Abstract
The newer immunotherapies for multiple sclerosis (fingolimod, natalizumab, dimethyl fumarate, teriflunomide, alemtuzumab) offer advantages of efficacy or tolerability over the injectable therapies of the 1990s. But they also have greater risks. As further treatments emerge (daclizumab and ocrelizumab are likely to be licensed in the next two years), the physician needs to be able to place them within a complex landscape of drugs and a specific treatment strategy, which may be an “escalation” or “induction” approach. Whilst on treatment, neurologist and patient need to be vigilant to signs of disease breakthrough or adverse effects.
Keywords: Multiple sclerosis, Newer drugs, efficacy, safety, disease burden
Introduction
There are four eras in the history of disease-modifying therapies for multiple sclerosis. In the first era of nihilism, before 1993, there was no effective treatment and many neurologists thought the disease to be untreatable. Then, in the second era of modest efficacy, interferon beta and glatiramer acetate were found to reduce the relapse rate and accumulation of disability in the short term, in people with relapsing-remitting multiple sclerosis. Over the next decade, we learnt that these drugs had a moderate impact on the disease and were very safe. For nearly all patients, they were preferable to the more efficacious drugs available then, such as cyclophosphamide or mitoxantrone, because of these drugs’ serious side effects. Then, in 2004, the era of complexity was introduced by the licensing of natalizumab. Clearly more effective than the drugs of the second era, the emergent adverse effect of progressive multifocal leukoencephalopathy soon tempered the early enthusiasm for the drug. Over the next 10 years, fingolimod, dimethyl fumarate, teriflunomide, and alemtuzumab have become licensed in many countries for the treatment of multiple sclerosis, and it is likely that daclizumab (a nondepleting antibody against CD25) and ocrelizumab (a depleting antibody against CD20, similar in effect to rituximab) will follow soon. Autologous bone marrow transplantation has also been reevaluated for the treatment of multiple sclerosis. New issues arise: What is the correct sequence of drugs to be offered to patients? How can people be safely switched from one sequence of drugs to another? For the patient and doctor, treating a relapsing-remitting disease has become more hopeful, intense, and complicated. All these drugs focus on this inflammatory phase of the disease, whereas attention in the fourth era of disease-modifying therapies will focus on slowing or halting the progressive phase of the disease.
Classifying The Newer Therapies for Multiple Sclerosis
It is increasingly important for the physician to have a system of categorizing the disease-modifying therapies of multiple sclerosis in order to organize his/her thoughts and communicate clearly to patients. The landscape of treatments is simply too complex for an unstructured list of individual drugs to be helpful. Each drug should be considered in three domains: efficacy, safety, and treatment burden (i.e., mode of administration, intensity of monitoring, and so on). A graphical attempt at such an analysis is shown in Figure 1. There is no international consensus but the Association of British Neurologists has recently divided treatments into drugs of moderate efficacy (category 1) and drugs of high efficacy (category 2).[1] In discussions with patients, these may be caricatured as “low risk, low gain” and “high risk, high gain” [Table 1].[2,3,4,5,6,7,8,9,10,11,12,13,14] Not all have accepted this classification; some have argued that fingolimod and dimethyl fumarate ought to occupy a third category, lying somewhere between 1 and 2, given their claimed greater efficacy than interferon beta and a signal that both may increase the risk of progressive multifocal leucocencephalopathy (PML) [Food and Drug Administration (FDA) reports of November 25, 2014 and August 5, 2015]. There is a further category of treatments, which might be regarded as “very high risk” and which include more than 2 years of natalizumab for John Cunningham virus (JC) virus-positive patients and autologous bone marrow transplantation. It is likely that daclizumab will be in category 1 and ocrelizumab in category 2, if licensed.
Figure 1.
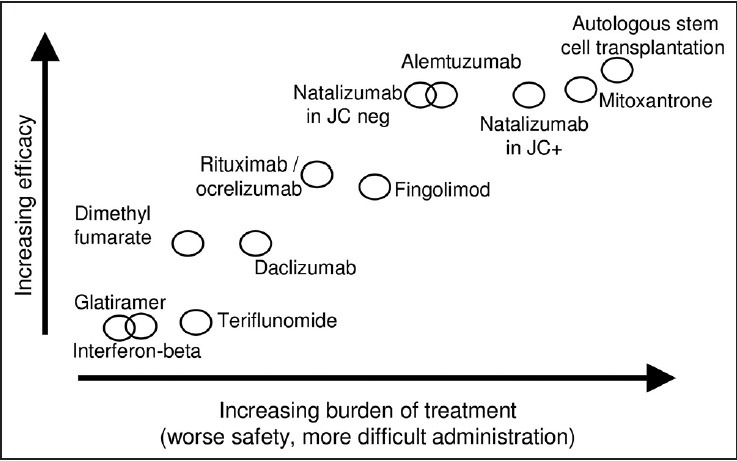
Classifying the newer therapies for multiple sclerosis according to efficacy, safety, and treatment burden
Table 1.
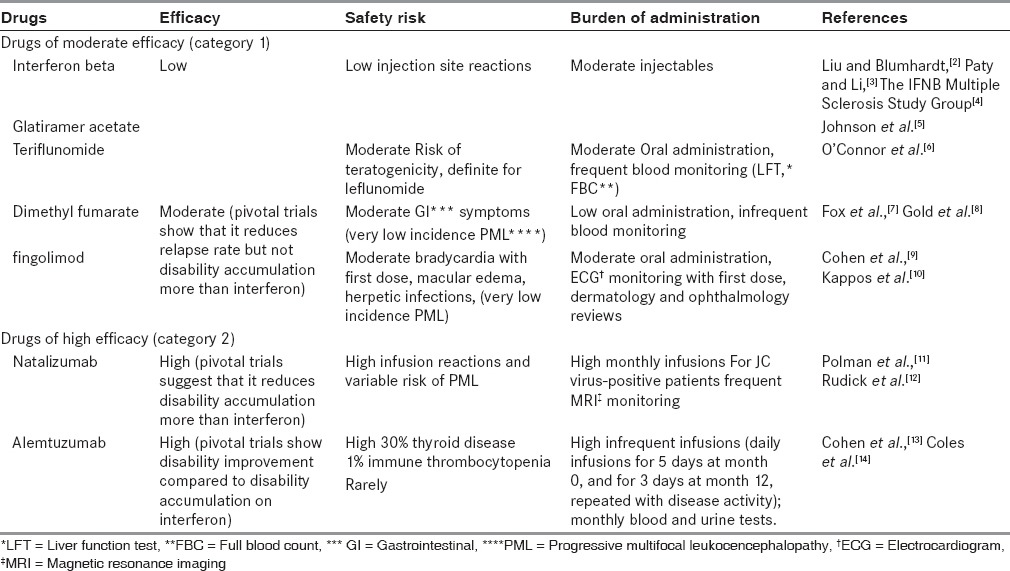
Efficacy, safety and treatment burden of MS drugs
Risk Assessment of Patients Before Starting Therapy
Someone with early relapsing-remitting multiple sclerosis is now likely to face a future of two or three decades of immunotherapy, with varying drugs of different risks. It is therefore, appropriate to assess their individual risks of immunotherapy at the outset. All patients should be assessed for latent infection with human immunodeficiency virus (HIV), treponema, hepatitis B and hepatitis C, cytomegalovirus (CMV), and tuberculosis (in endemic areas) before starting treatment. If patients are not immune to varicella, vaccination should be considered. All female patients should have recent cervical smears and annually on the more potent immunotherapies, such as alemtuzumab, to monitor for dysplastic change. A key early test should be anti-JC virus serology; where this is negative, patients may be reassured that their current risk of PML was low and natalizumab should be considered as a treatment option although this will change with treatment duration and serological status. Where patients have positive anti-JC virus serology, they can be given the soundest advice on the risks of natalizumab treatment. The incidence of PML on natalizumab is now estimated at 3.72/1,000 patients (95% CI 3.4-4.06/1,000 patients) (Biogen Idec data, Last accessed 2014 Sep 3, website http://www.biogenidec-international.com/tysabri.aspx); and this risk varies with serological index, prior use of immunotherapies and duration of natalizumab treatment Plavina et al.[15] Wherever alemtuzumab (and perhaps ocrelizumab in the future) is available, a reasonable starting position would be to offer this to JC virus-positive patients ahead of natalizumab.
More difficult is how to counsel patients with positive JC virus serology who are considering the other immunotherapies. There have now been case reports of PML in multiple sclerosis patients on fingolimod and dimethyl fumarate (as cited above), as well as people with other diseases treated using rituximab[16] and alemtuzumab[17] to date, teriflunomide has not been associated with PML but its parent compound, leflunomide.[18] Clearly, these are occurring at a very low incidence and so it is hard to quantify the risk of PML and communicate it appropriately to a patient.
Starting The Newer Therapies: Escalation and Induction
An unhelpful classification of the multiple sclerosis treatments is “first-line,” “second-line,” “third-line,” etc. Inherent in these terms is a particular treatment strategy, namely, “escalation” from the low-risk low-gain treatments to more efficacious, riskier therapies if the disease breaks through [Figure 2]. In many situations, this may be appropriate and indeed the regulated indication of some drugs, such as fingolimod in England, may allow no other approach. The advantage of this strategy is that only patients with a very active disease are exposed to the most risky drugs. The disadvantage is that they have almost certainly accumulated disability on the way, which cannot be recovered.
Figure 2.
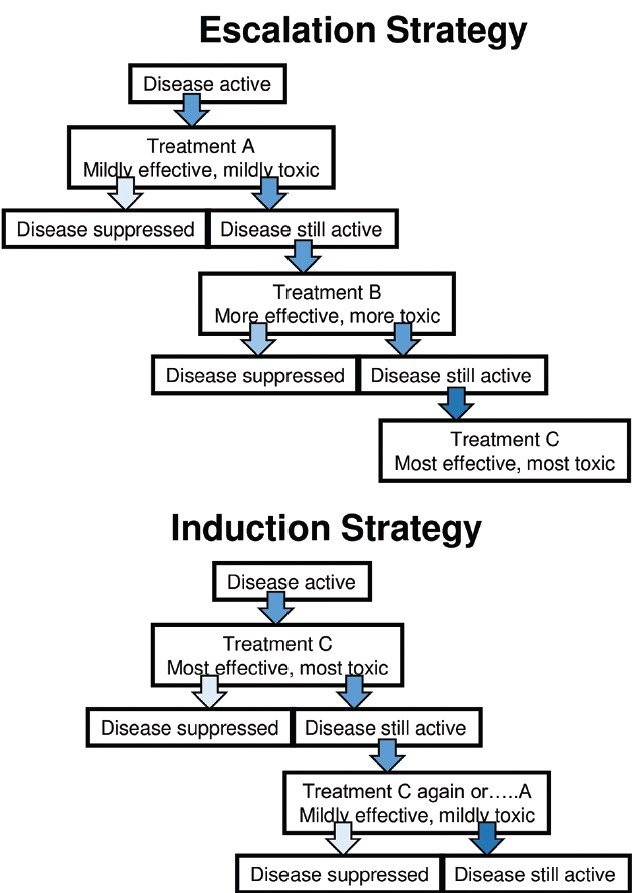
Escalation and induction strategy
However, it may be reasonable to start treatment with a more potent treatment, which is permitted under the indications for alemtuzumab and natalizumab (and fingolimod in Scotland and other jurisdictions). This approach is called “induction” therapy [Figure 2]. The straightforward rationale for this is aggressive disease activity such as defined by the “rapidly evolving severe” indication for natalizumab is as follows: two disabling relapses over 1 year, with gadolinium enhancement on a magnetic resonance imaging (MRI) of the brain.
However, there is also a trend toward induction therapy of patients who do not have aggressive disease. The alemtuzumab trials were focused on people with early disease (within 3-10 years from onset) low disability (EDSS < 3.0) and moderate relapse rate (at least two relapses in 2 years) (Cohen et al.,[13] Coles et al.,[14] Coles et al.)[19] The positive results of these trials, including seeing disability improvement, led to the liberal European license of “radiological or clinical evidence of active disease.”
Patient-related factors may suggest one treatment over another. Those with a greater tolerance of risk may opt for more potent therapies; those with a needle phobia may prefer tablets; and those contemplating pregnancy may be attracted to alemtuzumab, which allows safe conception 4 months after the last infusion, with continued suppression of multiple sclerosis disease activity throughout the pregnancy and beyond.
Sequencing The Newer Therapies
There are a few concerns when escalating from interferon beta or glatiramer to more potent agents. If there is any evidence of bone marrow suppression, such as leukopenia, most investigators will “wash out” these agents for 1 month or so. For people on fingolimod who wish to escalate to depleting therapies such as alemtuzumab, it is important to wait until the patient's total lymphocyte count returns to normal before administering alemtuzumab. If one administers alemtuzumab earlier, when the lymphocytes remain trapped in the lymph nodes, lymphocyte depletion may be suboptimal and alemtuzumab's efficacy may be compromised based on a single case. This usually takes 1 month but it may take longer. The most challenging switching relates to JC virus-positive patients on natalizumab who wish to move to other therapies such as alemtuzumab. The tension is between starting an alternative treatment early in order to minimize the risk of disease rebound or breakthrough and starting it late in order to ensure that the patient does not have incipient PML, which will be exacerbated by the novel therapy. Notably, there is one case report, surprisingly, where rituximab did not exacerbate established PML.[20] Patients may be kept off all treatment for an interval (3-6 months has been suggested by UK and German authorities respectively) or put on “bridging treatment” with monthly intravenous immunoglobulin or corticosteroids for some months. During this time, patients may need further estimates of JC virus serology or cerebrospinal fluid (CSF) JC virus DNA and further imaging. None of these strategies have been formally tested or compared.
Disease Monitoring
It is important to early identify a breakthrough of disease on therapy in order to consider switching to a more potent drug (if adopting an escalation strategy) or consider a further cycle of the same drug, as in the case of alemtuzumab after the first two cycles. All patients should be encouraged to report possible relapses in time for a competent assessment to distinguish these from pseudo-relapses. An acute access clinic is of great assistance here. The place of routine MRI monitoring is controversial although intuitively it would seem that the accumulation of new lesions on a scan, even if clinically silent, marks a poor prognosis. Evidence suggests that one or more gadolinium-enhancing MRI lesions in the first year of interferon beta treatment predicts higher chance of more than two relapses over 5 years but does not predict a higher chance of accumulating disability at 2 years.[21] MRI scans beyond the first year of treatment are less predictive. The Barcelona's group “Rio score” helpfully combines MRI and clinical markers for prognosticating although it is undermined by the fact that disability accumulation (which may mark the onset of the progressive phase) is taken as a marker of disease activity.[22]
From such data, the controversial idea has emerged that we should treat multiple sclerosis with the aim of establishing “no evidence of disease activity (NEDA)”; there is no evidence of any clinical and radiological disease activity.[23,24] Importantly, it is yet to be tested whether escalating therapy on the basis of achieving NEDA actually improves long-term outcome irrespective of how attractive the concept is.
Safety Monitoring
None of the newer agents is as safe as interferon beta or glatiramer acetate. So, neurologists now have to accept a greater need to monitor their patients for potentially serious adverse effects. An important component of this, aside from the specific requirements of individual agents, is effective communication between the physician and patient, perhaps facilitated by a multiple sclerosis nurse or other professional. For the more intensive drugs, monitoring may be more easily achieved in larger centers, with good administrative and nursing support but this inevitably leads to greater travel for patients. This has led to a debate about whether there should be a hierarchy of health care agencies providing multiple sclerosis care. Individual drug monitoring requirements are given in Table 1.
Neuroprotective and Remyelinating Therapies
There are no licensed therapies to protect neurons or promote remyelination in multiple sclerosis. But there is encouraging recent data to suggest that repurposed licensed therapies may be useful, for instance, amiloride[25,26] and phenytoin (Kapoor, AAN 2015) to protect neurons and bexarotene to promote remyelination.[27] These need to be tested more rigorously before they may be used routinely.
Conclusion
Multiple sclerosis is no longer an untreatable disease. Early in its course, before disability has been acquired, active treatment of the relapsing-remitting phase can yield long-lasting benefits. The newer therapies provide greater choice for the physician and patient, offering drugs of greater efficacy or greater tolerability than the treatments of the 1990s but also greater toxicity. Monitoring for adverse effects and breakthrough diseases are now an important part of caring for people with multiple sclerosis. Different strategies are emerging for the sequencing and timing of treatments, with little evidence to support one approach or other approaches. At present, there are no treatments to protect neurons or promote remyelination but these are realistic prospects within the next two decades.
Financial support and sponsorship
Nil.
Conflicts of interest
Alasdair Coles has received travel refunds and honoraria for speaking at events organised by Genzyme (a Sanofi company) and his department has also received research grants from Genzyme.
References
- 1.Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol. 2015;15:273–9. doi: 10.1136/practneurol-2015-001139. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Blumhardt LD. Randomised, double blind, placebo controlled study of interferon beta-1a in relapsing-remitting multiple sclerosis analysed by area under disability/time curves. J Neurol Neurosurg Psychiatry. 1999;67:451–6. doi: 10.1136/jnnp.67.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paty DW, Li DK. Interferon beta-1b is effective in relapsingremitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:662–7. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 4.Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43:655–61. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–76. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, et al. ; TEMSO Trial Group. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–303. [Google Scholar]
- 7.Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–97. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 8.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. DEFINE Study Investigators. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 10.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 11.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 12.Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. SENTINEL Investigators. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–23. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. CARE-MS I Investigators. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet. 2012;380:1819–28. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 14.Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. CARE-MS II Investigators. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet. 2012;380:1829–39. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 15.Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802–12. doi: 10.1002/ana.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaheer F, Berger JR. Treatment-related progressive multifocal leukoencephalopathy: Current understanding and future steps. Ther Adv Drug Saf. 2012;3:227–39. doi: 10.1177/2042098612453849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isidoro L, Pires P, Rito L, Cordeiro G. Progressive multifocal leukoencephalopathy in a patient with chronic lymphocytic leukaemia treated with alemtuzumab. BMJ Case Rep. 2014;8:2014. doi: 10.1136/bcr-2013-201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmedt N, Andersohn F, Garbe E. Signals of progressive multifocal leukoencephalopathy for immunosuppressants: A disproportionality analysis of spontaneous reports within the US Adverse Event Reporting System (AERS) Pharmacoepidemiol Drug Saf. 2012;21:1216–20. doi: 10.1002/pds.3320. [DOI] [PubMed] [Google Scholar]
- 19.Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, et al. CAMMS223 Trial Investigators. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 20.Asztely F, Gilland E, Wattjes MP, Lycke J. Rituximab treatment did not aggravate ongoing progressive multifocal leukoencephalopathy in a patient with multiple sclerosis. J Neurol Sci. 2015;353:155–7. doi: 10.1016/j.jns.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Dobson R, Rudick RA, Turner B, Schmierer K, Giovannoni G. Assessing treatment response to interferon- β: Is there a role for MRI? Neurology. 2014;82:248–54. doi: 10.1212/WNL.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tintore M, Rovira À, Río J, Otero-Romero S, Arrambide G, Tur C, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain. 2015;138:1863–74. doi: 10.1093/brain/awv105. [DOI] [PubMed] [Google Scholar]
- 23.Dadalti Fragoso Y. Why some of us do not like the expression “no evidence of disease activity” (NEDA) in multiple sclerosis. Mult Scler Relat Disord. 2015;4:383–4. doi: 10.1016/j.msard.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015;4:329–33. doi: 10.1016/j.msard.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Arun T, Tomassini V, Sbardella E, de Ruiter MB, Matthews L, Leite MI, et al. Targeting ASIC1 in primary progressive multiple sclerosis: Evidence of neuroprotection with amiloride. Brain. 2013;136:106–15. doi: 10.1093/brain/aws325. [DOI] [PubMed] [Google Scholar]
- 26.Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, et al. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134:571–84. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- 27.Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]


