Abstract
Patterns of starch hydrolysis in stem, equatorial, and calyx zones of ‘Honeycrisp’ and ‘Empire’ apples (Malus sylvestris (L.) Mill var. domestica (Borkh.) Mansf.) during maturation and ripening, and in ‘Gala’ apples in response to propylene or 1-methylcyclopropene (1-MCP) treatments after harvest, were studied. Differences in zonal starch concentrations were found for ‘Empire’ and ‘Gala’ fruits, but not for ‘Honeycrisp’. During maturation and ripening of ‘Empire’, the concentration of starch was highest in the calyx end and lowest in the stem region.</title> Differences in rates of starch hydrolysis among zones were not detected. ‘Honeycrisp’ and ‘Empire’ had the highest concentration of sorbitol in the calyx region, whereas it was highest in the stem-end region in ‘Gala’. The distribution differences of glucose, fructose, and sucrose were similar in all three cultivars; higher fructose and glucose concentrations in the stem region, and higher sucrose concentrations in the calyx end of the fruit. Postharvest treatment of ‘Gala’ with propylene did not affect the internal ethylene concentration of the fruit but 1-MCP markedly inhibited it. Starch concentrations were highest in the calyx end but gradients of starch among zones were not changed by postharvest treatment. The rate of hydrolysis was slowed by 1-MCP treatment, but was unaffected by propylene. Postharvest treatments influenced sorbitol, glucose, and fructose concentrations. Patterns of starch concentration among the zones did not confirm differences in ripening, but reflected its uneven distribution throughout the fruit during development. Therefore, measured differences in zonal starch are most likely related to starch accumulation during fruit development, rather than differences in rates of starch degradation during ripening.
Introduction
It is generally assumed that ripening is initiated in different parts of the fruit depending on species. Fruits such as tomato undergo uneven ripening across the latitudinal gradient.1 Based on color changes it is assumed that ripening starts in different zones, e.g. tomatoes from the calyx and bananas from distal end. In the case of apples, the popular belief is that apples ripen first at the stem end, although hitherto this has not been validated. In some respects it depends on the definition of ripening, the process by which a product becomes ready to eat or able to disperse seeds, and therefore the initiating zone may not be critical per se in terms of consumer preferences. However, it is important in terms of understanding how ripening is controlled.
Fruits developing on the tree utilize photoassimilates to accumulate carbohydrates in the form of starch, and once detached from the tree the starch is used to provide soluble sugars for metabolic processes of ripening and senescence. Also, when a certain stage of maturity is reached while on the tree, starch is hydrolyzed into soluble carbohydrates. Sugars released from starch are ultimately glucose, fructose, and sucrose, which are used for respiration and to enhance sweetness of the ripening fruit.
The apple fruit is comprised of different tissues, the hypanthial (cortical) and carpillary (core) tissues, surrounded by a waxy cuticle covering the epidermal and hypodermal layers.2 Rudell et al.3 found that ethylene production was generally highest in the core tissues. Likewise, concentrations of the precursor of ethylene, 1-aminocyclopropane-1-carboxylic acid, were much higher in core tissues, than the inner cortex and peduncular (shoulder) tissue, while calyx and outer cortex tissues were lowest.4 These studies support a view that the ripening signal might be initiated in the core tissues, either as a seed-controlled mechanism and/or as a ‘tree factor’.
Brookfield et al.5 found that starch concentrations were higher in outer cortical tissues than in the core before the onset of hydrolysis. While the rate of starch hydrolysis was slower in the core than in the cortex, its onset was simultaneous in all tissues. Differences in starch concentration between the opposite ends of the fruit were found for ‘Fuji’, which had higher starch concentrations in the calyx region, but not for ‘Royal Gala’.5 Starch granules were more abundant in the cytoplasm of the cells in the outer flesh of ‘Jonagold’, and granule size differences between the mid and outer flesh compared with the inner flesh might lead to starch composition differences between the tissue zones.6 Differences in structure and size of cells could lead to developmental differences within the fruit, although such differences vary by cultivar.7,8
Progress of starch degradation in apple fruit is commonly assessed using the starch pattern index (SPI), which is used as an indicator of when to harvest fruit for short- or long-term storage.9–11 While the SPI is used as a means of assessing starch hydrolysis in fruit, few studies are available on mechanisms underlying changes in starch concentration. Neuwald et al.12 compared on- and off-tree changes in the SPI in different cultivars and showed that starch loss was typically slower at 1 °C and faster at 20 °C than on the tree. Treatment of fruit with 1-methylcyclopropene (1-MCP), an inhibitor of ethylene perception, resulted in little effect on SPIs at 20 °C, but markedly inhibited fruit softening. 1-MCP treatment was found to reduce starch loss based on SPI readings.13,14 However, Thammawong and Arakawa15 found little influence of 1-MCP, and a cultivar-dependent influence of exogenous ethylene on starch degradation. Starch concentrations declined faster in ‘Tsugaru’ fruit treated with ethylene than in untreated fruit of the same cultivar.15,16 In contrast, rates of starch decline in ‘Fuji’ fruit, which generally produce low amounts of ethylene, were not affected by 1-MCP or ethylene treatments.15 ‘Tsugaru’ apples also showed few differences among treatments in total sugar concentrations among zones within the cortex tissue.16 Starch degradation appears to be very ethylene sensitive and starts before a substantial increase of internal ethylene concentration (IEC) is detectable.17
Differences between starch concentrations and the rate of starch degradation within the fruit can be cultivar specific,18 but little information about differences in metabolism in stem- and calyx-end tissue of apples is available. In addition to presumed metabolic differences, physiological differences include higher tissue density in calyx-end than stem-end tissue,19 which collectively may have relevance to the development of physiological disorders such as flesh browning in ‘Empire’ and ‘Gala’ apples, which is first visible in the stem-end part of the fruit.20–23
The objective of this study was to investigate changes in starch concentrations in stem-end, equatorial, and calyx-end zones of apple fruit. Two approaches have been taken. In the first, concentrations in different tissue zones of ‘Honeycrisp’ and ‘Empire’ fruit during maturation and ripening on the tree were measured. In the second, postharvest changes were measured in ‘Gala’ fruit kept at 20 °C after treatment with propylene, an ethylene analogue,24 or 1-MCP.
Material and methods
Maturation and ripening on the tree
‘Honeycrisp’ and ‘Empire’ apples (Malus sylvestris (L.) Mill var. domestica (Borkh.) Mansf.) were obtained from trees grown in the Cornell University Orchard, Ithaca, NY, USA. Five fruits of each cultivar were harvested weekly between August 16 and September 13, 2011, and thereafter twice a week until September 23 and October 18, respectively. Each fruit was analyzed separately. The IEC of each fruit was measured on each sampling date except the first (August 16), by injecting a 1-mL gas sample taken from the core cavity into a Hewlett-Packard 5890 series II gas chromatograph (Hewlett-Packard, Wilmington, DE, USA).25
Tissue sampling
Tissue samples were then taken for analysis of soluble sugars and starch. Each fruit was cut latitudinally parallel to the core to leave an approximately 1-cm wide section around the core, stem, and calyx. Tissue samples were taken from the shoulder (stem), the equatorial cortex centered between core and skin, and the calyx-end of the fruit, using a core borer (diameter 1.9 cm). Tissues from the blushed and green sides of the fruit were combined to provide three samples per fruit: stem, equatorial, or calyx. The samples were frozen in liquid nitrogen and stored at −20 °C until they were lyophilized. Dried samples were ground in a Wiley mill through a 20-mesh screen.26
Postharvest manipulation of ripening
‘Gala’ apples were harvested from potted trees at the Cornell University Orchard in Ithaca, NY, USA, on August 29, 2012. In the lab they were split equally into three lots of ∼60 fruit, and either untreated or treated with 1-MCP or propylene on the day of harvest. One set of fruit was put into a 4000-L plastic tent and treated with 1 µL L−1 1-MCP (SmartFresh, AgroFresh Inc., Springhouse, PA, USA) for 24 h using a release and fan system supplied by the manufacturer. A second set was treated for 24 h with 200 µL L−1 propylene in a 280-L gas tight plexiglass chamber. Both treatments and the untreated control fruit were kept at 20 °C. After removal from the chambers the fruit were allowed to vent before sampling 1, 2, 3, 5, 7, 9, and 13 days after harvest (DAH). Five randomly selected fruits were used at each sampling date, and analyzed separately. The IEC was measured as described above. IAD was measured using a handheld non-destructive instrument (DA meter, Sinteleia, Bologna, Italy).27 Readings were taken on the opposite green and blushed sides and the average noted.28 Flesh samples were taken by cutting two wedges, each approximately one-eighth of the fruit. The wedge was peeled and placed on the edge of the cutting board where a 1-cm wide area was marked to ensure even cutting of the sections. The sections were frozen in liquid nitrogen and stored at −20 °C until they were lyophilized and ground as described above.
Soluble carbohydrate and starch determination
For determination of sugars and starch, 50 mg of ground sample was washed with 3 mL 80% ethanol and incubated for 30 min at 70 °C,26,29 centrifuged (Eppendorf Model 5810 – Eppendorf North America, Inc., Westbury, NY, USA) and the aqueous phase separated into glass culture tubes. This was repeated three times, the extracts bulked and the aqueous phase containing all the soluble carbohydrates was run through columns of 1 mL Dowex 50-W, 100–200 mesh layered on 1 mL Amberlite IRA-45, 16–50 mesh.26,29 The column exudate was vacuum evaporated using a Rapidvap Vacuum Evaporation System (Labconco, Kansas City, MO, USA)30 for the maturation experiment (‘Honeycrisp’ and ‘Empire’), and an Evapo-Mix (Buchler Instruments, Fort Lee, NJ, USA) for the postharvest experiment (‘Gala’). The dry sugar samples were kept at −20 °C until preparation for ionic chromatography. Maturation samples were dissolved in 10 mL high-performance liquid chromatography (HPLC) grade water and postharvest ‘Gala’ samples in 20 mL HPLC grade water.
In both experiments, the samples were further diluted by adding 400 µL HPLC grade water to 100 µL sample aliquots. Samples were analyzed using Dionex (Sunnyvale, CA, USA) high-performance anion exchange chromatography with pulsed amperometric detection (ED50; Dionex) equipped with a Carbopac PA-1 column (CarboPac PA1 Analytical, 4 × 250 mm; Dionex). Carbohydrates were eluted with 100 mM NaOH at a flow rate of 1.0 mL min−1 for 20 min.30 Sorbitol, glucose, fructose, and sucrose were quantified by comparison with known standards.
The non-soluble carbohydrates obtained after extraction with ethanol were dried overnight at 60 °C in the drying oven. Enzymatic starch determination with amyloglucosidase was performed.18,26,29
Statistics
All statistical calculations were done in JMP Pro 11.0.0 (SAS Institute, Cary, NC, USA) using simple least-square models with inclusion of interactions. For regressions and analysis of variance (ANOVA) individual samples were used as replicates. ANOVA was performed on log-transformed IEC data, but back transformed and averaged IEC is presented within the graphs. Regressions within the graphs were calculated using OriginPro 8 SRO (OriginLab Corp., Northampton, MA, USA).
Results
Maturation and ripening on the tree
Low levels of IECs were measured in ‘Honeycrisp’, except for a spike in ethylene on September 6 (Figure 1). ‘Empire’ IECs remained low throughout the experiment, and never exceeded 2.8 µL L−1 with the exception of one fruit on September 27 (120 µL L−1). This fruit had likely abscised (‘push off’) and was not included in the analysis.
Figure 1.
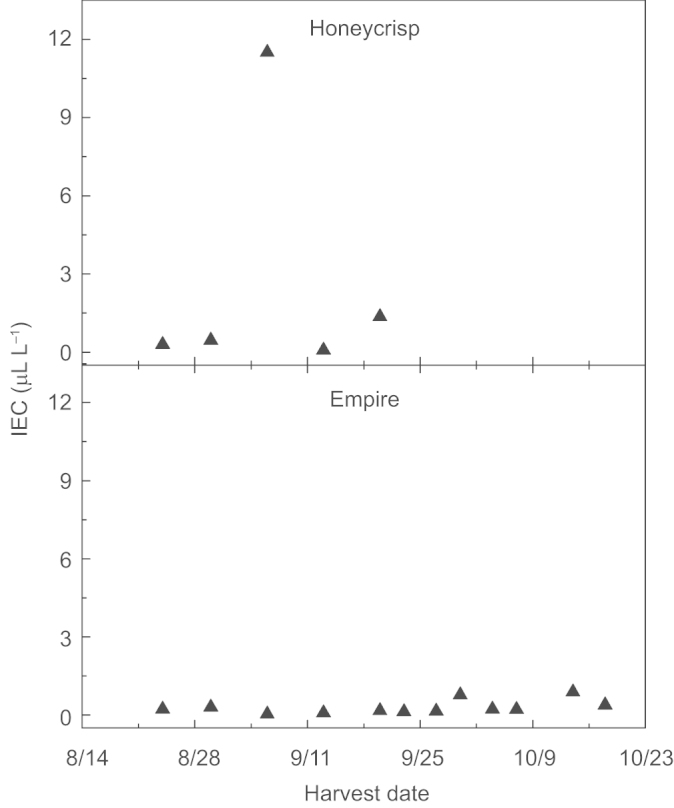
IEC in fruit of ‘Honeycrisp’ and ‘Empire’ during harvest. Each mean is the average of five fruits. Effects of harvest date for each cultivar P < 0.0001.
Starch concentrations per g dry matter in the stem-end, equatorial, and calyx-end zones of ‘Honeycrisp’ and ‘Empire’ decreased linearly during maturation and ripening (Figure 2). Differences in starch concentrations between zones were detected only for ‘Empire’ fruit. In this cultivar, concentrations were higher overall in the calyx-end (180 mg g−1) than in the equatorial- and stem-end zones (171 mg g−1 and 141 mg g−1, respectively). The daily decrease of starch was similar in all zones for each cultivar, ranging from −6.33 mg g−1 to −6.96 mg g−1 for ‘Honeycrisp’, and −4.24 mg g−1 to −4.29 mg g-1 for ‘Empire’ (Figure 2).
Figure 2.
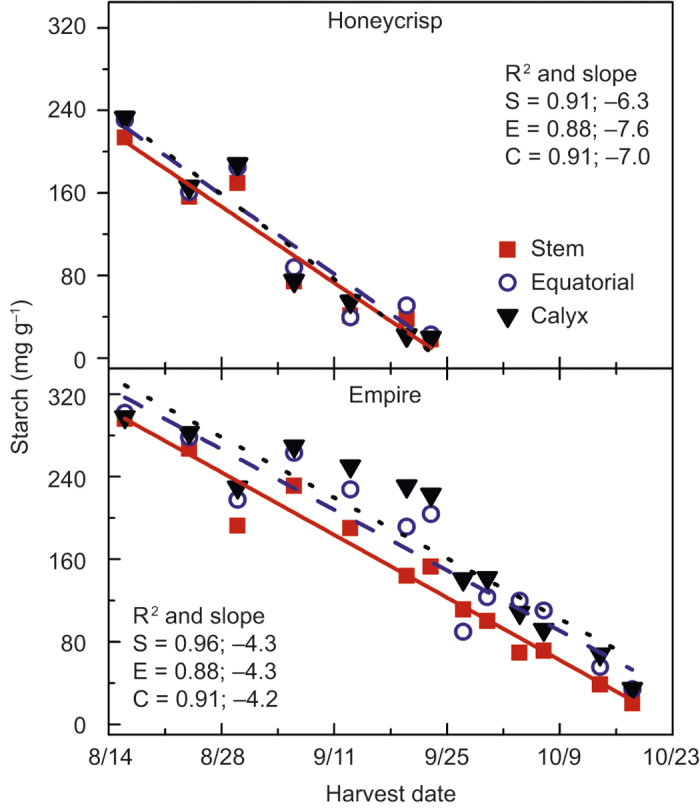
Starch concentration (mg g−1) of ‘Honeycrisp’ and ‘Empire’ of stem (S; ▪), equatorial (E; ○), and calyx (C; ▴) at each harvest date. Each mean is the average of five fruits. The R2 represents the linear fit of the data over harvest date; P values: ‘Honeycrisp’ harvest date P < 0.0001, no interaction detected; ‘Empire’ harvest date P < 0.0001, and zone P < 0.0001, no interaction detected.
Although there was variability among harvest dates, overall the concentrations of sorbitol, glucose, and sucrose increased in ‘Honeycrisp’ tissue zones over the harvest period, while those of fructose decreased (Table 1). Sorbitol and sucrose concentrations were highest in the calyx tissues, whereas glucose and fructose were highest in stem tissues. Similarly, the concentrations of sorbitol, glucose, and sucrose increased in ‘Empire’ tissue zones over the harvest period (Table 2). However, fructose concentrations changed over time, with a quadratic relationship being detected. Sorbitol and sucrose concentrations were highest in the calyx tissues, whereas glucose was highest in stem tissues. Fructose was not affected by tissue zone.
Table 1. Sorbitol, glucose, fructose, and sucrose (mg g−1 dry weight) in stem (S), equatorial (E), and calyx (C) tissues of ‘Honeycrisp’ at each harvest date.
| Harvest date (2011) | Sorbitol |
Glucose |
Fructose |
Sucrose |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg g−1) | ||||||||||||
| S | E | C | S | E | C | S | E | C | S | E | C | |
| 8/16 | 8.2 | 10.4 | 10.3 | 45.3 | 29.9 | 17.3 | 303.7 | 293.1 | 281.9 | 202.7 | 196.8 | 228.3 |
| 8/24 | 8.9 | 10.1 | 13.7 | 57.8 | 51.6 | 30.7 | 274.7 | 286.0 | 280.0 | 215.1 | 225.8 | 252.2 |
| 8/30 | 11.9 | 14.7 | 15.2 | 52.0 | 40.8 | 34.4 | 282.1 | 270.5 | 272.9 | 241.9 | 259.3 | 272.6 |
| 9/06 | 12.7 | 15.2 | 18.7 | 60.4 | 42.4 | 30.5 | 318.7 | 285.5 | 274.1 | 288.9 | 292.3 | 291.0 |
| 9/13 | 11.7 | 15.0 | 18.7 | 61.8 | 39.9 | 31.3 | 282.5 | 273.1 | 288.2 | 279.0 | 319.6 | 357.7 |
| 9/20 | 14.6 | 21.0 | 22.6 | 48.4 | 40.6 | 33.5 | 266.9 | 247.4 | 253.9 | 324.4 | 333.2 | 350.5 |
| 9/23 | 10.2 | 12.0 | 13.4 | 62.6 | 51.0 | 43.3 | 249.2 | 236.6 | 239.2 | 379.8 | 397.1 | 407.3 |
| Overall means | 11.2 | 14.1 | 16.4 | 55.0 | 40.0 | 31.7 | 284.6 | 271.8 | 271.8 | 269.7 | 284.2 | 305.9 |
| Regression | L* | L* | L* | ns | L* | L** | L* | L** | L* | L*** | L*** | L*** |
|
P values | ||||||||||||
| Date | <0.001 | 0.051 | <0.001 | <0.001 | ||||||||
| Zone | 0.002 | <0.001 | 0.071 | 0.009 | ||||||||
| Date × zone | 0.743 | 0.908 | 0.673 | 0.819 | ||||||||
Each mean is the average of five fruits.
ns, *, **, *** = not significant or significant at P<0.05, 0.01, or 0.001, respectively
Table 2. Sorbitol, glucose, fructose, and sucrose concentrations (mg g−1 dry weight) in stem (S), equatorial (E), and calyx (C) tissues of ‘Empire’ at each harvest date.
| Harvest Date (2011) | Sorbitol |
Glucose |
Fructose |
Sucrose |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg g−1) | ||||||||||||
| S | E | C | S | E | C | S | E | C | S | E | C | |
| 8/16 | 10.5 | 12.3 | 14.4 | 44.2 | 23.2 | 22.3 | 266.1 | 277.7 | 263.1 | 137.1 | 154.5 | 164.0 |
| 8/24 | 10.6 | 14.6 | 17.7 | 43.6 | 32.5 | 31.4 | 301.0 | 299.7 | 286.8 | 149.2 | 165.0 | 169.0 |
| 8/30 | 12.7 | 14.8 | 17.7 | 61.0 | 43.0 | 40.4 | 279.0 | 283.8 | 278.1 | 165.1 | 170.1 | 176.9 |
| 9/06 | 9.5 | 12.6 | 13.9 | 59.8 | 39.2 | 35.8 | 280.7 | 281.6 | 272.3 | 164.9 | 192.3 | 200.8 |
| 9/13 | 15.5 | 16.7 | 17.7 | 53.5 | 38.6 | 37.4 | 253.4 | 287.1 | 278.9 | 154.6 | 190.6 | 196.9 |
| 9/20 | 18.6 | 24.6 | 25.6 | 75.6 | 56.7 | 46.6 | 256.1 | 282.4 | 260.2 | 209.5 | 236.7 | 240.3 |
| 9/23 | 11.2 | 16.4 | 16.1 | 54.9 | 35.5 | 30.5 | 275.6 | 281.7 | 274.3 | 220.6 | 239.4 | 251.6 |
| 9/27 | 13.6 | 17.4 | 20.7 | 63.1 | 46.1 | 43.1 | 279.2 | 267.9 | 279.6 | 232.2 | 236.3 | 261.3 |
| 9/30 | 12.3 | 14.7 | 15.0 | 55.0 | 42.3 | 36.9 | 277.9 | 256.0 | 255.3 | 237.0 | 254.6 | 258.1 |
| 10/04 | 13.6 | 19.4 | 20.2 | 78.6 | 55.7 | 48.6 | 322.6 | 320.3 | 310.4 | 216.8 | 255.8 | 278.8 |
| 10/07 | 16.5 | 25.4 | 24.6 | 69.6 | 57.2 | 49.8 | 309.5 | 302.0 | 285.2 | 254.4 | 287.0 | 288.8 |
| 10/14 | 15.5 | 24.9 | 23.6 | 64.8 | 51.1 | 46.6 | 257.0 | 274.6 | 251.4 | 252.8 | 293.2 | 289.8 |
| 10/18 | 17.1 | 18.2 | 18.2 | 56.4 | 52.7 | 46.1 | 248.7 | 245.0 | 229.7 | 287.0 | 284.0 | 282.8 |
| Overall means | 13.7 | 17.8 | 18.8 | 60.2 | 43.9 | 39.5 | 279.5 | 281.5 | 271.4 | 208.3 | 227.6 | 236.2 |
| Regression | L*** | L*** | L** | L** | L*** | L*** | Q* | Q* | Q* | L*** | L*** | L*** |
|
P values | ||||||||||||
| Date | <0.001 | <0.001 | 0.039 | <0.001 | ||||||||
| Zone | <0.001 | <0.001 | 0.089 | <0.001 | ||||||||
| Date × zone | 0.377 | 0.438 | 0.992 | 0.855 | ||||||||
Each mean is the average of five fruits.
ns, *, **, *** = not significant or significant at P<0.05, 0.01, or 0.001, respectively
Postharvest manipulation of ripening
Propylene and 1-MCP were applied to ‘Gala’ apples with the objective of increasing and decreasing the rate of ripening, respectively. The IECs of fruit from all treatments remained low for the first 5 DAH (Figure 3). The IECs of untreated and propylene-treated fruit then increased, but no differences between them were detected. In contrast, the IECs of 1-MCP-treated fruit remained low throughout the experiment.
Figure 3.
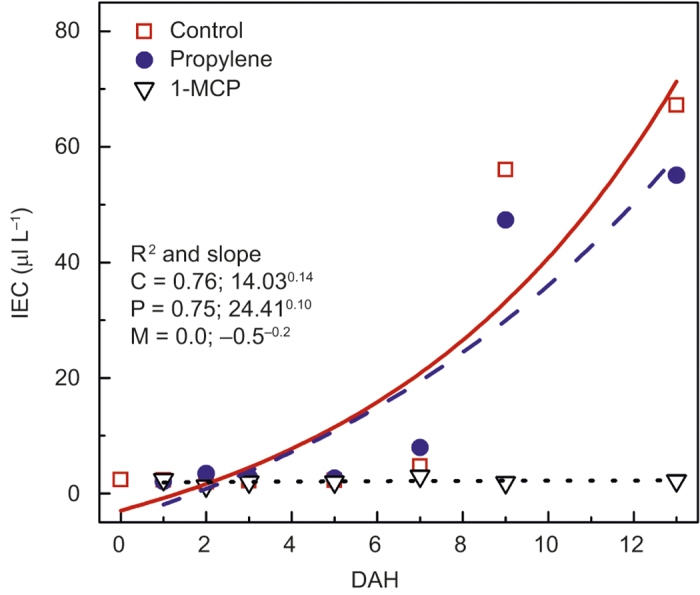
IEC (µL L−1) of ‘Gala’ in untreated control (C; □), propylene treated (P; •), or 1-MCP-treated (M; ▿) fruit from at harvest (0) to 13 DAH. Each mean is the average of five fruits. The R2 describes the exponential fit; P-values: treatment < 0.001, DAH < 0.001, and treatment × DAH < 0.001.
The decline in IAD values was slower in 1-MCP-treated fruit, indicating slower loss of chlorophyll a levels, compared with untreated fruit and those treated with propylene (Figure 4).
Figure 4.
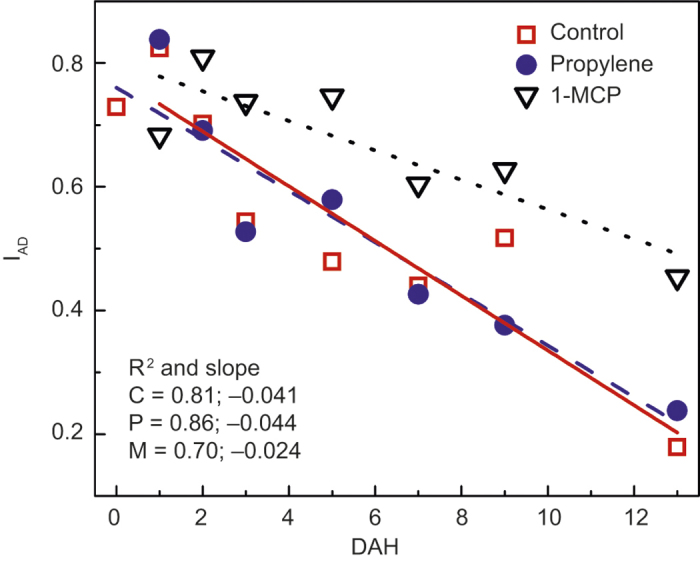
IAD of ‘Gala’ in untreated control (C; □), propylene treated (P; •), or 1-MCP-treated (M; ▿) fruit from at harvest (0) to 13 DAH. Each mean is the average of five fruits. The R2 describes the exponential fit; P-values: treatment = 0.010, DAH < 0.001, no interaction detected.
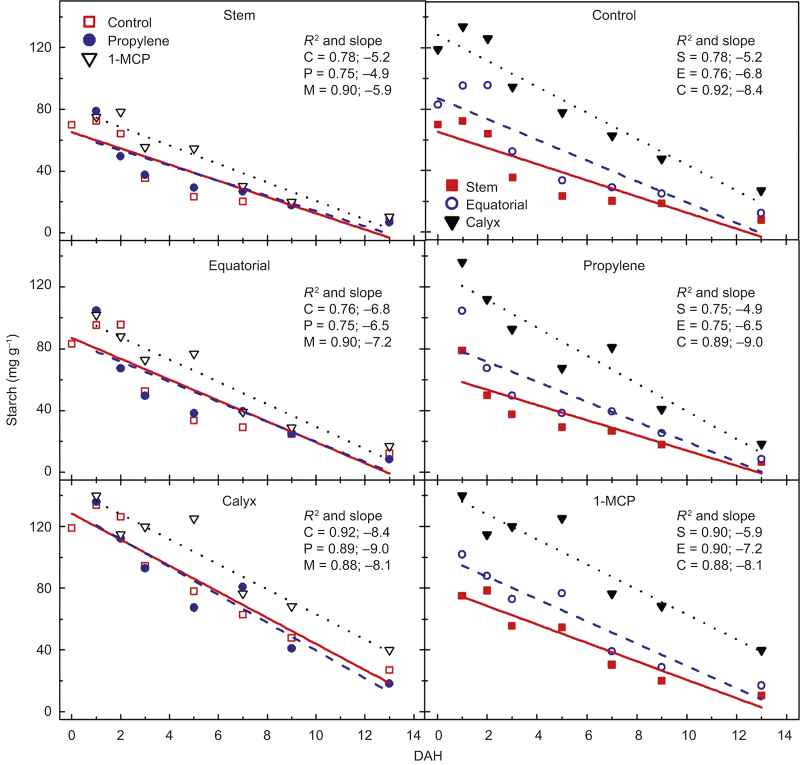
Regardless of treatment, starch concentrations were lowest in the stem-end tissues and highest in the calyx-end tissues, and these decreased linearly over time (Figure 5). 1-MCP-treated fruit generally had the highest concentration in each zone, while there was little difference between control and propylene-treated fruit. Although treatment with propylene led to lower starch concentrations per g dry matter combined for all zones and sampling dates with 53 mg g−1 compared with 57 mg g−1 and 69 mg g−1 for control and 1-MCP-treated fruit, respectively; a statistical difference between the propylene-treated fruit and the untreated was not detected. Starch concentrations in the calyx-end of the fruit over all treatments were 34% higher compared with the equatorial zone, and 74% higher compared with the stem-end tissues.
Figure 5.
Starch concentration (mg g−1 dry wt) in stem, equatorial, and calyx tissues of ‘Gala’ in untreated control (C; □), propylene-treated (P; •), or 1-MCP-treated (M; ▿) fruit from at harvest (0) to 13 DAH (left side) and (ride side) starch concentration in fruit zones of either untreated control, propylene, or 1-MCP-treated fruit; stem-end (S; ▪), equatorial (E; ○), and calyx-end (C; ▴). Each mean is the average of five fruits. The R2 describes the linear fit of the data; P-values: treatment < 0.001, zone < 0.001, DAH < 0.001, zone × DAH < 0.001, no other interactions detected.
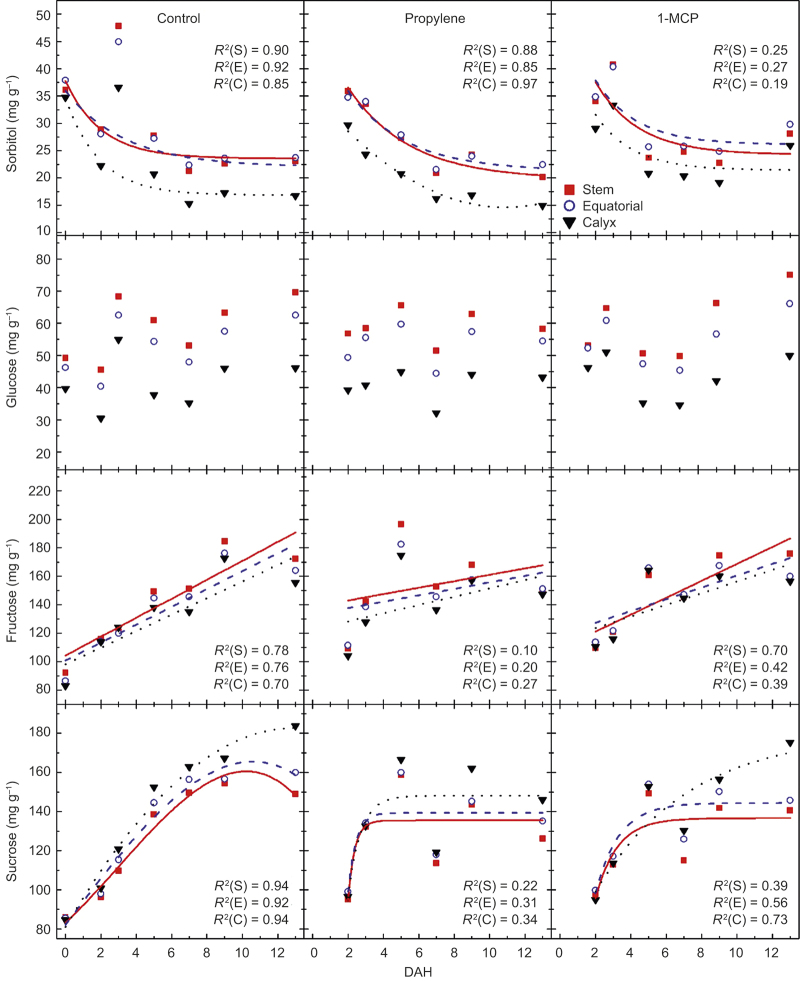
Concentrations of sorbitol generally decreased, while those of sucrose generally increased over time, although the patterns were curvilinear (Figure 6; Table 3). Sorbitol in untreated fruit decreased between 1 DAH and 13 DAH in the stem, equatorial and calyx zones decreased by 36%, 67% and 50%, respectively. Propylene-treated fruit had lower sorbitol concentrations: 43%, 37% and 50% for stem-, equatorial-, and calyx-end zones, respectively. Sorbitol in 1-MCP-treated fruit decreased until 9 DAH, but increased at the last sampling point (13 DAH). In contrast to the general trend of a continuous concentration gradient, the equatorial zone sorbitol was slightly higher than stem-end concentrations on most sampling days for all treatments. Glucose increased in untreated control and 1-MCP-treated fruit, but remained relatively level in propylene-treated fruit. The glucose concentrations of control and 1-MCP-treated fruit on day 13 were higher (average of zones being 60 mg g−1 and 64 mg g−1, respectively) compared with that in propylene-treated fruit (52 mg g−1). Fructose concentrations increased linearly over time. Zonal effects on fructose concentrations were most pronounced in 1-MCP-treated fruit at day 13 with equatorial and calyx at 160 mg g−1 and 157 mg g−1 compared with the stem-end with 176 mg g−1. Little differences in fructose concentrations were found between control- and propylene-treated fruit on day 13, but overall concentrations were higher in the stem-end zone compared with the equatorial zone, and lowest concentrations were in calyx, averaging 135 mg g−1, 135 mg g−1, and 130 mg g−1 in stem, equatorial, and calyx zone of control, respectively, and 146 mg g−1, 141 mg g−1, and 135 mg g−1 in the zones of propylene-treated fruit. Sucrose concentrations varied among zones with the highest concentration in the calyx, and they reached maximum values at different times depending on treatment. Zones of propylene-treated fruit all reached the maximum at 9 DAH. Equatorial and calyx zones of control and 1-MCP-treated fruit also reached highest sucrose concentrations on 9 DAH, but the calyx-end concentration increased until 13 DAH. The effect of tissue zone was significant for all sugars and an interaction between tissue zone and DAH was detected for sucrose (Table 3). The interaction between treatment and DAH was significant for sorbitol, fructose, and sucrose.
Figure 6.
Sorbitol, glucose, fructose, and sucrose in stem (S; ▪), equatorial (E; ○), and calyx-end (C; ▴) tissues of ‘Gala’ in untreated control, propylene- or 1-MCP-treated fruit from at harvest (0) to 13 DAH. Each mean is the average of five fruits. The R2 describes the exponential or linear fit of the data.
Table 3. ANOVA for sorbitol, glucose, fructose, and sucrose tissue zones (stem, equatorial, and calyx) of ‘Gala’ with treatment (untreated (control), propylene or 1-MCP), zone, and days after harvest (DAH).
| Sorbitol | Glucose | Fructose | Sucrose | |
|---|---|---|---|---|
| Treatment | 0.001 | 0.031 | 0.049 | 0.288 |
| Zone | <0.001 | <0.001 | 0.011 | 0.002 |
| DAH | <0.001 | <0.001 | <0.001 | <0.001 |
| Treatment × zone | 0.944 | 0.989 | 0.952 | 0.938 |
| Treatment × DAH | <0.001 | 0.131 | <0.001 | <0.001 |
| Zone × DAH | 0.879 | 0.072 | 0.597 | 0.001 |
| Treatment × zone × DAH | 0.986 | 0.653 | 0.874 | 0.964 |
Discussion
Starch concentrations in all tissue zones declined in a linear fashion over time, regardless of cultivar, and whether fruit were on or off the tree. These patterns of change are therefore consistent with those found for starch concentrations in whole fruit.18 Rates of decrease were similar between the same zones in the different treatments, but were different within fruit of the same treatment. Nevertheless, this might not indicate differences in rates of ripening per se, but rather may reflect differences in starch accumulation in different tissue zones during fruit development. Although starch is known to accumulate during fruit development to a maximum before net starch loss occurs,5,31 little is known about rates of deposition in the different tissues. Starch concentrations in the calyx-end tissue in all treatments of ‘Gala’ were higher at 1 DAH compared with those in the equatorial and stem zones (Figure 5). ‘Empire’ fruit also had a higher starch concentrations in the calyx-end than in the stem-end (Figure 2). Higher amounts of starch in the outer cortex compared to the core and the vascular bundle region were previously found by Brookfield et al.5
The effect of higher IEC on starch has been shown previously, with a hastening effect of increased ethylene on starch hydrolysis.17 However, Thammawong and Arakawa15 found that exogenous ethylene increased the rate of starch hydrolysis only in ‘Tsugaru’ not in ‘Fuji’ fruit. Relative independence of SPI changes in fruits treated with ethylene was also observed by Blankenship and Unrath32 in ‘Golden Delicious’. Starch concentrations in ‘Gala’ treated with propylene were slightly lower in all three tissue zones compared with the untreated control fruit. Propylene treatment did not elevate IEC, and a higher dose of, or prolonged exposure to, propylene might have been needed to increase IEC sufficiently and affect starch hydrolysis. Little effect of 1-MCP on starch degradation was detected in ‘Jonagold’ and ‘Golden Delicious’ apples supporting the idea that starch hydrolysis is relatively ethylene independent.12 Initiation of starch hydrolysis is very ethylene sensitive; however, and therefore even a very small increase in IEC can trigger the onset of starch hydrolysis.17 Once initiated the progression is relatively independent of ethylene concentrations within the fruit tissue. In ‘Gala’, starch degradation occurred even with low IECs in 1-MCP-treated fruit; hydrolysis may have been already initiated on the tree. The effects of 1-MCP on decreasing starch hydrolysis in ‘Gala’ were stronger than those observed for ‘Jonagold’ and ‘Golden Delicious’,12 but those observations were based on SPI readings and not chemical analyses of starch.
Starch hydrolysis has been shown to initiate and proceed simultaneous throughout the fruit5 and higher initial values of starch in the calyx-end of the fruit (Figures 2 and 5) supports the assumption that starch is not evenly distributed within the fruit during development.6 Such distributional differences within the fruit earlier in development with changes in granule size or granule structure have been documented.33,34 Granules are degraded from the inside out,6 leading to a rate of degradation not related to granule size. The actual structure and composition of starch was not analyzed here, but changes in starch composition of all three cultivars were previously studied in the whole fruit.18 The percentage amylose (linear starch) in total starch decreases as the fruit matures. This also supports the assumption that differences within the starch granule cause differences in starch degradation.6 Therefore, the differences in granule size and/or number of starch granules in different fruit tissue zones could lead to differences in starch distribution within the fruit, even if starch degradation is initiated simultaneously in the entire fruit. Hence, the measured differences could be artifacts from earlier events during fruit development rather than differentiated starch degradation.
Low glucose and sorbitol, and increasingly higher concentrations of sucrose and fructose during fruit ripening and storage have been described.35 Changes in sugar concentrations in the whole fruit (on a fresh weight basis) have been seen in ‘Honeycrisp’ during development.36 Similar trends of sugar accumulation were measured in the current study; all three cultivars had low concentrations of sorbitol and glucose, and much higher concentrations of fructose and sucrose, which increased as the fruit ripened. Sorbitol is the preferred transport sugar in Rosaceae fruit trees,37 but generally represents a relatively small proportion of the total carbohydrates in the fruit.15,38 Sorbitol is not stored, but is converted into other sugars, mostly fructose by NAD-dependent sorbitol dehydrogenase.39,40 Therefore, the already low levels of sorbitol decrease after harvest, since there is no longer an influx of sorbitol. Fluctuations in glucose levels could be explained since glucose is used for respiration and is transformed into fructose and subsequently sucrose (sucrose-6-phosphate-syhthase). This could explain the small increase in glucose compared with the hydrolysis of starch and the much greater increase in fructose and sucrose. Glucose is not as sweet in taste as fructose and sucrose41 and, therefore, does not contribute as much to flavor characteristics of the fruit. The sucrose concentration gradient between tissue zones was similar to the starch concentration gradient, with higher concentrations in the calyx region. The other sugars show an opposite trend in ‘Gala’, with higher levels in the stem region. Differences in sugar concentration between parts of the apple fruit have been documented previously,42,43 with higher total sugar concentrations in the stem-end vs. the calyx-end of the fruit,44 similar to those sugar concentrations found in ‘Honeycrisp’, ‘Empire,’ and ‘Gala’. However, variations between fruits are much greater than variance of total sugars within the fruit.42,43
‘Gala’ fruit treated with 1-MCP maintained higher IAD values, indicating slower chlorophyll a degradation by the treatment. Hue angle of skin has been found to have low sensitivity, and therefore stronger dependence on ethylene.17 Propylene-treated fruit decreased in IAD at a similar rate as untreated fruit, but the values of IEC were also very similar. Delay of chlorophyll a degradation by 1-MCP during storage has been found previously.45,46
A zonal gradient of starch and sugar concentration between the stem- and calyx-end of the fruit was shown in ‘Empire’ and ‘Gala’ fruit. The results for ‘Honeycrisp’ were not as conclusive. Whether these differences are purely due to a distributional difference in starch accumulation during development and therefore an artifact of starch distribution during fruit growth or whether this proves a differentiation in maturation among tissue zones cannot be answered based on these data alone. More research needs to be carried out to understand the developmental differences between the stem and calyx region of the fruit. Changes in starch and soluble sugars cannot verify true maturity differences, and may be artifacts from the earlier development of larger starch granules in the lower part of the fruit. Also, IECs of ‘Gala’ fruit treated with propylene or 1-MCP alone could not explain the differences in starch decline between the zones and/or treatments, so there still could be a linkage between IEC and starch hydrolysis. Very small amounts of ethylene trigger the onset of starch hydrolysis,17 and increasing the amount of ethylene hastens the progression. Such effects of ethylene could not be detected in our study but the propylene treatment, without affecting overall IECs, did lead to a lower starch concentration after 13 days at 20 °C. The differences between zones might be due to accumulation differences earlier in development rather than differences in hydrolysis of starch, and the differences between the propylene treatment and untreated fruit, and 1-MCP treatment, could be due to miniscule changes in ethylene concentrations within the fruit.
Acknowledgments
F.C.D. was supported by a Department of Horticulture Graduate Assistantship. Funding for this research was provided by the NY Apple Research and Development Program and AgroFresh. This work was also supported by the USDA National Institute of Food and Agriculture, Hatch project 2013-14-483, Improving Quality and Reducing Losses in Specialty Fruit Crops through Storage Technologies (NE-1336). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the National Institute of Food and Agriculture (NIFA) or the United States Department of Agriculture (USDA). We thank Rose Harmon for technical assistance, and Dr. Nigel Gapper for assistance with propylene treatment.
References
- Nguyen CV, Vrebalov JT, Gapper NE et al. Tomato GOLDEN2-LIKE transcription factors reveal molecular gradients that function during fruit development and ripening. Plant Cell Online 2014; 26: 585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C. Apple flower and fruit: morphology and anatomy. Hort Rev 1988; 10: 273–308. [Google Scholar]
- Rudell DR, Mattinson DS, Fellman JK et al. The progression of ethylene production and respiration in the tissues of ripening ‘Fuji’ apple fruit. HortScience 2000; 35: 1300–1303. [Google Scholar]
- Mansour R, Latché A, Vaillant V et al. Metabolism of 1-aminocyclopropane-1-carboxylic acid in ripening apple fruits. Physiol Plant 1986; 66: 495–502. [Google Scholar]
- Brookfield P, Murphy P, Harker R et al. Starch degradation and starch pattern indices; interpretation and relationship to maturity. Postharvest Biol Technol 1997; 11: 23–30. [Google Scholar]
- Ohmiya A, Kakiuchi N. Quantitative and morphological studies on starch of apple fruit during development. J Japan Soc Hort Sci 1990; 59: 417–423. [Google Scholar]
- Bain JM, Robertson RN. The physiology of growth in apple fruits I. Cell size, cell number, and fruit development. Aus J Biol Sci 1951; 4: 75–91. [DOI] [PubMed] [Google Scholar]
- Leshem YY, Ferguson IB, Grossman S. On ethylene, calcium and oxidative mediation of whole apple fruit senescence by core control. In Fuchs Y, Chalutz E, editors. Ethylene. Dordrecht: Springer; 1984; 9: 111–120. [Google Scholar]
- Blanpied GD, Silsby KJ. Predicting harvest date windows for apples. Cooperative Extension Information Bulletin No. 221, Ithaca, NY: Cornell University; 1992: 2. [Google Scholar]
- Reid M, Padfield CAS, Watkins CB et al. Starch iodine pattern as a maturity index for Granny Smith apples. 1. Comparison with flesh firmness and soluble solids content. NZ J Agric Res 1982; 25: 239–243. [Google Scholar]
- Travers I, Jacquet A, Brisset A et al. Relationship between the enzymatic determination of starch and the starch iodine index in two varieties of cider apple. J Sci Food Agric 2002; 82: 983–989. [Google Scholar]
- Neuwald DA, Streif J, Kittemann D. Fruit starch degradation patterns in apple cultivars on-tree and off-tree at different holding temperatures. Acta Hort 2010; 858: 263–266. [Google Scholar]
- Fan X, Blankenship SM, Mattheis JP. 1-Methylcyclopropene inhibits apple ripening. J Am Soc Hort Sci 1999; 124: 690–695. [Google Scholar]
- Pre-Aymard C, Weksler A, Lurie S. Responses of ‘Anna’, a rapidly ripening summer apple, to 1-methylcyclopropene. Postharvest Biol Technol 2003; 27: 163–170. [Google Scholar]
- Thammawong M, Arakawa O. Starch degradation of detached apple fruit in relation to ripening and ethylene. J Japan Soc Hort Sci 2007; 76; 345–350. [Google Scholar]
- Thammawong M, Arakawa O. Starch to sugar conversion in “Tsugaru” apples under ethylene and 1-methylcyclopropene treatments. J Agric Sci Tech 2010; 12: 617–626. [Google Scholar]
- Johnston JW, Gunaseelan K, Pidakala P et al. Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J Exp Bot 2009; 60: 2689–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger FC, Miller WB, Nock JF et al. Relationships between starch pattern indices and starch concentrations in four apple cultivars. Postharvest Biol Technol 2015; 110: 86–95. [Google Scholar]
- James HJ, Jobling JJ. Contrasting the structure and morphology of the radial and diffuse flesh browning disorders and CO2 injury of ‘Cripps Pink’ apples. Postharvest Biol Technol 2009; 53: 36–42. [Google Scholar]
- Lee J, Mattheis JP, Rudell DR. Antioxidant treatment alters metabolism associated with internal browning in ‘Braeburn’ apples during controlled atmosphere storage. Postharvest Biol Technol 2012; 68: 32–42. [Google Scholar]
- Lee J, Mattheis JP, Rudell DR. Fruit size affects physiological attributes and storage disorder in cold-stored ‘Royal Gala’ apples. HortScience 2013; 48: 1518–1524. [Google Scholar]
- Lee J, Rudell D, Davies P et al. Metabolic changes in 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apple fruit during storage. Metabolomics 2012; 8: 742–753. [Google Scholar]
- Doerflinger FC, Rickard BJ, Nock JF et al. An economic analysis of harvest timing to manage the physiological storage disorder firm flesh browning in ‘Empire’ apples. Postharvest Biol Technol 2015; 107: 1–8. [Google Scholar]
- Abdi N, Holford P, McGlasson WB et al. Ripening behaviour and responses to propylene in four cultivars of Japanese type plums. Postharvest Biol Technol 1997; 12: 21–34. [Google Scholar]
- Watkins CB, Nock JF, Whitaker BD. Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol Technol 2000; 19: 17–32. [Google Scholar]
- Miller WB, Langhans RW. Carbohydrate changes of Easter lilies during growth in normal and reduced irradiance environments. J Am Soc Hort Sci 1989; 114: 310–315. [Google Scholar]
- Costamagna F, Giordani L, Costa G et al. Use of IAD index to define harvest time and characterize ripening variability at harvest in ‘Gala’ apple fruit. Acta Hort 2013; 998: 117–123. [Google Scholar]
- Nyasordzi J, Friedman H, Schmilovitch Z et al. Utilizing the IAD index to determine internal quality attributes of apples at harvest and after storage. Postharvest Biol Technol 2013; 77: 80–86. [Google Scholar]
- Ranwala AP, Miller WB. Analysis of nonstructural carbohydrates in storage organs of 30 ornamental geophytes by high-performance anion-exchange chromatography with pulsed amperometric detection. New Phytol 2008; 180: 421–433. [DOI] [PubMed] [Google Scholar]
- Hou JY, Miller WB, Chang YCA. Effects of simulated dark shipping on the carbohydrate status and post-shipping performance of Phalaenopsis. J Am Soc Hort Sci 2011; 136: 364–371. [Google Scholar]
- Berüter J, Studer Feusi ME. The effect of girdling on carbohydrate partitioning in the growing apple fruit. J Plant Physiol 1997; 151: 277–285. [Google Scholar]
- Blankenship SM, Unrath CR. Internal ethylene levels and maturity of ‘Delicious’ and ‘Golden Delicious’ apple destined for prompt consumption. J Am Soc Hort Sci 1988; 113: 88–91. [Google Scholar]
- Pérez S, Baldwin PM, Gallant DJ. Chapter 5 – structural features of starch granules I. In BeMiller J, Whistler R, editors. Starch (3rd edn.), San Diego: Academic Press; 2009: 149–192. [Google Scholar]
- Smith AM. The biosynthesis of starch granules. Biomacromolecules 2001; 2: 335–341. [DOI] [PubMed] [Google Scholar]
- Ackermann J, Fischer M, Amado R. Changes in sugars, acids, and amino acids during ripening and storage of apples (cv. Glockenapfel). J Agric Food Chem 1992; 40: 1131–1134. [Google Scholar]
- Zhang Y, Li P, Cheng L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem 2010; 123: 1013–1018. [Google Scholar]
- Bieleski R, Redgwell R. Sorbitol versus sucrose as photosynthesis and translocation products in developing apricot leaves. Aus J Plant Physiol 1985; 12: 657–668. [Google Scholar]
- Yamaki S, Ishikawa K. Role of four sorbitol related enzymes and invertases in the seasonal alternation of sugar metabolism in apple tissue. J Am Soc Hort Sci 1986; 111: 134–137. [Google Scholar]
- Kanayama Y, Yamada K, Kato K et al. Biochemical and molecular aspects of sorbitol metabolism in Rosaceae fruit trees and other plants. In Matsumoto T, editor, Phytochemical Research Progress, New York: Nova Science; 2008: 75–86. [Google Scholar]
- Teo G, Suzuki Y, Uratsu SL et al. Silencing leaf sorbitol synthesis alters long-distance partitioning and apple fruit quality. Proc Natl Acad Sci USA 2006; 103: 18842–18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz HR. Ratio scales of sugar sweetness. Percept Psychophys 1970; 7: 315–320. [Google Scholar]
- Harding PL. Distribution of total soluble solids and catalase in different parts of Jonathan apples. J Agric Res 1936; 53: 43–48. [Google Scholar]
- Smock RM. Apples and Apple Products. New York, NY: Interscience Publishers; 1950. [Google Scholar]
- Archbold HK, Barter AM. Chemical studies in the physiology of apples: XV. The relation of carbon dioxide output to the loss of sugar and acid in Bramley’s seedling apples during storage. Ann Bot 1934; 48: 957–966. [Google Scholar]
- Toivonen PMA, Hampson CR. Relationship of IAD index to internal quality attributes of apples treated with 1-methylcyclopropene and stored in air or controlled atmospheres. Postharvest Biol Technol 2014; 91: 90–95. [Google Scholar]
- Zanella A. Control of apple superficial scald and ripening—a comparison between 1-methylcyclopropene and diphenylamine postharvest treatments, initial low oxygen stress and ultra low oxygen storage. Postharvest Biol Technol 2003; 27: 69–78. [Google Scholar]