Abstract
Background
The Centers for Disease Control and Prevention monitors vibriosis through 2 surveillance systems: the nationwide Cholera and Other Vibrio Illness Surveillance (COVIS) system and the 10-state Foodborne Diseases Active Surveillance Network (FoodNet). COVIS conducts passive surveillance and FoodNet conducts active surveillance for laboratory-confirmed Vibrio infections.
Methods
We summarized Vibrio infections (excluding toxigenic V. cholerae O1 and O139) reported to COVIS and FoodNet from 1996 through 2010. For each system, we calculated incidence rates using US Census Bureau population estimates for the surveillance area.
Results
From 1996 to 2010, 7700 cases of vibriosis were reported to COVIS and 1519 to FoodNet. Annual incidence of reported vibriosis per 100 000 population increased from 1996 to 2010 in both systems, from 0.09 to 0.28 in COVIS and from 0.15 to 0.42 in FoodNet. The 3 commonly reported Vibrio species were V. parahaemolyticus, V. vulnificus, and V. alginolyticus; both surveillance systems showed that the incidence of each increased. In both systems, most hospitalizations and deaths were caused by V. vulnificus infection, and most patients were white men. The number of cases peaked in the summer months.
Conclusions
Surveillance data from both COVIS and FoodNet indicate that the incidence of vibriosis increased from 1996 to 2010 overall and for each of the 3 most commonly reported species. Epidemiologic patterns were similar in both systems. Current prevention efforts have failed to prevent increasing rates of vibriosis; more effective efforts will be needed to decrease rates.
Vibrios are gram-negative, rod-shaped bacteria that occur naturally in estuarine or marine environments. Roughly a dozen species are known to cause disease in humans [1], and infection is usually from exposure to seawater or consumption of raw or undercooked seafood [2, 3]. Vibriosis is characterized by diarrhea, primary septicemia, wound infections, or other extra-intestinal infections [2–6]. Cholera has been reportable in the United States for more than a century; however, vibriosis did not become nationally notifiable until 2007. Laboratory criteria for diagnosis include isolation of a species of the family Vibrionaceae (other than toxigenic Vibrio cholerae O1 or O139, which is reportable as cholera) from a clinical specimen.
Vibrio infection results in an estimated 80 000 illnesses, 500 hospitalizations, and 100 deaths each year in the United States [7]. Two national surveillance systems monitor cases: the national Cholera and Other Vibrio Illness Surveillance (COVIS) system and the Foodborne Diseases Active Surveillance Network (FoodNet). COVIS is a passive surveillance system to which all states can report laboratory-confirmed Vibrio infections. FoodNet conducts active, population-based surveillance in 10 states for all laboratory-confirmed Vibrio infections, as well as other enteric infections transmitted commonly through food.
We reviewed all cases of vibriosis reported to the Centers for Disease Control and Prevention (CDC) through COVIS and FoodNet from 1996 to 2010 to compare patterns in reports to the 2 surveillance systems.
METHODS
Surveillance
COVIS (http://www.cdc.gov/nationalsurveillance/cholera_vibrio_surveillance.html) was established in 1988 by the CDC; the Gulf Coast states of Alabama, Florida, Louisiana, and Texas (states with high incidences of vibriosis); and the US Food and Drug Administration (FDA) to conduct surveillance of illnesses caused by Vibrio species (Table 1). Other states began reporting to COVIS; by the late 1990s, about half of all states were reporting each year. Other reporting jurisdictions, such as Guam, Puerto Rico, and the District of Columbia, also report to COVIS and are counted as states for this analysis. Vibriosis became nationally notifiable in 2007 and was notifiable at the state level in all but 5 states by 2010; however, 3 of these 5 states reported at least 1 case to COVIS in 2010. State and local health officials submit COVIS report forms for laboratory-confirmed cases of human infection. The report form captures demographic and isolate information, as well as clinical and exposure-related information. Isolate information includes Vibrio species and the source of the specimen from which Vibrio was isolated. Although it occurs rarely, >1 isolate can be reported from a single patient. Clinical information includes hospitalization and death.
Table 1.
Overview of COVIS and FoodNet
| COVIS | FoodNet | |
|---|---|---|
|
|
||
| Year Started | 1988 | 1996 |
|
| ||
| Type of Surveillance | Passive | Active |
| Reporting states | 1988: Gulf Coast states only (Alabama, Florida, Louisiana, Mississippi, Texas) |
California, Connecticut, Georgia, Minnesota, Oregon, then added Colorado (2001), Maryland (1998), New Mexico (2004), New York (1998), Tennessee (2000) |
| 1988–2006: Gulf Coast states and voluntary reporting from other states |
||
| 2007: Nationally notifiable and all 50 states reporting | ||
| Variables captured | ||
| Demographic | Age, sex, race, ethnicity, occupation | Age, sex, race, ethnicity |
| Isolate | Species, isolates (all), and specimen source | Species, isolate (most invasive only), and specimen source |
| Clinical | Symptoms, hospitalization, sequelae, death, antibiotic treatment, preexisting conditions, and medications |
Hospitalization, patient outcome |
| Epidemiologic | Travel, seafood consumption, and recreational water exposure |
Travel history, outbreak status |
| Seafood investigation |
Seafood trace back | Not applicable |
Abbreviations: COVIS, Cholera and Other Vibrio Illness Surveillance; FoodNet, Foodborne Diseases Active Surveillance Network.
FoodNet (http://www.cdc.gov/foodnet/) is a collaborative project that includes the CDC, 10 participating state health departments, the US Department of Agriculture’s Food Safety and Inspection Service, and the FDA. Since 1996, FoodNet has conducted active surveillance for cases of laboratory-confirmed foodborne infections transmitted commonly through food (Campylobacter, Listeria, Salmonella, Shiga toxin–producing Escherichia coli [STEC] O157 and non-O157, Shigella, Vibrio, Yersinia, Cryptosporidium, and Cyclospora) by regularly contacting all clinical laboratories serving the surveillance area to ensure that all cases are reported. FoodNet initially included 2 states—Minnesota and Oregon—and selected counties in California, Connecticut, and Georgia. The FoodNet surveillance area has expanded and in 2010 included 7 states—Connecticut, Georgia, Maryland, Minnesota, New Mexico, Oregon, and Tennessee—and selected counties in California, Colorado, and New York. FoodNet sites also report Vibrio infections to COVIS. The FoodNet surveillance area includes 46.9 million people, or 15.3% of the US population. If a given pathogen is isolated from >1 specimen from a patient, only the most invasive isolate is reported. Hospitalizations occurring within 7 days of specimen collection date are recorded, as is the patient’s vital status at hospital discharge or at 7 days after the specimen collection date, if not hospitalized. Deaths and hospitalizations meeting these criteria are attributed to the infection.
Analysis
We examined Vibrio infections (excluding toxigenic V. cholerae O1 and O139) reported to COVIS and FoodNet from 1996 through 2010. We compared patient demographics (age, sex, race, and ethnicity) and clinical information (species, specimen source, hospitalization, and death). We categorized ages into 10-year age groups, with the oldest group consisting of persons aged ≥80 years. We defined the month of infection as the month of illness onset or, if onset date was not available, the specimen collection date. For each system, we calculated incidence rates by dividing the annual number of laboratory-confirmed infections by US Census Bureau population estimates for the surveillance area. For COVIS, we considered the surveillance area to include all states that reported at least 1 case in a given year. To assess changes in incidence, we compared the average annual incidence for 1996–2000 with 2006–2010 in each system. Based on COVIS data for 1996–2010 and for each state including only years in which at least 1 case was reported, we classified states as higher (≥0.30 cases per 100 000) or lower (<0.30) incidence. Analyses were conducted using SAS version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
From 1996 through 2010, 7700 cases of vibriosis were reported through COVIS and 1519 through FoodNet; since 1996, an average of 19% of COVIS cases have also been reported to FoodNet. Seven states, all coastal (Connecticut, Delaware, Maryland, Florida, Washington, Louisiana, and Hawaii), were categorized as higher incidence. Reported incidence varied greatly between states; for example, in 2010, state-specific incidence ranged >50-fold, from 0.03 per 100 000 population in Oklahoma to 1.7 per 100 000 population in Hawaii.
Demographic characteristics of patients reported to the 2 systems were similar. In both, 68% of illnesses were in men, the age group with the largest percentage of cases was 40–49 years (19% in COVIS, 21% in FoodNet), and most patients with reported race were white (74% in COVIS, 64% in FoodNet). Of patients with reported ethnicity, 18% were Hispanic in COVIS and 6% in FoodNet. Data on race and ethnicity were missing less often in COVIS than in FoodNet (race: 8% missing in COVIS, 19% in FoodNet; ethnicity: 14% missing in COVIS, 28% in FoodNet). In both systems, cases peaked in the summer months, with the highest proportion in July (20% COVIS, 21% FoodNet) and August (20% COVIS, 26% FoodNet). However, the summer peak occurred slightly earlier in COVIS than in FoodNet.
The 3 most commonly reported Vibrio species causing infection in both COVIS and FoodNet were V. parahaemolyticus, V. vulnificus, and V. alginolyticus (Table 2), which accounted for 75% of reports to both systems. Vibrio parahaemolyticus infection was reported most commonly but was rarely fatal, with a case-fatality ratio (CFR) <1% in both systems. Vibrio vulnificus infection, by contrast, had the highest CFR, >30% in both systems, with >80% of patients hospitalized. Hospitalization and death rates for infection with V. alginolyticus were similar to those of V. parahaemolyticus. Because V. vulnificus infection accounted for a higher proportion of COVIS reports than FoodNet reports, the overall proportion hospitalized in COVIS (41% of 2925 patients) was higher than in FoodNet (28% of 432) and the overall CFR was 8.2% in COVIS and 4.7% in FoodNet, although species-specific hospitalization rates and CFRs were similar.
Table 2.
Number of Vibriosis Infections and Selected Outcomes, by Species, Reported to COVIS and FoodNet, 1996–2010
| COVIS |
FoodNet |
|||||
|---|---|---|---|---|---|---|
| Vibrio Species | Infections, No. (%) |
Hospitalized, No. (%) |
Died, No. (%) |
Infections, No. (%) |
Hospitalized, No. (%) |
Died, No. (%) |
| V. parahaemolyticus | 3460 (44.9) | 714 (20.6) | 24 (0.7) | 820 (54.0) | 131 (16.0) | 4 (0.5) |
| V. vulnificus | 1446 (18.8) | 1250 (86.4) | 462 (31.9) | 193 (12.7) | 157 (81.3) | 58 (30.1) |
| V. alginolyticus | 884 (11.5) | 168 (19.0) | 11 (1.2) | 132 (8.7) | 17 (12.9) | 1 (0.8) |
| V. cholerae non-O1, non-O139 | 697 (9.1) | 278 (39.9) | 35 (5.0) | 78 (5.1) | 23 (29.5) | 2 (2.6) |
| V. fluvialis | 394 (5.1) | 156 (34.2) | 11 (2.8) | 104 (6.2) | 41 (39.4) | 1 (1.0) |
| V. mimicus | 173 (2.3) | 73 (42.2) | 3 (1.7) | 30 (2.0) | 14 (46.7) | 2 (6.7) |
|
Grimontia hollisae (formerly known as V. hollisae) |
121 (1.6) | 67 (53.1) | 1 (0.8) | 18 (1.2) | 10 (55.6) | 0 (0) |
| V. cholerae O1 | 40 (0.5) | 22 (55.0) | 2 (0.5) | 10 (0.7) | 4 (40.0) | 0 (0) |
| Vibrio species not identified | 284 (3.7) | 97 (34.2) | 9 (3.2) | 90 (5.9) | 19 (21.1) | 2 (2.2) |
| Multiple | 98 (1.3) | 52 (53.1) | 8 (8.2) | … | … | … |
| Othera | 103 (1.3) | 48 (46.6) | 4 (3.9) | 44 (2.9) | 16 (36.4) | 2 (0.2) |
| Total | 7700 | 2925 (40.5) | 570 (8.2) | 1519 | 432 (28.4) | 72 (4.7) |
Abbreviations: COVIS, Cholera and Other Vibrio Illness Surveillance; FoodNet, Foodborne Diseases Active Surveillance Network.
Includes Photobacterium damselae subsp damselae (formerly known as V. damsela), V. furnissii, V. metschnikovii, V. cincinnatiensis, V. cholerae O139, V. cholerae non-O1, V. cholerae unspecified, and V. harveyi.
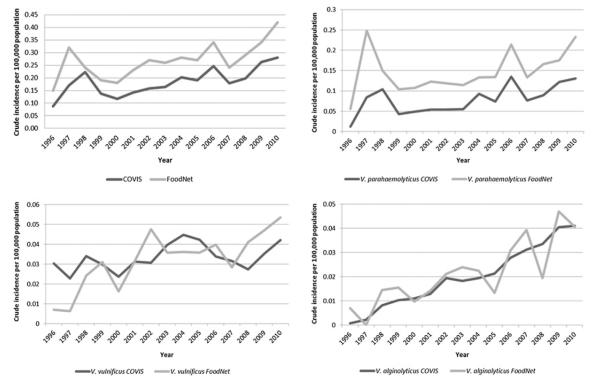
The annual incidence of vibriosis per 100 000 population increased from 1996 to 2010 (Figure 1). In COVIS, it increased from 0.09 to 0.28, peaking in 2010 at 0.28. In FoodNet, it increased from 0.15 to 0.42, peaking in 2006 at 0.42. The incidence of V. parahaemolyticus infection increased from 1996 to 2010, in COVIS from 0.01 to 0.13 and in FoodNet from 0.06 to 0.23. The incidence of V. vulnificus infection increased slightly from 1996 to 2010, in COVIS from 0.03 to 0.04, but more in FoodNet, from 0.01 to 0.05 (Figure 1). The incidence of V. alginolyticus infection increased from 1996 to 2010, in COVIS from 0.001 to 0.04 and in FoodNet from 0.01 to 0.04. These patterns were consistent when the COVIS analysis was limited to states not in FoodNet, the contiguous United States (ie, excluding Hawaii, Alaska, and Guam), and states in which Vibrio infection is notifiable at the state level (data not shown).
Figure 1.
Crude vibriosis incidence per 100 000 population, Cholera and Other Vibrio Illness Surveillance system and Foodborne Diseases Active Surveillance Network, 1996–2010. Abbreviations: COVIS, Cholera and Other Vibrio Illness Surveillance; FoodNet, Foodborne Diseases Active Surveillance Network.
DISCUSSION
Our analysis of surveillance data from the 2 US national systems that monitor Vibrio infection indicate that the incidence of vibriosis increased during the 15 years from 1996 through 2010. This increase has been driven primarily by increases in V. parahaemolyticus, the species most commonly reported, but is also seen for V. vulnificus and V. alginolyticus, the second and third most commonly reported species. Increases of V. vulnificus are particularly concerning, given the high mortality rate associated with this pathogen. The causes of this increase are not known, but warming of coastal waters, which contributes to growth and persistence of Vibrio, has been posited as a factor that could contribute to increases in human illness [8]. If Vibrio contamination rates did not change, increased exposure to seafood or seawater could lead to increased risk of exposure to Vibrio, but we are not aware of evidence for such changes. Changes in surveillance for Vibrio infection could also affect reported rates, although, as discussed below, they are unlikely to account entirely for the observed increases.
Before vibriosis became nationally notifiable in 2007, FoodNet provided the most complete picture of vibriosis in the United States. However, FoodNet includes none of the Gulf Coast states that were founders of COVIS and, given the wide variation in rates between states, might not provide an accurate view of the nation as a whole. Also, FoodNet surveillance for isolates from specimen sources other than stool may have been incomplete in the early years of the program (P. M. Griffin, CDC, oral communication, December 2011), which may explain, at least in part, the larger increase in reported V. vulnificus incidence in FoodNet when compared with COVIS.
COVIS, on the other hand, expanded substantially over the study period and 96% of states have reported since 2007. Most of the few states where vibriosis reporting is not mandated have reported cases to COVIS. COVIS data show that all of the higher-incidence states are in coastal areas where consumption of shellfish and exposure to seawater would be expected to be most common. Because COVIS is a passive system, it is not known whether states not reporting cases each year actually had no cases. However, in recent years, this has applied to only a few states and would not likely change overall patterns of incidence.
Thus, the completeness of FoodNet data for the sites under active surveillance gives credence to trends seen in COVIS, and the national coverage of COVIS validates the representativeness of FoodNet. Taken as a whole, data from the 2 systems credibly demonstrates increasing incidence of vibriosis. The 2 systems also show similarities in demographic and seasonal patterns of laboratory-confirmed Vibrio infections in the United States, extending observation of previous reports [3, 6, 9, 10] to more recent years.
To facilitate public health action to prevent and control vibriosis, COVIS collects information about seafood and seawater exposures of patients. In an era of increasing incidence, this information can help to improve education and control measures. Raw shellfish, especially oysters, are the most common foodborne source of vibriosis [2, 6, 11]. Our data indicate that efforts to control vibriosis by educating the public about the hazards of raw oyster and other shellfish consumption have not been effective [12, 13] and that measures to decrease contamination of oysters, such as postharvest decontamination by freezing, heat treatment, or high hydrostatic pressure [14], may need to be implemented routinely to decrease rates of illness. It will also be important to understand the complex and dynamic factors affecting Vibrio persistence and growth in marine and estuarine environments in order to develop targeted strategies for decreasing Vibrio exposure through other routes. For example, potential strategies could include educational outreach to persons at higher risk for severe Vibrio disease, such as those with liver disease, and to avoid exposure of skin wounds to seawater.
Acknowledgments
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC; Cooperative Agreement U60/CD303019). FoodNet is funded by the Food Safety Office and the Emerging Infections Program of the CDC, the US Department of Agriculture Food Safety and Inspection Service, and the US Food and Drug Administration.
Supplement sponsorship. This article was published as part of a supplement entitled “Studies From the Foodborne Diseases Active Surveillance Network,” sponsored by the Division of Foodborne, Waterborne, and Environmental Diseases of the National Center for Emerging and Zoonotic Infectious Diseases from the Centers for Disease Control and Prevention, and the Association of Public Health Laboratories.
Notes
Disclaimer. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Austin B. Vibrios as causal agents of zoonoses. Vet Microbiol. 2009;140:310–7. doi: 10.1016/j.vetmic.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, Bishop RD, Baldy LM, et al. Vibrio gastroenteritis in the US Gulf of Mexico region: the role of raw oysters. Epidemiol Infect. 2000;124:489–95. doi: 10.1017/s0950268899003714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dechet AM, Yu PA, Koram N, Painter J. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997–2006. Clin Infect Dis. 2008;46:970–6. doi: 10.1086/529148. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics . Other vibrio infections. In: Pickering LK, Baker C, Kimberlin DW, Long SS, editors. Red book: 2009 report of the committee on infectious diseases. 28th ed American Academy of Pediatrics; Elk Grove Village, IL: 2009. pp. 729–30. [Google Scholar]
- 5.Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2010;15:e157–66. doi: 10.1016/j.ijid.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Daniels NA, MacKinnon L, Bishop R, et al. Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis. 2000;181:1661–6. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 7.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vezzulli L, Brettar I, Pezzati E, et al. Long-term effects of ocean warming on the prokaryotic community: evidence from the vibrios. ISME J. 2011;6:21–30. doi: 10.1038/ismej.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Cholera and other vibrio illness surveillance (COVIS), summary data, 2008. US Department of Health and Human Services, CDC; Atlanta, GA: 2011. [Google Scholar]
- 10.Daniels NA, Shafaie A. Review of pathogenic Vibrio infections for clinicians. Infect Med. 2000;17:665–85. [Google Scholar]
- 11.Daniels NA. Vibrio vulnificus oysters: pearls and perils. Clin Infect Dis. 2011;52:788–92. doi: 10.1093/cid/ciq251. [DOI] [PubMed] [Google Scholar]
- 12.Matyas B, Cronquist A, Cartter M, et al. Preliminary FoodNet data on the incidence of infections with pathogens transmitted commonly through food—10 states, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:16. [PubMed] [Google Scholar]
- 13.Ralston EP, Kite-Powell H, Beet A. An estimate of the cost of acute health effects from food- and water-borne marine pathogens and toxins in the USA. J Water Health. 2011;9:680–94. doi: 10.2166/wh.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Depaola A, Jones JL, Noe KE, Byars RH, Bowers JC. Survey of postharvest-processed oysters in the United States for levels of Vibrio vulnificus and Vibrio parahaemolyticus. J Food Prot. 2009;72:2110–3. doi: 10.4315/0362-028x-72.10.2110. [DOI] [PubMed] [Google Scholar]