Abstract
Introduction:
Menopausal is a normal physiologic aging process in women characterized by decreasing estrogen levels. The skin is an organ dependant on hormones, estrogen being the most important in case of females, thereby influencing both the biology of skin and composition. Studies show that the systemic effects of estrogen deprivation occur years after attaining menopausal, however cutaneous features have been noticed earlier. The purpose of this study is to evaluate the common disorders occurring in perimenopausal women of Indian ethnicity.
Materials and Methods:
A retrospective observational study of outpatient records from Dermatology clinic between 2005 and 2012. All female patients between 45-55 years of age from an outpatient register that outlines the final diagnosis made by a qualified dermatologist after investigations. The data was entered according to the pattern of dermatoses and their seasonal variation and analyzed were included.
Results:
A total of 8,156 cases were found. After analysis of the many variables, the most common dermatoses in the perimenopausal population were eczematous disorders (23.6%), followed by urticaria (12.4%) and papulosquamous disorders (10.7%). Of the eczematous disorders, allergic and photosensitive disorders were found to be more frequent.
Conclusion:
The leading dermatoses being eczema and urticaria in the perimenopausal population probably accounts for a tendency of exaggerated response to external factors. The population studied in the current study might be of significance due to complete lack of treatment in the form of hormone replacement therapy (HRT), while routine sun exposure and cultural practices predominate. However, evaluation with respect to individual factors is beyond the scope of the current study and may be necessary to define a causal relationship.
Keywords: Eczematous disorders, estrogen, perimenopausal dermatoses
INTRODUCTION
Menopausal is a normal physiologic aging process in women characterized by decreasing estrogen levels. The skin is known to be an organ dependant on hormones, estrogen being the most important in case of females, which influences both the biology of skin as well as skin composition.[1] It is known from studies that the systemic effects of estrogen deprivation take its toll after years of attaining menopausal, however, cutaneous features have been noticed to occur far earlier. Hall and Philips described the following cutaneous features: Dryness, atrophy, wrinkling and poor wound healing.[1] Although certain dermatoses are known to occur during perimenopause, there is lack of a study about types of dermatoses occurring in perimenopausal women in India. The purpose of this study is to evaluate the common disorders occurring in perimenopausal women in India, and to document probable effects of hormonal fluctuations on the skin.
MATERIALS AND METHODS
The study model was that of an observational retrospective study conducted in a tertiary care hospital in South India during 2005-2012. Data of all female patients aged 45-55 years who were diagnosed with a skin condition was recorded into a predesigned proforma. All demographic details, the month and year of presentation and the final diagnosis were entered. A qualified dermatologist made the final diagnosis after necessary investigations. Descriptive statistics were used to analyze the data using Epi Info software.
RESULTS
A total of 8,156 female subjects aged 45-55 years and diagnosed with a skin disease during 2005-2012 period were included. All patients hailed from rural areas. Out of a total of 184 dermatoses that were divided into 15 categories, the most common dermatoses were eczematous (23.9%) followed by urticaria (12.3%) and papulosquamous (10.9%) as outlined in Table 1. On evaluating the occurrence of dermatoses with respect to age of presentation, it can be noted that higher proportion was noted in the early period, 45-50 years, as shown in Table 2. The dermatoses under miscellaneous category are outlined in Table 3.
Table 1.
Pattern of dermatoses presenting in perimenopausal women over 8 year time frame
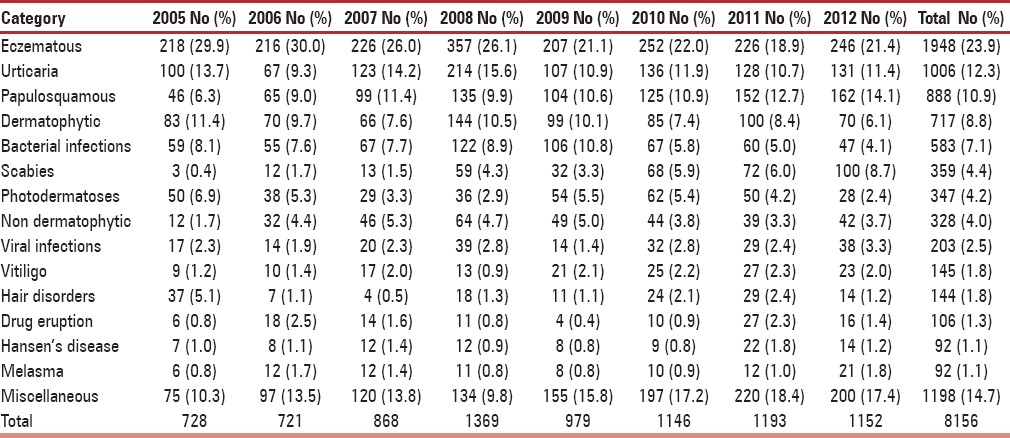
Table 2.
Age distribution of perimenopausal cases
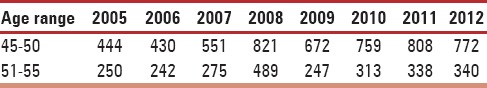
Table 3.
Disorders belonging to miscellaneous category

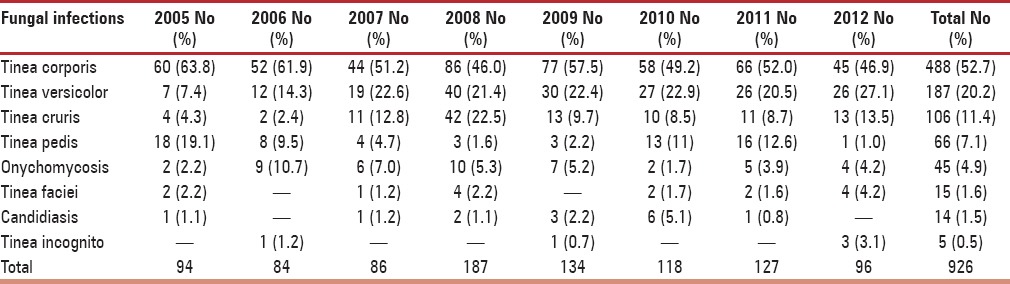
On further analysis of subtypes, among eczematous disorders presenting in perimenopausal women, the most common type was allergic contact dermatitis (32.3%) followed by asteatosis (13.0%) and irritant dermatitis (11.6%) as mentioned in Table 4. The proportion of photodermatoses presenting in perimenopausal women was considerable (4.2%), and a recent decline in incidence may suggest a sun avoidance factor as seen in Table 1. Among patients suffering from urticaria, the most frequent was chronic urticaria (47.7%), followed by acute urticaria (42.0%) and papular urticaria (10.2%). On evaluating papulosquamous disorders, the condition diagnosed most frequently was psoriasis vulgaris (40.7%), followed by palmoplantar psoriasis (25.1%) and lichen planus (15.4%), as outlined in Table 5. On examining reports of vitiligo, a considerable peak in incidence of vitiligo vulgaris (81.3%) can be appreciated. On evaluating leprosy cases, a significantly higher incidence of borderline tuberculoid Hansen's disease (58.4%) was observed, followed by treated Hansen's disease (18.2%), as noted in Table 6. On analyzing hair disorders reported in perimenopausal women, the most reported was alopecia areata (67.9%), followed by telogen effluvium (24.8%). On looking at types of drug reactions, lichenoid type was commonest. On further evaluation of dermatophytic fungal infections, tinea corporis had highest occurrence followed by tinea versicolor as noted in Table 7. The incidence of melasma (1.1%) is not considerable in comparison to other dermatoses.
Table 4.
Pattern of eczematous disorders over 8-year period
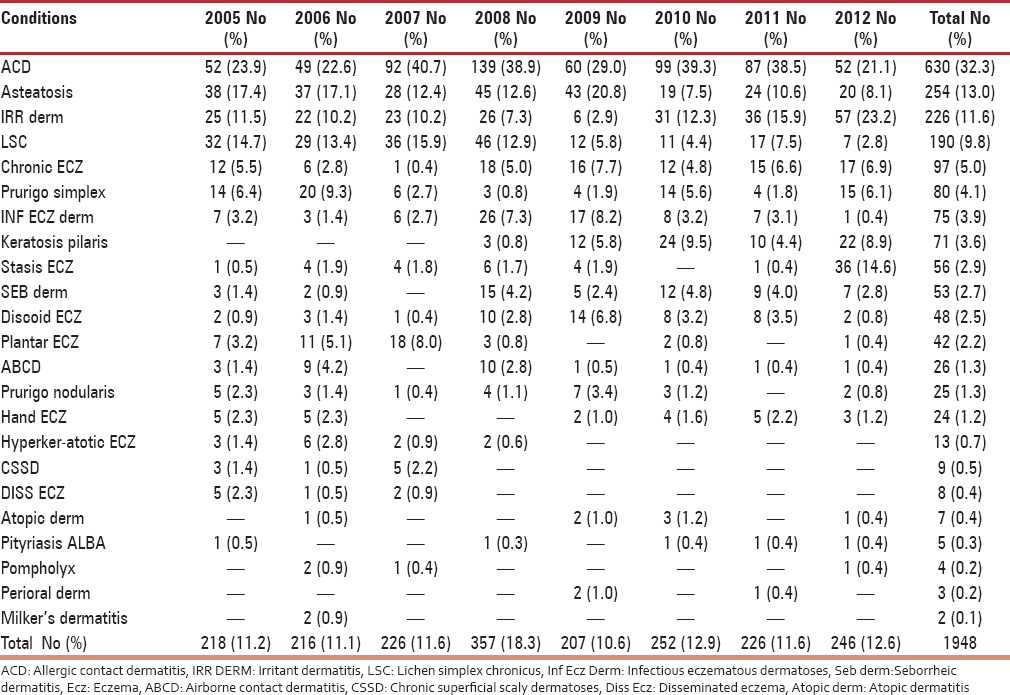
Table 5.
Pattern of papulosquamous disorders
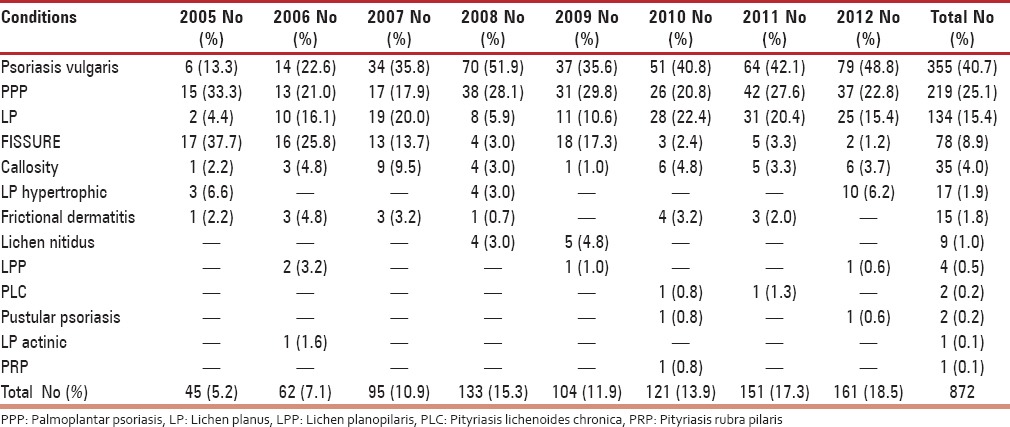
Table 6.
Incidence of Hansen's disease in perimenopausal women
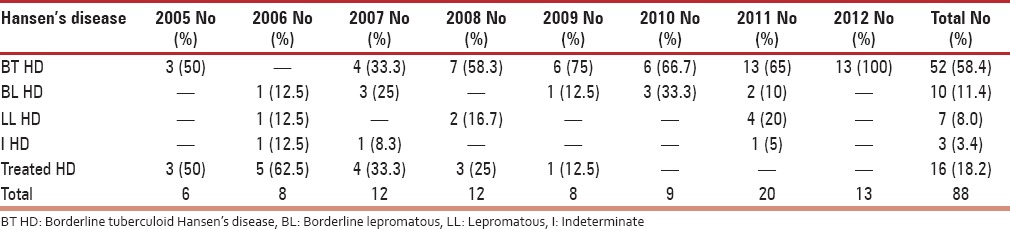
Table 7.
List of fungal infections presenting in perimenopausal women
After analyzing the trend of dermatoses occurring in the perimenopausal age group, a prominent increased frequency of eczematous disorders was noted in 2008 and 2010. A similar trend was seen for cases of urticaria and bacterial infections.
On observing the pattern of leprosy cases this was found to occur uniformly (0.8-1.8%) within this time period.
The occurrence of scabies in perimenopausal period showed low incidence in the early years that exhibited a gradual increase in following years as shown in Table 1. The overall incidence is ranked as the sixth most common dermatoses and the second infectious disease occurring in perimenopausal population.
On comparing incidence of melasma to other hyperpigmented disorders, a considerable difference can be appreciated (1.7:1). Among hair disorders, only a single case of scarring alopecia has been reported in the current study.
DISCUSSION
Menopausal occurs when normal follicular maturation and ovulation ceases, resulting in reduced estrogen levels and increased follicle stimulating hormone. The results of these effects on skin have been studied coupled with hormone measurement. Perimenopausal women are identified to be susceptible to skin disorders because of the disturbed physiological effects exerted by hormonal changes. In a tropical country like India, particularly the southern region, where the disease pattern can be influenced by factors such as occupation, nutrition and sun exposure, we studied the dermatoses in perimenopausal women of rural population.
Hall and Philips conducted a systematic review in 2005 revealing increased sensitivity of skin and progressive loss of dermal collagen in postmenopausal skin.[1] The physiological effects resulting from estrogen deficit include impaired wound healing and increased transepidermal water loss.
On studying the action of estrogen on skin, it was concluded that it is mediated by estrogen receptors that are expressed in increasing numbers over face, groin area and lower extremities.[2] The estrogen receptors are classified as two types: Alpha and beta. The alpha-receptors are expressed on fibroblasts and macrophages, while beta receptors occur on basal keratinocytes, melanocytes and dendritic cells.[3] Decreased estrogen stimulation on skin has been described to cause reduced epidermal differentiation, diminished melanin secretion, decreased production of collagen IV and VII and reduced lipogenesis.[4] It has to be studied as to how these effects translate to a change in dermatoses occurring in this age group.
Previous studies have outlined the skin disorders related to menopausal as atrophic vulvo-vaginitis, flushing, lichen sclerosus et atrophicus, hirsutism, androgenetic alopecia, vulvovaginal candidiasis, Paget's disease of nipple and keratoderma climactericum.[5,6] However in our study, reports of these conditions were negligible in comparison to other disorders. It may seem appropriate to classify these disorders as occurring in late menopausal, whereas this study evaluated the perimenopausal period alone.
Few authors have highlighted the dermatoses linked to aging process as pruritus, asteatosis, atopic eczema, seborrheic dermatitis, pemphigoid, flexural psoriasis, leg ulcers, decubitus ulcers, post herpetic neuralgia, seborrheic keratosis, acrochordons, actinic keratoses, basal cell carcinoma, squamous cell carcinoma, lentigo maligna and scabies. Apart from the occasional diagnosis, the majority of dermatoses does not seem related to intrinsic aging in the perimenopausal period.[7]
After analysis, eczematous disorders were the most common dermatoses noted in the perimenopausal period in the present study, however a notable change noted was a gradual decrease in incidence over the years 2008-2010. The increased occurrence of eczema can be linked to a finding in a study conducted by Paquet et al., which revealed disturbed moisture content of epidermis in relation to menopausal.[8] The most common eczematous disorder was found to be allergic contact dermatitis that can be attributed to the changes of increased skin sensitivity and transepidermal water loss noted in perimenopausal skin. Reported increased skin drying to be associated with menopausal supports this finding.[9] Most of our patients are agricultural laborers exposed to plant allergens including parthenium. This is reflected in the higher incidence of allergic contact dermatitis in our study.
The pattern of dermatoses is influenced by other factors such as geography, climate, occupation etc. This is reflected in our study with high incidence of bacterial and fungal infections. The tropical climate predisposes every individual to these infections and perimenopausal age group is no exception.
With regard to the uniform incidence of leprosy in the 7-year period this may have no relation with hormonal change but rather point to an external factor. This in turn points to an absence of a decreased immunity in perimenopausal period. The incidence of Hansen's disease is quite high considering the small group of the study population. The occurrence of Hansen's disease cases does not auger well for leprosy eradication program.
The pattern of hair disorders showed increased occurrence of alopecia areata. Previous studies have described frontal fibrosing alopecia to be related to menopausal,[10,11,12] however, in the present study there was a single case of the condition. Another finding of importance is the lowest incidence of diffuse hair fall. This can particularly be explained by the estrogen role of reducing telogen phase whereby increasing the anagen phase.[13]
In the present study, a total of 92 (1.1%) patients were diagnosed with melasma and the finding of higher incidence in comparison to other pigmentation disorders denotes relation to menopausal. However, there was no report of extra facial melasma that is presumably seen in menopausal skin.[14]
A number of studies have shown a benefit of hormone replacement therapy (HRT) on skin, particularly related to aging,[15,16,17] whereas in a rural population where our study was conducted, this was inconsiderable. As our study involved the rural population, they were not likely to be exposed to HRT and hence we cannot comment on this aspect.
Even though this study has enlightened on the incidence of specific dermatoses presenting in perimenopausal women, further studies are needed to evaluate the causative role, varied presentations and the outcome of preventive measures.
CONCLUSION
The leading dermatoses being eczema and urticaria in the perimenopausal population probably accounts for a tendency of exaggerated response to external factors. The population studied in the current study might be of significance due to complete lack of treatment in the form of HRT, while routine sun exposure and cultural practices predominate. However, evaluation with respect to individual factors is beyond the scope of the current study and may be necessary to define a causal relationship.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hall G, Philips TJ. Estrogen and skin: The effects of estrogen, menopause and hormone replacement therapy on the skin. J Am Acad Dermatol. 2005;53:555–68. doi: 10.1016/j.jaad.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Strupf WE, Sur M, Joshi S. Oestrogen target cells in the skin. Experimentalia. 1976;30:176–7. [Google Scholar]
- 3.Schmidt JB. Perimenopausal influence on skin, hair and appendages. In: Fischl F, editor. Menopause — andropause: Hormone replacement therapy through the ages. 1st ed. Gablitz: Krause and Pachernegg GmbH; 2001. pp. 145–51. [Google Scholar]
- 4.Zegarska B, Woźniak M. Wplyw estrogenu na zmiany zachodzace w skoórze [The influence of estrogen on skin changing] Przegląd Menopauzalny. 2007;4:233–8. [Google Scholar]
- 5.Graham-Brown R. Later life. Dermatologic problems of the menopause. Clin Dermatol. 1997;15:143–5. doi: 10.1016/s0738-081x(96)00116-2. [DOI] [PubMed] [Google Scholar]
- 6.Brenner S, Politi Y. Dermatologic diseases and problems of women throughout the life cycle. Int J Dermatol. 1995;34:369–79. doi: 10.1111/j.1365-4362.1995.tb04434.x. [DOI] [PubMed] [Google Scholar]
- 7.Nair AP. Dermatosis associated with menopause. J Midlife Health. 2014;5:168–75. doi: 10.4103/0976-7800.145152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sator PG, Schmidt JB, Rabe T, Zouboulis CC. Skin aging and sex hormones in women – Clinical perspectives for intervention by hormone replacement therapy. Exp Dermatol. 2004;13:36–40. doi: 10.1111/j.1600-0625.2004.00259.x. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair R. Hair structure and function. In: Sinclair R, Banfield C, Dawber R, editors. Handbook of disease of the hair and scalp. 4th ed. New York: Blackwell Oxford Press; 1999. pp. 3–23. [Google Scholar]
- 10.Paquet F, Pierard-Franchimont C, Fumal I, Goffin V, Paye M, Pierard GE. Sensitive skin at menopause; dew point and electrometric properties of the stratum corneum. Maturitas. 1998;28:221–7. doi: 10.1016/s0378-5122(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 11.Dawn G, Holmes SC, Moffat D, Munro CS. Post-menopausal frontal fibrosing alopecia. Clin Exp Dermatol. 2003;28:43–5. doi: 10.1046/j.1365-2230.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 12.Tosti A, Piraccini BM, Iorizzo M, Misciali C. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55–60. doi: 10.1016/j.jaad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Tchernev G, Tronnier M. Steven Kossards postmenopausal frontal fibrosing alopecia (PFFA) – A therapeutic dilemma. Akush Ginekol (Soflia) 2010;49:46–9. [PubMed] [Google Scholar]
- 14.Ritter CG, Fiss DV, Borges da Costa JA, de Carvalho RR, Bauermann G, Cestari TF. Extra facial melasma: Clinical, histopathological, and immunohistochemical case control study. J Eur Acad Dermatol Venereol. 2013;27:1088–94. doi: 10.1111/j.1468-3083.2012.04655.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt JB, Binder M, Macheiner W, Kainz C, Gitsch G, Bieglmayer C. Treatment of skin ageing symptoms in perimenopausal females with estrogen compounds: A pilot study. Maturitas. 1994;20:25–30. doi: 10.1016/0378-5122(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 16.Pierard-Franchimont C, Letawe C, Goffin V, Pierard GE. Skin water-holding capacity and transdermal estrogen therapy for menopause: A pilot study. Maturitas. 1995;22:151–4. doi: 10.1016/0378-5122(95)00924-a. [DOI] [PubMed] [Google Scholar]
- 17.Pierard-Franchimont TC, Cornil F, Dehavay J, Deleixe-Mauhin F, Letot B, Pierard GE. Climacteric skin ageing of the face – A prospective longitudinal comparative trial on the effect of oral hormone replacement therapy. Maturitas. 1999;32:87–93. doi: 10.1016/s0378-5122(99)00019-5. [DOI] [PubMed] [Google Scholar]


