Abstract
Recent advances in the understanding of neuromyelitis optica spectrum of disorders (NMOSD) have expanded. Diagnostic criteria have changed over the years. The clinical spectrum of disease manifestations are now understood to include sites outside the spinal cord and optic nerve. A variety of autoimmune diseases may coexist with this disorder. Non neurological manifestations have been recently reported. Novel biomarkers other than aquoporin 4 Immunoglobulin G (anti AQP4-IgG) have been discovered which may have clinical relevance. In particul myelin associated oligoglycoprotein antibody (MOG-Ab) associated NMOSD may be relatively benign. This update describes some of these new findings highlighting the clinical manifestations, biomarkers associated with the disease and magnetic resonance imaging characteristics of brain and spinal cord.
Keywords: Anti aquaporin 4 -IgG, clinical features, MRI, NMOSD
Introduction
Neuromyelitis optica (NMO) is an autoimmune disorder of the central nervous system, which until recently was thought to be a variant of multiple sclerosis (MS). As the name suggests it targets the spinal cord and optic nerve predominantly, is most often relapsing and has a female preponderance. The discovery of a novel biomarker anti aquoporin 4 immunoglobulin G (anti AQP4-IgG) vastly improved the specificity of diagnosis.[1] A variety of conditions were discovered with anti AQP4-IgG positive state necessitating the coining of the term NMO spectrum disorders (NMOSD).[2] Recently greater efforts have been made to improve the diagnostic criteria of the disease that would allow the inclusion of ever increasing clinical manifestations of the disease. Brain MRI features suggestive of NMOS have been described and additional biomarkers are being identified, which may produce an “NMO phenotype” disorder.
This review highlights the clinical features particularly the “extra opticospinal” cord manifestations and the non-neurological associations of this disorder. It briefly touches on new biomarkers associated with NMOS and the clinical relevance of the same. Lastly an attempt is made to describe the NMOS-specific lesions on magnetic resonance imaging (MRI) of both spinal cord and brain.
Epidemiology
In India, there is a dearth of epidemiological data for demyelinating disorders. A population-based survey in urban Mangalore has shown a prevalence of 2.6/100,000 for NMO while the same for MS was 8.3/100,000.[3] The spectrum of NMO disorders is likely to constitute approximately 20% of all demyelinating disorders in India.[4] The clinical presentations of NMO and the disease course is similar worldwide. The mean age at onset (32.6-45.7) and median time to first relapse (8-12 months) is similar in different populations studied.[5] Female preponderance is seen particularly in the relapsing form of the disease.
Etiopathogenesis
It is noteworthy that nearly a third of attacks in NMOSD are preceded by fever or vaccination.[6] However no specific environmental agent has been associated with NMOS. Genetic susceptibility studies have shown that HLA-DRB1*03 may be associated with NMOS in Indian population.[7] A similar result was reported from Brazil,[8] the Caribbean islands[9] and France.[10] Most cases of NMOSD are sporadic in occurrence though familial forms have been rarely reported.
In nearly 70-80% of cases, anti AQP4-IgG is associated with NMOS. Aquoporin 4 is a water transport protein highly expressed at the astrocyte end feet at the blood brain barrier. It is expressed widely in all neural tissues with the highest concentration in optic nerves and spinal cord. Anti AQP4-IgG is produced mainly by plasma cells in the peripheral blood by unknown mechanisms and gains access to the central nervous system through a blood brain barrier breach. They target the aquoporin rich astrocyte foot processes and the subsequent antigen antibody interaction leads to activation of complement cascade with complement dependent cellular cytotoxicity. Granulocytes (neutrophils, eosinophils) migrate to the region by chemotaxis and there is antibody-dependent cellular cytotoxicity. When unchecked, the inflammation is severe and associated with necrosis.
Diagnostic criteria
The original criteria proposed by Wingerchuk et al., in 1996[6] was revised in 2006 after the discovery of anti AQP4-IgG. The 2006 criteria[11] included optic neuritis (OPN), acute myelitis, and at least two out of three supportive criteria: Contiguous spinal cord MRI lesion extending over three or more vertebral segments; brain MRI not meeting MS diagnostic criteria; and seropositivity for NMO-IgG [Table 1]. Seropositive patients may manifest limited forms of the disease, which includes isolated unilateral/bilateral recurrent OPN, recurrent transverse myelitis, myelitis associated with collagen vascular disorders and many more and is loosely termed as NMOSD.[2] Currently a revision of diagnostic criteria is underway. Some of the salient features include the addition of area postrema syndrome (presentation with nausea, vomiting and hiccups), other brain stem syndromes, symptomatic narcolepsy and symptomatic cerebral syndrome with MRI findings.
Table 1.
Revised criteria for diagnosis of NMO

Clinical Features
The classical features of NMO are acute severe OPN and longitudinally extensive transverse myelitis (LETM) defined as longitudinal cord lesions extending >three vertebral segments. Most often the first attack is monosymptomatic. The concomitant appearance of both OPN and TM is seen in 15-40% of cases. It is difficult to differentiate OPN associated with NMOSD from that seen in MS. Severe visual impairment, bilateral simultaneous or sequential OPN in rapid succession is suggestive of NMOSD rather than MS. Spinal cord lesions typically present as complete transverse myelitis leading to total quadriplegia or paraplegia with a definite sensory level and bladder involvement. Occasionally seropositive patients have been noted to have spinal lesions that fall short of the prescribed three segments. The timing of MRI is very crucial. When imaging is performed early in the disease course and also when disease is resolving, the classical tumefactive and longitudinal lesion may be missed. Spinal cord may even harbor asymptomatic lesions in patients presenting with OPN.[12] Neuropathic pain, Lhermitte's sign and painful tonic spasms[13] are common. Extension of cervical spinal cord lesions into the brainstem can cause hiccups, vomiting and occasionally respiratory failure. It is to be remembered that not all long cord lesions are NMOSD. They may be due to idiopathic transverse myelitis, acute disseminated encephalomyelitis (ADEM) or rarely infective causes.
Extra optico spinal manifestations of NMOSD
The clinical manifestations of NMOSD may involve sites outside the spinal cord and optic nerve. They may be the presenting and sometimes the only features of the disease. Brain stem involvement in the form of intractable vomiting[14] (area postrima syndrome), intractable hiccups due to peri-aquiductal lesions in the midbrain[15] and narcolepsy,[16] hypothermia and hypersomnolence due to diencephalic involvement[17] are typical examples. In a large series of 258 patients reported by Kremer et al.,[18] brainstem involvement was seen in one-third of the patients. In 54.3% patients, it was the initial manifestation. Vomiting and hiccups were more common followed by occulomotor dysfunction, pruritis, deafness, facial palsy, vertigo, trigeminal neuralgia and other cranial nerve signs. Brainstem involvement is more common in anti AQP4-IgG-positive patients and particularly among non-Caucasians.
Other symptoms/syndromes that have been reported include seizures, hyposmia,[19] posterior reversible encephalopathy syndrome (PRES),[20] meningoencephalitis,[21] myeloradiculopathy,[22] cognitive dysfunction, and ophthalmoplegia. Skeletal and smooth muscle involvement in the form of muscle edema and myocarditis respectively have been noted.[23] HyperCKemia has been detected during attacks.[24] The growing number of clinical manifestations most often backed by neuroimaging and pathological studies suggests that these extra optico spinal manifestations may be explained by the distribution and expression of AQP4.[25]
Non-neurological manifestations of NMO
Recently placentitis with risk of abortion,[26] internal otitis[27] and gastritis[28] have been described with seropositive NMOSD. These findings suggest that anti AQP4-IgG antibodies may target extra neural tissues that express aquoporin 4. Nearly 30-40% of patients with NMOSD have coexisting autoimmune disorders such as Sjögren's syndrome, systemic lupus erythematosus, autoimmune thyroid disease, myasthenia gravis, autoimmune-mediated vitamin B12 deficiency, autoimmune encephalitis and several more.
The clinical course of the disease and particularly mortality has changed remarkably in recent years. Mortality rates have improved from 30% at 5 years[6] to 9% at 6 years.[29] It is likely that greater awareness of the disease, access to anti AQP4-IgG testing and use of long-term immunosuppressants have all contributed to improved outcome.
Investigations
Biomarkers in NMOSD
In 2004 an antibody specific to NMO was discovered, initially called as NMO immunoglobulin (NMO-IgG), which later became known as anti-aquaporin 4 antibody.[1] Various assays have shown high specificity (91-100%) and varying sensitivity (83-91%) with cell-based assays being the most optimal.[30] Immunosuppressive therapy dramatically reduces serum levels of anti AQP4-IgG and hence testing has to be done prior to therapy. Retesting may be necessary and preferably at the onset of a relapse in such situations. Anti AQP4-IgG levels are stable at room temperature for up to 8 days. Levels may not be significantly affected by repeat freeze/thaw cycles in the lab. Therefore shipment at room temperature is justified when no other options are available. The exception may be in borderline or hemolytic samples where storage conditions matter.[31]
Overall anti AQP4-IgG positive patients have a relapsing disease, are overwhelmingly female, have a strong association with other connective tissue disorders and have more severe clinical attacks. An important proportion of cases are seronegative.[32] Seronegative patients have more often a monophasic course and affect males equally as females. Recent studies showed that anti AQP4-IgG negative disorders may be positive for anti-myelin associated oligoglycoprotein (anti-MOG).[33] Anti MOG associated NMOS may be seen equally in women and men, and have a monophasic course.[34] OPN and particularly simultaneous and recurrent OPN may be more common. Myelitis involving the caudal portions of the cord is more likely. Coexisting systemic autoimmunity is less common than AQP4 antibody associated NMOS. Most importantly these patients may have a more benign course. Other auto antibodies such as anti-CV2/CRMP5 and NMDA receptor antibody have been shown to mimic NMOSD in isolated cases.
Paraclinical tests
Cerebrospinal fluid (CSF) evaluation may show a variable degree of pleocytosis and raised proteins. Oligoclonal bands (restricted to the CSF) may be evident in approximately 20% of anti AQP4-IgG seropositive patients. It is not unusual to find eosinophils and neutrophils in the CSF.
Optical coherence tomography (OCT)
It is a non-invasive method of evaluating the unmyelinated retinal axons otherwise called retinal nerve fiber layer (RNFL) and their neurons (retinal ganglion cells). In NMOSD, OCT studies have consistently showed severe RNFL thinning associated with poor visual outcome. The role of OCT in differentiating NMOSD from MS and other inflammatory diseases, its role in monitoring disease progress and therapy is currently being investigated.
MRI in NMO
MRI of the spine
Spinal cord MRI is crucial for the diagnosis of NMOSD especially since brain lesions indistinguishable from MS are seen in up to 27% of patients.[35] The hallmark lesion is the long cord lesions extending more than three vertebral segments. It is edematous in the acute phase, most often centrally placed and involves the thoracic and cervical cord commonly [Figure 1]. In contrast, spinal cord lesions in MS are short, situated posteriorly and most often in the cervical cord. NMOSD lesions show patchy enhancement in 33-71% of cases and during an acute relapse.[36] It is to be noted that failure to enhance sometimes prevents the distinguishing of a recent attack from a pseudo relapse. After steroid therapy lesions tend to fragment into shorter segments, chronic lesions may show cavitation and cord atrophy.
Figure 1.
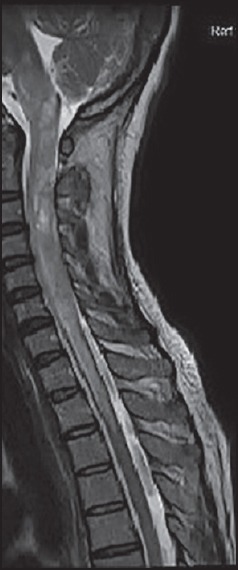
Spinal cord Image in NMOS, T2-weighted MRI scan showing longitudinally extensive transverse myelitis involving the cervical and upper thoracic cord. Cranial extension of cord lesion into the medulla is seen
MRI of the Brain
Brain lesions are present in more than half of the patients at onset and increases during disease course.[37,38] It is increasingly clear that in many instances the MRI of the brain shows lesions in NMOSD that are indistinguishable from MS. Absence of Dawson's finger, “U” fiber lesions, paucity of lateral ventricle and inferior temporal lobe lesions are strongly suggestive of NMOSD.[35] In doubtful situations the cord lesions should be carefully scrutinized to obtain additional supportive evidence. The so-called NMO-specific lesions are seen at sites of high AQP4 expression, such as the diencephalon, the hypothalamus and the aqueduct and are present in <10% of NMOS.[39] Conversely patients presenting with typical NMOSD symptoms such as intractable vomiting and hiccups need not show corresponding lesions on conventional MRI sequences. NMOSD lesions are frequent in the corpus callosum. Infratentorial lesions commonly involve the medulla where they may be contiguous with cervical cord lesions. Lesions may be seen in the pons, cerebellar peduncles and midbrain. Tumefactive brain lesions, peri-ependymal lesions involving third, fourth and lateral ventricles and involvement of corticospinal tracts [Figure 2] have been described.[40] Brain lesions in NMOSD may show variable enhancement.
Figure 2.
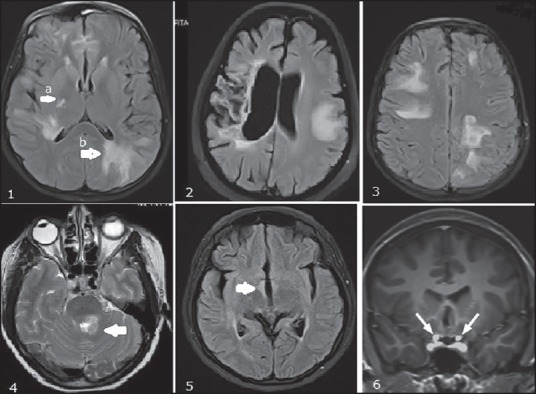
Brain Images in anti-aquaporin-IgG-positive NMOS, FLAIR images of the brain showing 1a. Lesion in posteriorlimb of internal capsule; 1b. Periependymal lesions around the lateral ventricle. 2. T2W image of brain showing a tumefactive demyelinating lesion with evidence of contralateral gliosis and atrophy from a previous attack. 3. PRES like brain lesions. 4. Lesion near fourth ventricle extending to middle cerebellar peduncle. 5. Diencephalic involvement. 6. Bilateral optic nerve (intracranial segment) involvement
MRI of the orbit
It is important to include optic nerve imaging as part of the MRI protocol for investigation of a suspected case of CNS demyelinating disorder. Optic nerve lesions are frequently extensive, bilateral, involving intracranial portions of the nerve and frequently the optic chiasm.[41]
Conclusions
Our understanding of the NMOSD is incomplete and we are only beginning to comprehend the diverse clinical manifestations of the disease. Literature worldwide supports the view that anti AQP4-IgG associated disorders are severely disabling without early treatment interventions. With the rapid expansion of the spectrum of anti AQP4-IgG associated disorders, the real challenge that faces the clinician is the recognition of this condition in settings unaccompanied by OPN and/or myelitis. Disability in NMOSD is attack-related unlike in MS. Therefore, early diagnosis offers a window of opportunity for treatment and induction of remission.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Lennon V, Wingerchuk D, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet. 2004;364:2106–12. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 3.Pandit L, Kundapur R. Prevalence and patterns of demyelinating central nervous system disorders in urban Mangalore, South India. Mult Scler. 2014;20:1651–3. doi: 10.1177/1352458514521503. [DOI] [PubMed] [Google Scholar]
- 4.Pandit L, Mustafa S, Kunder R, Shetty R, Misri Z, Pai S, et al. Optimizing the management of neuromyelitis optica and spectrum disorders in resource poor settings: Experience from the Mangalore demyelinating disease registry. Ann Indian Acad Neurol. 2013;16:572–6. doi: 10.4103/0972-2327.120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite MI, et al. GJCF International Clinical Consortium and Biorepository for Neuromyelitis Optica. Demographic and clinical features of neuromyelitis optica: A review. Mult Scler. 2015;21:845–53. doi: 10.1177/1352458515572406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–14. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 7.Pandit L, Malli C, D’Cunha A, Mustafa S. Human leukocyte antigen association with neuromyelitis optica in a south Indian population. Mult Scer. 2015 doi: 10.1177/1352458515574149. [DOI] [PubMed] [Google Scholar]
- 8.Brum DG, Barreira AA, dos Santos AC, Kaimen-Maciel DR, Matiello M, Costa RM, et al. HLA-DRB association in neuromyelitis optica is different from that observed in multiple sclerosis. Mult Scler. 2010;16:21–9. doi: 10.1177/1352458509350741. [DOI] [PubMed] [Google Scholar]
- 9.Deschamps R, Paturel L, Jeannin S, Chausson N, Olindo S, Béra O, et al. Different HLA class II (DRB1 and DQB1) alleles determine either susceptibility or resistance to NMO and multiple sclerosis among the French Afro-Caribbean population. Mult Scler. 2011;17:24–31. doi: 10.1177/1352458510382810. [DOI] [PubMed] [Google Scholar]
- 10.Zéphir H, Fajardy I, Outteryck O, Blanc F, Roger N, Fleury M, et al. Is neuromyelitis optica associated with human leukocyte antigen? Mult Scler. 2009;15:571–9. doi: 10.1177/1352458508102085. [DOI] [PubMed] [Google Scholar]
- 11.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan EP, Weinshenker BW, Krecke KN, Pittock S. Asymptomatic myelitis in neuromyelitis optica and autoimmune aquaporin4 channelopathy. Neurol Clin Pract. 2015;5:175–77. doi: 10.1212/CPJ.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SM, Go MJ, Sung JJ, Park KS, Lee KW. Painful tonic spasm in neuromyelitis optica: Incidence, diagnostic utility, and clinical characteristics. Arch Neurol. 2012;69:1026–31. doi: 10.1001/archneurol.2012.112. [DOI] [PubMed] [Google Scholar]
- 14.Popescu BF, Lennon VA, Parisi JE, Howe CL, Weigand SD, Cabrera-Gomez JA, et al. Neuromyelitis optica unique area postrema lesions: Nausea, vomiting, and pathogenic implications. Neurology. 2011;76:1229–37. doi: 10.1212/WNL.0b013e318214332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misu T, Fujihara K, Nakashima I, Sato S, Itoyama Y. Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology. 2005;65:1479–82. doi: 10.1212/01.wnl.0000183151.19351.82. [DOI] [PubMed] [Google Scholar]
- 16.Kanbayashi T, Shimohata T, Nakashima I, Yaguchi H, Yabe I, Nishizawa M, et al. Symptomatic narcolepsy in patients with neuromyelitis optica and multiple sclerosis: New neurochemical and immunological implications. Arch Neurol. 2009;66:1563–6. doi: 10.1001/archneurol.2009.264. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Nakamura T, Hashimoto K, Miyamoto M, Komagamine T, Nagashima T, et al. Hypothermia, hypotension, hypersomnia, and obesity associated with hypothalamic lesions in a patient positive for the anti-aquaporin 4 antibody: A case report and literature review. Arch Neurol. 2012;69:1355–9. doi: 10.1001/archneurol.2012.300. [DOI] [PubMed] [Google Scholar]
- 18.Kremer L, Mealy M, Jacob A, Nakashima I, Cabre P, Bigi S, et al. Brainstem manifestations in neuromyelitis optica: A multicenter study of 258 patients. Mult Scler. 2014;20:843–47. doi: 10.1177/1352458513507822. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt F, Goktas O, Jarius S, Wildemann B, Ruprecht K, Paul F, et al. Olfactory dysfunction in patients with neuromyelitis optica. Mult Scleros Int 2013. 2013 doi: 10.1155/2013/654501. 654501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magan˜;a SM, Matiello M, Pittock SJ, McKeon A, Lennon VA, Rabinstein AA, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72:712–7. doi: 10.1212/01.wnl.0000343001.36493.ae. [DOI] [PubMed] [Google Scholar]
- 21.Wang JY, Wang K, Chen XW, Wang JW, Zhang K, Xu MW, et al. Meningoencephalitis as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler. 2012;19:639–43. doi: 10.1177/1352458512459785. [DOI] [PubMed] [Google Scholar]
- 22.Takai Y, Misu T, Nakashima I, Takahashi T, Itoyama Y, Fujihara K, et al. Two cases of lumbosacral myeloradiculitis with anti-aquaporin-4 antibody. Neurology. 2012;79:1826–8. doi: 10.1212/WNL.0b013e3182703ff7. [DOI] [PubMed] [Google Scholar]
- 23.Cosgrove J, Alli S, Ramadan H, Ford HL. Myocarditis and diffuse skeletal muscle oedema: New features of neuromyelitis optica spectrum disorder? A case report. Mult Scler. 2014;20:120–2. doi: 10.1177/1352458513495939. [DOI] [PubMed] [Google Scholar]
- 24.Deguchi S, Deguchi K, Sato K, Yunoki T, Omote Y, Morimoto N, et al. HyperCKemia related to the initial and recurrent attacks of neuromyelitis optica. Intern Med. 2012;51:2617–20. doi: 10.2169/internalmedicine.51.7898. [DOI] [PubMed] [Google Scholar]
- 25.Weinshenker BG, Wingerchuk DM. The two faces of neuromyelitis optica. Neurology. 2014;82:466–7. doi: 10.1212/WNL.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 26.Saadoun S, Waters P, Leite MI, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica IgG causes placental inflammation and fetal death. J Immunol. 2013;191:2999–3005. doi: 10.4049/jimmunol.1301483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarius S, Lauda F, Wildemann B, Tumani H. Steroid-responsive hearing impairment in NMO-IgG/aquaporin-4-antibody-positive neuromyelitis optica. J Neurol. 2013;260:663–4. doi: 10.1007/s00415-012-6755-4. [DOI] [PubMed] [Google Scholar]
- 28.Jarius S, Paul F, Ruprecht K, Wildemann B. Low vitamin B12 levels and gastric parietal cell antibodies in patients with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorders. J Neurol. 2012;259:2743–5. doi: 10.1007/s00415-012-6677-1. [DOI] [PubMed] [Google Scholar]
- 29.Kitley J, Leite MI, Nakashima I, Waters P, McNeillis B, Brown R, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain. 2012;135:1834–49. doi: 10.1093/brain/aws109. [DOI] [PubMed] [Google Scholar]
- 30.Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A, et al. Serologic diagnosis of NMO: A multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78:665–71. doi: 10.1212/WNL.0b013e318248dec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarius S, Wildemann B. Effect of storage conditions and freeze/thaw cycles on aquaporin-4 antibody (NMO-IgG) serum levels. Clin Chem Lab Med. 2011;49:2121–2. doi: 10.1515/CCLM.2011.717. [DOI] [PubMed] [Google Scholar]
- 32.Jiao Y, Fryer JP, Lennon VA, McKeon A, Jenkins SM, Smith CY, et al. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology. 2013;81:1197–204. doi: 10.1212/WNL.0b013e3182a6cb5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitley J, Woodhall M, Waters P, Leite MI, Devenney E, Craig J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. 2012;79:1273–7. doi: 10.1212/WNL.0b013e31826aac4e. [DOI] [PubMed] [Google Scholar]
- 34.Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, Jorge FM, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody positive NMO spectrum disorders. Neurology. 2014;82:474–81. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathews L, Marasco R, Jenkinson M, Kuker W, Luppe S, Leite MS, et al. Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology. 2013;80:1330–7. doi: 10.1212/WNL.0b013e3182887957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kýyat-Atamer A, Ekizoğlu E, Tuzun E, Kürtüncü M, Shugaiv E, Akman-Demir G, et al. Long-term MRI findings in neuromyelitis optica: Seropositive versus seronegative patients. Eur J Neurol. 2013;20:781–7. doi: 10.1111/ene.12058. [DOI] [PubMed] [Google Scholar]
- 37.Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006;63:390–6. doi: 10.1001/archneur.63.3.390. [DOI] [PubMed] [Google Scholar]
- 38.Cabrera-Gomez JA, Kister I. Conventional brain MRI in neuromyelitis optica. Eur J Neurol. 2012;19:812–9. doi: 10.1111/j.1468-1331.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 39.Huh SY, Min JH, Kim W, Kim SH, Kim HJ, Kim BJ, et al. The usefulness of brain MRI at onset in the differentiation of multiple sclerosis and seropositive neuromyelitis optica spectrum disorders. Mult Scler. 2014;20:695–704. doi: 10.1177/1352458513506953. [DOI] [PubMed] [Google Scholar]
- 40.Kim W, Park MS, Lee SH, Kim SH, Jung IJ, Takahashi T, et al. Characteristic brain magnetic resonance imaging abnormalities in central nervous system aquaporin-4 autoimmunity. Mult Scler. 2010;16:1229–36. doi: 10.1177/1352458510376640. [DOI] [PubMed] [Google Scholar]
- 41.Khanna S, Sharma A, Huecker J, Gordon M, Naismith RT, Van Stavern GP. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol. 2012;32:216–20. doi: 10.1097/WNO.0b013e318254c62d. [DOI] [PubMed] [Google Scholar]


