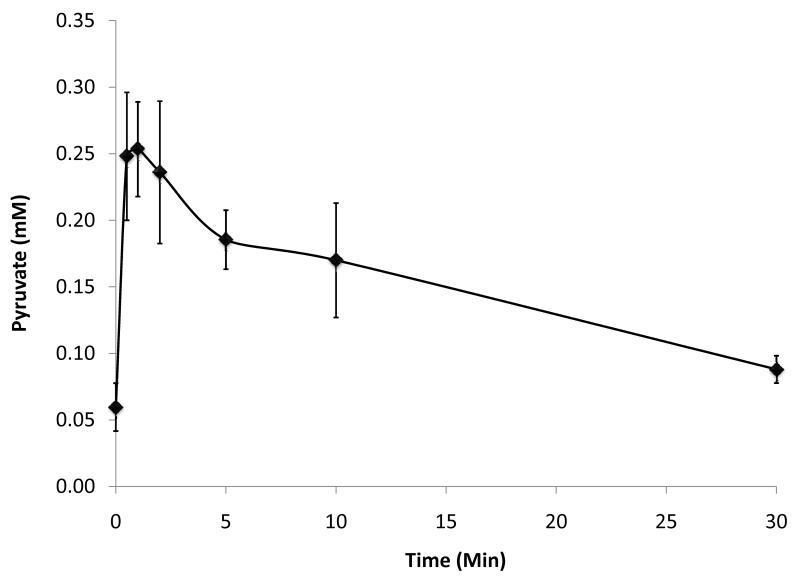
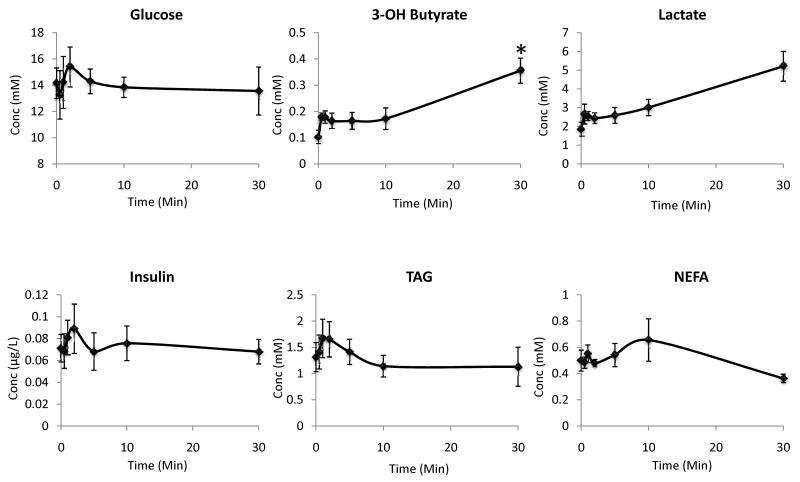
A second aim of this work was to investigate the metabolic effects of infusing pyruvate during hyperpolarized MRS experiments. The results are summarised in Figures 3 and 4. At baseline, the pyruvate concentration was found to be 60 ± 20 μM, which increased to 250 ± 40 μM at 1 min post 1 ml infusion of 80 mM sodium pyruvate (Figure 3). Pyruvate concentrations remained high until 10 min post infusion, by which point plasma pyruvate levels were no longer significantly different from baseline values (Figure 3). The circulating concentrations of glucose, insulin, lactate, TAG and NEFA did not alter significantly within 30 min of pyruvate infusion (Figure 4). The only significant metabolic change observed was a 3.5-fold increase in β-hydroxybutyrate at 30 min post pyruvate infusion (P < 0.01; Figure 4).
Figure 3.
Plasma pyruvate concentration post infusion of 1 ml 80 mM sodium pyruvate (*P < 0.01 versus baseline value)
Figure 4.
Plasma metabolic profiles post infusion of 1 ml 80 mM sodium pyruvate (*P < 0.01 versus baseline value)
Discussion
This study was designed to verify that non-invasive hyperpolarized MRS measurements of PDH flux accurately reflected the metabolic status of the heart. Using interventions known to modulate PDH activity we have for the first time shown that in vivo PDH flux, measured using hyperpolarized MRS, correlated significantly with ex vivo measurements of PDHa, thereby confirming that PDH activity directly influences in vivo flux of [1-13C]pyruvate through cardiac PDH. However, Table 1 and Figure 2F indicate that our measurement of PDH flux may be fundamentally different from PDH activity measurements determined ex vivo. Data in Table 1 shows that the increase in PDHa in response to DCA infusion, is much greater than the measured increase in in vivo PDH flux. Figure 2F demonstrates that, despite a strong correlation between the in vivo and ex vivo measurements, the correlation line between the two variables fails to intercept the origin. The finding that in vivo PDH flux and ex vivo PDH activity represent two distinct measurements is in many ways unsurprising. The ex vivo biochemical assay is designed to quantify the proportion of total PDH present in the active de-phosphorylated form as determined by PDK and PDP activity/expression. Consequently, the extraction protocol preserves the phosphorylation status of the enzyme complex, but disrupts any transient effects on enzyme regulation. Therefore, it cannot account for all mechanisms affecting PDH flux in vivo. For instance, in healthy control rats with relatively high levels of PDH activity, end product inhibition resulting from high concentrations of acetyl CoA or NADH may reduce PDH flux without affecting PDH phosphorylation or PDP/PDK expression [21, 22]. Furthermore, in rats fed a high fat diet, pyruvate can covalently inhibit PDK, thus transiently increasing both PDHa and PDH flux [23]. Other important physiological factors such as pyruvate delivery and uptake into cells are also ignored by ex vivo assays. In contrast, in vivo measurements of hyperpolarized [13C]bicarbonate production reveal the effects of short term constraints on PDH flux, and account for alterations in substrate delivery. Measuring PDH flux non-invasively also avoids potential effects from tissue disruption caused during tissue preparation and enzyme extraction processes.
In this study we evaluated PDH activity in control animals, as well as animals infused with DCA and animals fed a high fat diet for 4 weeks. DCA is a potent inhibitor of PDK, which increases PDH activity and promotes glucose oxidation [24, 25]. In contrast, provision of a high fat diet for 4 weeks has been shown to reduce PDH activity, with increased activity and expression of the lipid-responsive PDK isoform, PDK4 [26-28]. Our results confirmed that DCA administration markedly increased PDH flux. Our in vivo hyperpolarized MRS experiments revealed a 2.6-fold increase in PDH flux. This finding is consistent in magnitude with literature values showing a 2-3-fold increase in PDH activity upon DCA treatment [29-31]. In contrast to this, our ex vivo assay revealed a 6.1-fold increase in PDH activity. This discrepancy may be due to the absence of additional regulatory mechanisms ex vivo, as well as differences in the PDHa assay protocol used in the various studies. Our in vivo experiments revealed a 31% reduction in PDH flux, relative to control levels, following 4 weeks of high fat feeding. This was similar to literature values showing a reduction in PDH activity of ~40-65% in fat fed rats [26, 27]. However, the ex vivo biochemical assay did not detect a significant reduction in the activity of the active PDH fraction. These results imply that the noninvasive in vivo assessment of metabolic parameters made by hyperpolarized MRS may offer an improvement in sensitivity over invasive biochemical assays.
One disadvantage of the hyperpolarized MRS approach is that it requires infusion of pyruvate at levels which may alter the metabolic system being examined. Upon dilution in the blood, a 1 ml infusion of 80 mM pyruvate can potentially generate a maximum plasma pyruvate concentration of ~ 4 mM [32]. Based on previous biochemical studies of the PDH enzyme complex it is unlikely that changes to the enzyme’s phosphorylation state will have occurred within the 60 s experimental timeframe [27, 33-35]. However, if circulating concentrations of pyruvate and its associated metabolites alter dramatically during hyperpolarized MRS experiments, processes such as end product inhibition could have profound effects on enzymatic fluxes. For this reason, we infused a set of animals with 1 ml of 80 mM pyruvate and collected blood samples at various time points up to 30 minutes post infusion in order to define the effect on circulating metabolite levels. It was found that circulating pyruvate concentrations peaked at ~ 250 μM. This is in line with the findings of Otonkoshi and co-workers who infused humans with 13.9 mmol pyruvate into a total blood pool of ~ 5 L, which could have resulted in a maximum plasma concentration of ~ 2.3 mM. However, analysis revealed plasma pyruvate levels peaked at only ~ 150 μM [36]. In our study and in the study by Otonkoshi et al., the measured circulating concentration of pyruvate was considerably lower than the calculated potential maximum concentration, suggesting that the injected pyruvate was rapidly removed from the circulation, either through rapid uptake into various organs or through rapid metabolism into other substrates in the blood.
In our study, the baseline plasma concentration of pyruvate was ~ 60 μM, therefore a circulating concentration of 250 μM during hyperpolarized MRS was significantly higher than physiological levels. However, physiological plasma pyruvate concentrations of 250 μM are possible and such levels have been measured during exercise [37, 38] and with certain dietary modifications, such as high fat feeding [39]. This therefore indicates that the pyruvate concentrations reached during hyperpolarized MRS experiments should not perturb systemic metabolism beyond the normal physiological. Consistent with this, no significant alterations in the concentration of glucose, insulin, lactate, β-hydroxybutyrate, TAG or NEFA were detected up to 60 s post pyruvate infusion, which corresponds to the time during which the hyperpolarized MRS data would be collected. Furthermore, there were no alterations in circulating levels of glucose, insulin, lactate, TAG or NEFA up to 30 min post pyruvate infusion. The only significant difference in circulating metabolite levels was detected at 30 min post infusion, at which time the concentration of β-hydroxybutyrate was increased. This increase in β-hydroxybutyrate may indicate that the metabolism of pyruvate via PDH resulted in a high concentration of acetyl CoA as it is possible that any excess acetyl CoA which could not be immediately processed via the Krebs cycle could have been converted to this ketone body in the liver. There was also an increase in lactate at 30 min, consistent with LDH mediated processing of the excess pyruvate however this failed to reach significance.
Limitations of the study
Although the study here has demonstrated the usefulness of hyperpolarized 13C-MRS for investigations of in vivo metabolism, there were some limitations associated with the methodology which should be addressed. Most importantly, is the evidence to suggest that the in vivo PDH flux measurements and the ex vivo PDHa measurements were fundamentally different. Although possible physiological reasons for this have been discussed, experimental factors may also contribute. Following DCA infusion, the delivered hyperpolarized [1-13C]pyruvate concentration may have been insufficient to maximise flux through PDH. This could account for the smaller increase in PDH flux detected by this technique relative to the ex vivo measurements. It is also possible that in control and HFF animals, the delivery of pyruvate resulted in stimulation of PDH flux which would no longer be detected at the point of tissue collection for ex vivo analysis. This could account for the failure of the correlation line to pass through the origin in Figure 2F. A further limitation of this study was that a limited sweep width was utilised in these experiments preventing the quantitation of 13CO2 and pH. As the quantified bicarbonate is a marker of intracellular pH and therefore sensitive to the pH of the cell, this information would have been useful to ascertain that the observed changes in PDH flux were not a consequence of altered pH. In future studies, a wider sweep width will be used to enable pH information to be collected.
In summary, this study has shown that hyperpolarized MRS represents a novel, sensitive method to accurately assess PDH activity, through the quantification of hyperpolarized [13C]bicarbonate formation, and that the infusion of pyruvate during such experiments has minimal effects on metabolism during the 60 s data acquisition period. Longer term alterations in circulating metabolite concentrations are evident post infusion of pyruvate, which should be a consideration when designing hyperpolarized MRS experiments, particularly studies using multiple injections of hyperpolarized tracers.
Acknowledgements
We thank Dr. Anne-Marie Seymour for her advice regarding the PDH activity assay and Dr. Jan-Henrik Ardenkjaer-Larsen for advice regarding hyperpolarization procedures. M. S. gratefully acknowledges the Newton Abraham Scholarship Foundation for her D.Phil. studentship and NIH grant 1-F31-EB006692-01A1. This work was funded by research grants from the Medical Research Council (MRC grant G0601490) and the British Heart Foundation (BHF grant PG/07/070/23365) and through equipment support from GE Healthcare.
Abbreviations
- ATP
Adenosine Triphosphate
- DCA
Dichloroacetic Acid
- LDH
Lactate Dehydrogenase
- MRS
Magnetic Resonance Spectroscopy
- NEFA
Non-Esterified Fatty Acid
- PDH
Pyruvate Dehydrogenase
- PDK
Pyruvate Dehydrogenase Kinase
- PDP
Pyruvate Dehydrogenase Phosphatase
- SNR
Signal-to-Noise Ratio
- TAG
Triacylglyceride
References
- 1.Opie LH. The heart : physiology, from cell to circulation. Lippincott-Raven; Philadelphia: 1998. [Google Scholar]
- 2.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 3.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Current problems in cardiology. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 4.Randle PJ. Fuel selection in animals. Biochemical Society transactions. 1986;14:799–806. doi: 10.1042/bst0140799. [DOI] [PubMed] [Google Scholar]
- 5.Reed LJ. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Current topics in cellular regulation. 1981;18:95–106. doi: 10.1016/b978-0-12-152818-8.50012-8. [DOI] [PubMed] [Google Scholar]
- 6.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiological reviews. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 7.Seymour AM, Chatham JC. The effects of hypertrophy and diabetes on cardiac pyruvate dehydrogenase activity. Journal of molecular and cellular cardiology. 1997;29:2771–2778. doi: 10.1006/jmcc.1997.0512. [DOI] [PubMed] [Google Scholar]
- 8.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golman K, 't Zandt R, Thaning M. Real-time metabolic imaging. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD, Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19773–19777. doi: 10.1073/pnas.0706235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder MA, Cochlin LE, Heather LC, Clarke K, Radda GK, Tyler DJ. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12051–12056. doi: 10.1073/pnas.0805953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phelps ME, Hoffman EJ, Selin C, Huang SC, Robinson G, MacDonald N, Schelbert H, Kuhl DE. Investigation of [18F]2-fluoro-2-deoxyglucose for the measure of myocardial glucose metabolism. J Nucl Med. 1978;19:1311–1319. [PubMed] [Google Scholar]
- 13.Sharma N, Okere IC, Brunengraber DZ, McElfresh TA, King KL, Sterk JP, Huang H, Chandler MP, Stanley WC. Regulation of pyruvate dehydrogenase activity and citric acid cycle intermediates during high cardiac power generation. The Journal of physiology. 2005;562:593–603. doi: 10.1113/jphysiol.2004.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyler DJ, Schroeder MA, Cochlin LE, Clarke K, Radda GK. Application of hyperpolarized magnetic resonance in the study of cardiac metabolism. Applied Magnetic Resonance. 2008;34:523–531. [Google Scholar]
- 15.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Computers in Biology and Medicine. 2001;31:269–286. doi: 10.1016/s0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 16.Zierhut ML, Yen YF, Chen AP, Bok R, Albers MJ, Zhang V, Tropp J, Park I, Vigneron DB, Kurhanewicz J, Hurd RE, Nelson SJ. Kinetic modeling of hyperpolarized 13C1-pyruvate metabolism in normal rats and TRAMP mice. J Magn Reson. 202:85–92. doi: 10.1016/j.jmr.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder MA, Atherton HJ, Ball DR, Cole MA, Heather LC, Griffin JL, Clarke K, Radda GK, Tyler DJ. Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. Faseb J. 2009;23:2529–2538. doi: 10.1096/fj.09-129171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewandowski ED. Metabolic heterogeneity of carbon substrate utilization in mammalian heart: NMR determinations of mitochondrial versus cytosolic compartmentation. Biochemistry. 1992;31:8916–8923. doi: 10.1021/bi00152a031. [DOI] [PubMed] [Google Scholar]
- 19.Peuhkurinen KJ, Hiltunen JK, Hassinen IE. Metabolic compartmentation of pyruvate in the isolated perfused rat heart. The Biochemical journal. 1983;210:193–198. doi: 10.1042/bj2100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peuhkurinen KJ, Nuutinen EM, Pietilainen EP, Hiltunen JK, Hassinen IE. Role of pyruvate carboxylation in the energy-linked regulation of pool sizes of tricarboxylic acid-cycle intermediates in the myocardium. The Biochemical journal. 1982;208:577–581. doi: 10.1042/bj2080577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins TA, Sugden MC, Holness MJ, Kozak R, Dyck JR, Lopaschuk GD. Control of cardiac pyruvate dehydrogenase activity in peroxisome proliferator-activated receptor-alpha transgenic mice. American journal of physiology. 2003;285:H270–276. doi: 10.1152/ajpheart.00852.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hansford RG, Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feed-back inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Archives of biochemistry and biophysics. 1978;191:65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- 23.Sugden MC, Orfali KA, Fryer LG, Holness MJ, Priestman DA. Molecular mechanisms underlying the long-term impact of dietary fat to increase cardiac pyruvate dehydrogenase kinase: regulation by insulin, cyclic AMP and pyruvate. Journal of molecular and cellular cardiology. 1997;29:1867–1875. doi: 10.1006/jmcc.1997.0425. [DOI] [PubMed] [Google Scholar]
- 24.Leiter AB, Weinberg M, Isohashi F, Utter MF. Relationshiop between phosphorylation and activity of pyruvate dehydrogenase in rat liver mitochondria and the absence of such a relationship for pyruvate carboxylase. The Journal of biological chemistry. 1978;253:2716–2723. [PubMed] [Google Scholar]
- 25.Whitehouse S, Randle PJ. Activation of pyruvate dehydrogenase in perfused rat heart by dichloroacetate (Short Communication) The Biochemical journal. 1973;134:651–653. doi: 10.1042/bj1340651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryson JM, Cooney GJ, Wensley VR, Phuyal JL, Hew M, Denyer GS, Caterson ID. High-fat feeding alters the response of rat PDH complex to acute changes in glucose and insulin. The American journal of physiology. 1995;268:E752–757. doi: 10.1152/ajpendo.1995.268.4.E752. [DOI] [PubMed] [Google Scholar]
- 27.Orfali KA, Fryer LG, Holness MJ, Sugden MC. Long-term regulation of pyruvate dehydrogenase kinase by high-fat feeding. Experiments in vivo and in cultured cardiomyocytes. FEBS letters. 1993;336:501–505. doi: 10.1016/0014-5793(93)80864-q. [DOI] [PubMed] [Google Scholar]
- 28.Sugden MC, Bulmer K, Holness MJ. Fuel-sensing mechanisms integrating lipid and carbohydrate utilization. Biochemical Society transactions. 2001;29:272–278. doi: 10.1042/0300-5127:0290272. [DOI] [PubMed] [Google Scholar]
- 29.Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Greenhaff PL. Muscle acetyl group availability is a major determinant of oxygen deficit in humans during submaximal exercise. The American journal of physiology. 1998;274:E377–380. doi: 10.1152/ajpendo.1998.274.2.E377. [DOI] [PubMed] [Google Scholar]
- 30.Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Hultman E, Kaijser L, Chwalbinska-Moneta J, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. Substrate availability limits human skeletal muscle oxidative ATP regeneration at the onset of ischemic exercise. The Journal of clinical investigation. 1998;101:79–85. doi: 10.1172/JCI1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke B, Wyatt KM, McCormack JG. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. Journal of molecular and cellular cardiology. 1996;28:341–350. doi: 10.1006/jmcc.1996.0032. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder MA, Atherton HJ, Cochlin LE, Clarke K, Radda GK, Tyler DJ. The effect of hyperpolarized tracer concentration on myocardial uptake and metabolism. Magn Reson Med. 2009;61:1007–1014. doi: 10.1002/mrm.21934. [DOI] [PubMed] [Google Scholar]
- 33.Cooper RH, Randle PJ, Denton RM. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975;257:808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- 34.Holness MJ, Sugden MC. Pyruvate dehydrogenase activities during the fed-to-starved transition and on re-feeding after acute or prolonged starvation. The Biochemical journal. 1989;258:529–533. doi: 10.1042/bj2580529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. The Biochemical journal. 1998;329(Pt 1):197–201. doi: 10.1042/bj3290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otonkoski T, Kaminen N, Ustinov J, Lapatto R, Meissner T, Mayatepek E, Kere J, Sipila I. Physical exercise-induced hyperinsulinemic hypoglycemia is an autosomal-dominant trait characterized by abnormal pyruvate-induced insulin release. Diabetes. 2003;52:199–204. doi: 10.2337/diabetes.52.1.199. [DOI] [PubMed] [Google Scholar]
- 37.Freund H, Marbach J, Ott C, Lonsdorfer J, Heitz A, Zouloumian P, Kehayoff P. Blood pyruvate recovery curves after short heavy submaximal exercise in man. European journal of applied physiology and occupational physiology. 1980;43:83–91. doi: 10.1007/BF00421358. [DOI] [PubMed] [Google Scholar]
- 38.Carlson LA, Pernow B. Studies on the peripheral circulation and metabolism in man. 1. Oxygen utilization and lactate-pyruvate formation in the legs at rest and during exercise in healthy subjects. Acta physiologica Scandinavica. 1961;52:328–342. doi: 10.1111/j.1748-1716.1961.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 39.Mehlman MA, Tobin RB, Johnston JB. Metabolic control of enzymes in normal, diabetic, and diabetic insulin-treated rats utilizing 1,3 butanediol. Metabolism: clinical and experimental. 1971;20:149–167. doi: 10.1016/0026-0495(71)90089-8. [DOI] [PubMed] [Google Scholar]