Abstract
Purpose
We studied the implications of corneal endothelial dysfunctions on oxidative stress in the anterior segment via in vivo measurements of oxygen partial pressure (pO2) in the anterior chamber (AC) of human eyes.
Methods
We recruited 51 patients undergoing cataract surgery and/or endothelial keratoplasty (EK). Endothelial cell density (ECD; n = 33) and central corneal thickness (CCT; n = 41) were measured on patients with relatively clear corneas. Before surgery, an oxygen sensor was introduced into the AC via a peripheral corneal paracentesis. In all patients, seven measurements of pO2 were obtained by positioning the flexible tip near the endothelium at the central cornea, at four cardinal subendothelial locations near the midperipheral cornea, and in the mid-AC and AC angle. In patients with pseudophakia or eyes undergoing cataract surgery, pO2 also was measured near the lens surface and in the posterior chamber.
Results
Consistent with our previous reports, a steep oxygen gradient was noted in the anterior segment of normal controls (n = 24). In patients with endothelial dysfunctions (n = 27), there was a significant increase of pO2 at all five subendothelial locations without a significant increase of pO2 in the AC angle. By regression analyses, subendothelial pO2 correlated inversely with ECD and positively with CCT in patients with endothelial dysfunctions.
Conclusions
This study demonstrates an even steeper intraocular oxygen gradient in eyes with corneal endothelial dysfunctions. It suggests that the reduced oxygen consumption in corneal endothelial cells may increase oxidative stress in the AC and the existence of an alternative aqueous inflow pathway that maintains a relatively low and constant pO2 at the AC angle.
Keywords: corneal edema, corneal endothelial cells, oxidative damage, aqueous flow
There is a steep intraocular oxygen gradient in eyes with corneal endothelial dysfunctions and the reduced oxygen consumption in corneal endothelial cells may increase oxidative stress in the anterior chamber.
Cataract and glaucoma are two of the most common ocular morbidities. Previous studies by our group and others have suggested that exposure of the crystalline lens to excessive oxygen may have a critical role in the pathogenesis of age-related nuclear cataracts.1–9 Specifically, the oxygen level is relatively low in the vitreous gel and nuclear cataracts are prone to develop after vitrectomy by increasing lens exposure to oxygen from the posterior segment altering the normally hypoxic environment around the lens. Furthermore, Chang10 reported an increased risk of open angle glaucoma developing after vitrectomy and subsequent cataract surgery. The presence of the natural lens at the time of vitrectomy was associated with a 28-month delay in the onset of glaucoma.10,11 We hypothesized that exposure of the anterior segment to oxygen may increase the risk of glaucoma.
A large body of evidence indicates that increased oxidative stress or the accumulation of oxidative damage contributes to the pathogenesis of open angle glaucoma.12–22 Recent studies by our group on oxygen distribution in the human eyes clearly demonstrated that the oxygen level was significantly elevated in aqueous humor near the trabecular meshwork (TM) after combined vitrectomy and cataract surgery. Therefore, it further supports the notion that the elevated oxygen near the TM may contribute to the development of open-angle glaucoma.19–22
Our most recent study also has revealed a steep oxygen gradient in the anterior segment of human eyes with an inverse correlation between central corneal thickness (CCT) and oxygen partial pressure (pO2) exclusively in the anterior chamber (AC) angle.22 As the corneal endothelial pumps require active oxygen consumption, we speculated that corneal endothelial dysfunctions could impact the oxygen tension in the anterior segment of human eyes. This study further explored the oxygen consumption by corneal endothelium and its implications for oxidative stress in the anterior segment. Measurements of pO2 were performed in the anterior segment of patients undergoing either cataract surgery (normal control) or Descemet's stripping automated endothelial keratoplasty (DSAEK) due to Fuchs' endothelial corneal dystrophy (FECD) or pseudophakic bullous keratopathy (PBK).
Methods
Study Design
A cross-sectional study was designed to compare the oxygen distribution in different intraocular locations in three groups of patients. Patients scheduled to undergo routine phacoemulsification cataract surgery and intraocular lens implantation were designated as normal controls. Patients with FECD or PBK were scheduled to undergo DSAEK alone or combined with cataract surgery. The study protocol was approved by the Human Resource Protection Office and the Institutional Review Board of the Washington University School of Medicine in compliance with HIPAA guidelines and the tenets of the Declaration of Helsinki. Before surgery, informed consent was obtained from the subjects after an explanation of the nature and possible consequences of the study.
Patients and pO2 Measurements
We recruited 51 patients undergoing cataract surgery and/or DSAEK by AJWH for this study. Patients were excluded from the study if they had a history of prior ocular surgery other than cataract surgery, ischemic ocular disease, AC angle closure, inflammatory disease, ocular neoplasia, or monocular status. General medical and ophthalmic histories were obtained as well as a comprehensive ophthalmic examination. Preoperatively, semiautomated estimate of endothelial cell density (ECD) and CCT were measured on patients with relatively clear cornea using confocal specular microscopy (n = 33; Confoscan 4; Nidek, Freemont, CA, USA) and Pentacam HR (n = 41; Oculus, Lynnwood, WA, USA). Race was self-reported by a standardized questionnaire used in our previous studies.19–22
As per our routine protocol for intraocular surgeries, the patient was placed in the supine position and a peribulbar injection of 4 mL of 2% lidocaine and 0.375% bupivacaine (50:50) solution was performed to provide local anesthesia under intravenous sedation. Supplemental oxygen was provided by nasal cannula. After the surgical eye was prepped and draped, a lid speculum was placed. The surgical field was completely separated from the cannula using an adhesive surgical drape to avoid any additional oxygen exposure to the eye. Blood oxygen saturation (SaO2) was continuously monitored by pulse oximetry and maintained between 95% and 100%.
Before the planned surgical procedure, a peripheral corneal paracentesis was performed with a 30-gauge needle near the limbus for entry into the AC. A flexible Oxylab pO2 optical oxygen sensor (Optode; Oxford Optronix, Oxford, UK) was introduced carefully into the AC without leakage of aqueous humor. Instrument calibration was checked against solutions equilibrated to 0% and 5% oxygen before and following each set of measurements. On the basis of previous studies,19–22 we set the temperature compensation to a constant 32°C to minimize any variation of the measurements.
The tip of the flexible fiberoptic probe was positioned in the AC for pO2 measurements in all patients by the surgeon: (1) in the anterior AC subendothelially at the central cornea and four cardinal locations in the midperiphery, (2) in the mid-AC, and (3) in the AC angle. In patients with pseudophakia or undergoing cataract extraction (control eyes or phakic FECD undergoing combined DSAEK and cataract surgery), 2 additional measurements were taken (4) at the anterior surface of crystalline lens or intraocular lens and (5) in the posterior chamber just behind the iris. In patients with PBK undergoing DSAEK, pO2 was measured under the pseudophakic condition at the anterior surface of the IOL. Special care was taken to avoid damage to the lens in patients who were to remain phakic after the operative procedure. Patients were monitored postoperatively for any complications.
Statistical Analysis
Results are expressed as mean values ± SD. Statistical analyses were performed using SPSS software Version 17.0 (SPSS, Inc., Chicago, IL, USA). Student's t-test or ANOVA with multiple comparisons was performed to compare each variable among the groups. Multivariate regression analyses were performed with adjustment for all potential confounding variables measured. Probability values less than 0.05 were considered statistically significant.
Results
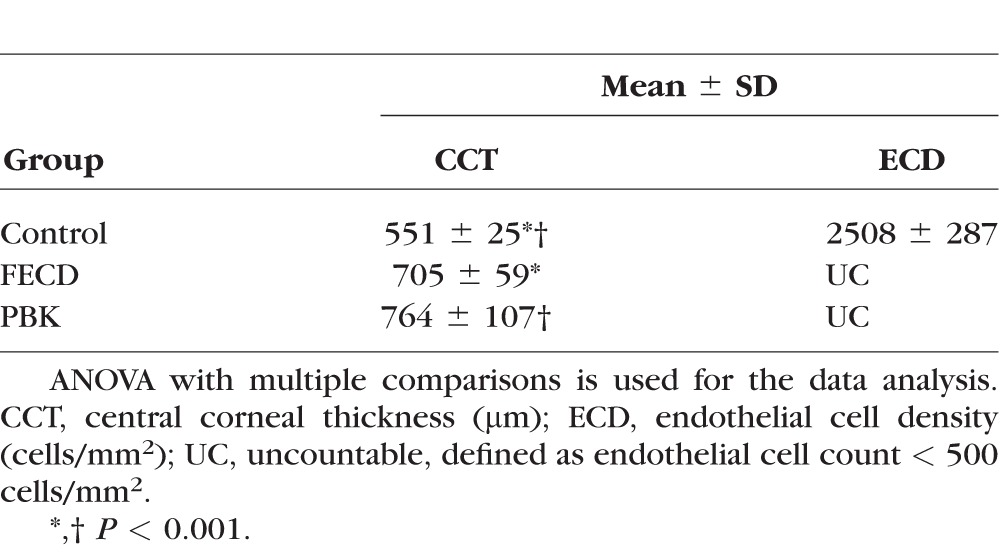
We included 51 eyes (one eye each from 51 patients) in the study. Table 1 shows the demographic characteristics of three groups (normal control, FECD, and PBK). Patients with FECD were all Caucasians. Patients with PBK were statistically older than those with FECD. The reference control group comprises patients undergoing cataract surgery with normal CCT (mean 551 μm) and ECD (mean 2508 cells/mm2), whereas the patients with FECD or PBK undergoing DSAEK surgery or combined with cataract surgery had statistically thicker CCT (mean 705 and 764 μm for FECD and PBK, respectively) and ECD below 500 cells/mm2 (Table 2).
Table 1.
Demographic Characteristics
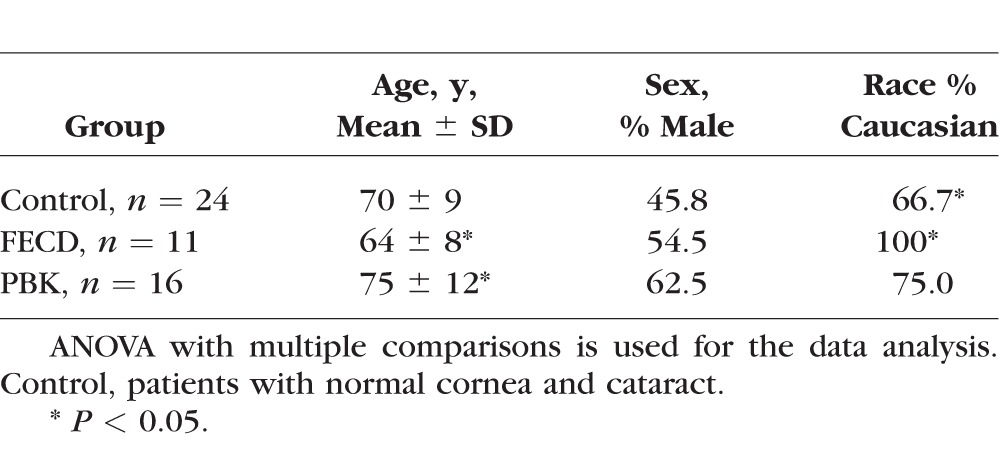
Table 2.
CCT and ECD of Each Group
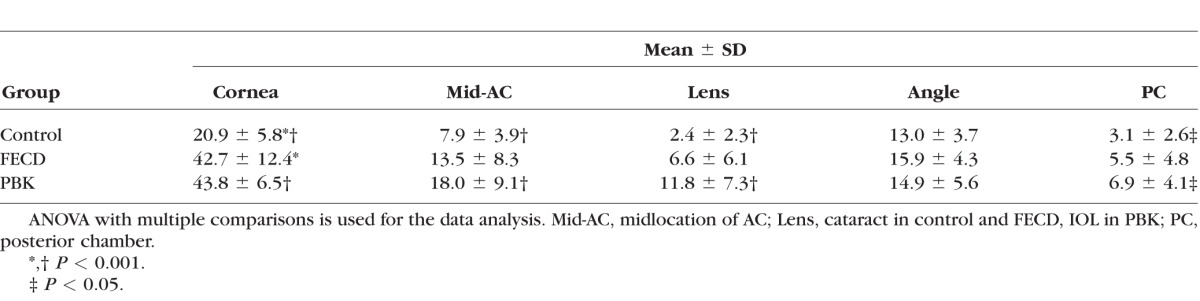
The pO2 measurements at five different intraocular locations of each group are listed in Table 3. The mean intraocular pO2 levels in the normal controls (n = 24) were 20.9 mm Hg near the central cornea, 7.9 mm Hg in the mid-AC, 13.0 mm Hg in the AC angle, 2.4 mm Hg at the anterior surface of the crystalline lens, and 3.1 mm Hg in the PC. In patients with FECD (n = 11) and PBK (n = 16), there was a statistically significant increase of pO2 near the central cornea (42.7 and 43.8 mm Hg for FECD and PBK, respectively, Table 3). Compared to the normal control, there was a significant increase of pO2 in the mid-AC, at the anterior surface of the IOL, and in the PC (18.0, 11.8, and 6.9 mm Hg, respectively) in patients with PBK.
Table 3.
Oxygen Measurements at Five Intraocular Locations (mm Hg)
The respective intraocular oxygen gradients of these three groups in Table 3 are further illustrated in Figure 1. Consistent with our recent reports,19–22 a steep oxygen gradient is present in the anterior segment of the reference normal group from central subendothelial AC to the anterior surface of the crystalline lens (Fig. 1, left). There also are steep oxygen gradients decreasing across the AC in FECD and PBK (Fig. 1, middle and right).
Figure 1.
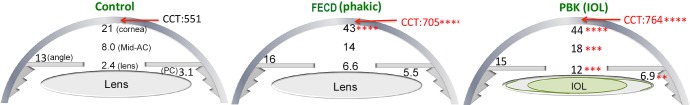
Average pO2 (mm Hg) at five different locations in the anterior segment of control with cataract, FECD with cataract, and PBK. Respective results of mean CCT for each group are also included. ANOVA with multiple comparisons is used for the data analysis. **P < 0.01; ***P < 0.001; ****P < 0.0001. (Please refer to Table 3 for detailed information.)
The mean pO2 at five subendothelial AC locations (central cornea and 4 cardinal positions) of these three groups is illustrated in Figure 2. There is no evident difference in pO2 at five different subendothelial locations in the normal controls (mean 20.9 mm Hg; Fig. 2, left; P > 0.05). In contrast, there is a generalized and significant increase of pO2 at all five subendothelial locations in patients with FECD (mean 42.7 mm Hg) and PBK (mean 44.8 mm Hg; Fig. 2, middle and right; P < 0.05 to 1 × 10−6). Overall, we note approximately a 2-fold increase of pO2 in the subendothelial AC in patients with FECD and PBK, when compared to the normal controls. Of significance, despite the significant elevation of the pO2 in the mid-AC and beneath the corneal endothelium of both groups, the mean pO2 in the AC angle of patients with FECD or PBK (mean 15.9 and 14.9 mm Hg, respectively; Table 3 and Figs. 1, 2) is not significantly affected and remains at levels similar to that of normal controls (mean 13.0 mm Hg).
Figure 2.
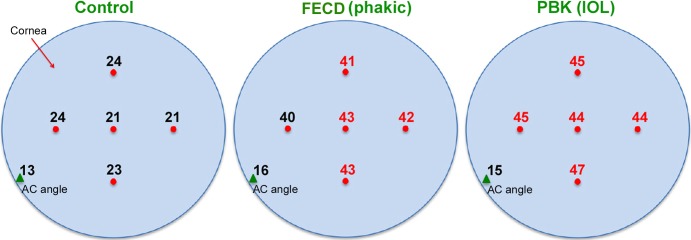
Average pO2 (mm Hg) in subendothelial AC at central cornea and 4 midperipheral locations (red dots), and at the AC angle (green triangle). The measurements of pO2 in red (in FECD and PBK) indicates significantly higher pO2 at those locations when compared to the control group. (P values ranged from <0.05 to <0.001 for each location by ANOVA with multiple comparisons.)
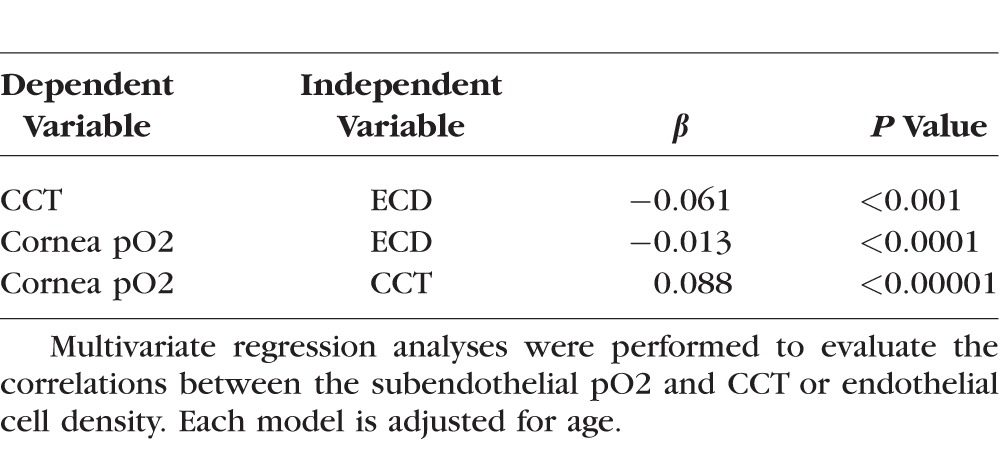
Multivariate regression analyses were performed to further explore the correlations between the subendothelial pO2 and CCT or ECD (Table 4). As expected, CCT correlated inversely with the ECD, but not with the patient's age. We also observed that subendothelial pO2 correlated inversely with ECD and positively with CCT, but was independent of patient's age.
Table 4.
Multivariate Regression Analyses
Discussion
The findings in this study are consistent with our previous reports19–22 that the normal human eye has a steep oxygen gradient in the aqueous humor of the AC from the subendothelial regions to the anterior surface of the lens. We found that there is a significant increase of pO2 in the subendothelial AC of FECD and PBK without impacting the pO2 in the AC angle. These observations may provide further insights into the oxidative stress induced by endothelial dysfunctions and its implications on aqueous humor dynamics.
Progressive or accelerated loss of corneal endothelium can lead to corneal decompensation with resultant corneal edema and vision impairment.23,24 Various endothelial keratoplasties, including DSAEK, have been developed to manage corneal endothelial dysfunctions effectively as seen in patients with FECD or PBK. Consistent with the prevailing knowledge, we demonstrated in this study that there is a significant decrease of ECD with a related increase of CCT in patients with FECD or PBK undergoing DSAEK for visual rehabilitation. Our in vivo pO2 measurements confirmed the steep oxygen gradient in normal human anterior segment and further supported our previous conclusion that oxygen consumption by crystalline lens significantly reduces intraocular oxidative stress. Our data also demonstrated that there is a generalized increase of subendothelial pO2 and an even steeper intraocular oxygen gradient in eyes with corneal endothelial dysfunctions. Thus, our data suggested that reduced oxygen consumption due to corneal endothelial dysfunctions increases the possible source of reactive oxygen species (ROS), molecular oxygen, in the AC.
The implication of oxidative damage in corneal endothelial dysfunctions has not been thoroughly investigated to our knowledge; however, evidence indicates that multiple processes, such as oxidative stress, unfolded protein response, and apoptosis, have a role in accelerated corneal endothelial cell loss.25–29 The generalized increase of subendothelial pO2 observed in FECD and PBK in this study further substantiated the presence of potential sources of ROS in endothelial dysfunctions. As corneal endothelial pumps are the major site of oxygen consumption in the anterior segment of the human eye, endothelial loss will result in reduced oxygen consumption.24,25 At the same time, as the cornea is the primary physical barrier against environmental assault on the eye,24 corneal endothelial dysfunctions also can lead to increased stromal hydration with augmented corneal oxygen permeability from the ambient air through the swollen collagen lamellae.24,25 These two factors can contribute to the increase of pO2 underneath the cornea. In turn, the increased oxidative stress in the AC further accelerates the endothelial damage. Nonetheless, the causal relationship between oxidative stress and corneal endothelial dysfunction awaits further investigations.
The cornea absorbs much of the ambient ultraviolet (UV) light entering the eye, suggesting that it would be highly susceptible to damage from light-induced ROS.28 Previous studies suggest that the cornea is rich in antioxidant enzymes such as superoxide dismutase (SOD), catalase, glutathione peroxidase, and glutathione reductase, all of which facilitate the removal of free radicals and ROS generated by constant UV absorption.30,31 It has been reported that the basal level of intraocular oxidative nucleotide modifications was higher in the cornea than in the iris and trabecular meshwork.14 Among them, the cornea is the least sensitive tissue, whereas the trabecular meshwork is the most sensitive tissue to oxidative damage.14 When compared to pO2 in the AC angle, our finding of higher oxygen tension near the inner corneal surface in normal patients may indirectly support the notion that the healthy cornea has a higher tolerance of oxidative stress than the trabecular meshwork.
At present, information is limited regarding the mechanism underlying the corneal endothelial damage by oxidants. As discussed, oxidative species in the aqueous humor have been suggested to be responsible for the corneal endothelial loss.32–34 The ROS in aqueous humor include hydrogen peroxide (H2O2),32,35,36 superoxide (O2−), singlet oxygen (O2), and hydroxyl radical (HO−), and are involved in various important biologic processes of the ocular system.32,37 Susceptibility of corneal endothelium to oxidative damage and the corneal endothelial loss associated with increasing age have been shown to be associated with continuous exposure of the endothelial cells to those light-induced free oxygen radicals.32–34,38,39 By using antibodies to detect various oxidative enzymes, such as isoenzymes for nitric oxide synthesis, malondialdehyde, and other products from lipid peroxidation, it also been has suggested that oxidative stress has a role in the onset of the corneal disease, particularly with regard to accelerated corneal endothelial loss.28
The present study as well as our previous studies19–22 on oxygen distribution in control human eyes (cataract only) also show that the oxygen levels beneath the cornea are approximately 1.5- to 2-fold of that in the angle and mid-AC as well as 5- to 6-fold of that in the PC and anterior to the lens, indicating the pO2 in the AC angle near the trabecular meshwork (TM) is lower than and independent of that in the subendothelial AC. Our previous studies also have demonstrated that the pO2 was significantly elevated in aqueous humor near the TM after combined vitrectomy and cataract surgery.19–22 Therefore, it supports the notion that the elevated oxygen tension near the TM may contribute to the development of open angle glaucoma.10,11,19,22 In addition, it is interesting to note that patients with FECD also have been shown in a recent secondary analysis of the FECD Genetics Multi-Center study to have an increased risk of open angle glaucoma.40 We have not studied oxygen levels in patients with FECD and open angle glaucoma to determine any correlations.
In one of our previous studies on oxygen distribution in the rabbit eye,41 the pO2 in the AC angle, but not that of the subendothelial AC, decreased with the lowering of the blood oxygen saturation. Previous studies by Freddo et al.42,43 as well as by others44–46 have revealed that most plasma proteins in the aqueous humor diffuse from the permeable vessels in the ciliary body, through the ciliary body and iris stroma, across the anterior face of the iris and into the aqueous via the AC angle, instead of directly diffusing across the ciliary epithelium. A recent study revealed that TGFβ-1 is elevated in the plasma of patients with primary open angle glaucoma,47 suggesting the possibility that TGFβ-1 from the plasma influences the cells of the outflow pathway. We further demonstrated that pO2 in the AC angle is unaffected by the significant increase of oxygen in the subendothelial AC and mid-AC in patients with corneal endothelial dysfunctions due to FECD or PBK. We surmised that the oxygen tension in the AC angle is regulated solely by oxygen from the blood in the ciliary body stroma, instead of being regulated by oxygen diffusing across the cornea. Taken together, these results supported the existence of an alternative inflow pathway in maintaining a relatively low and constant oxygen level at the AC angle in patients with normal physiology (Fig. 3). If this alternative inflow pathway is perturbed, the pO2 in the AC angle can be affected readily and lead to a potential damaging environment of oxidative stress in the TM. Even though we did not notice significant increase of pO2 in the AC angle in our patients with FECD and PBK, we speculated that extensive ocular surgery, such as penetrating keratoplasty or implantation of a keratoprosthesis, may affect the aqueous humor homeostasis at the AC angle and increase oxygen exposure to the TM, thereby increasing the risk of developing secondary glaucoma.
Figure 3.
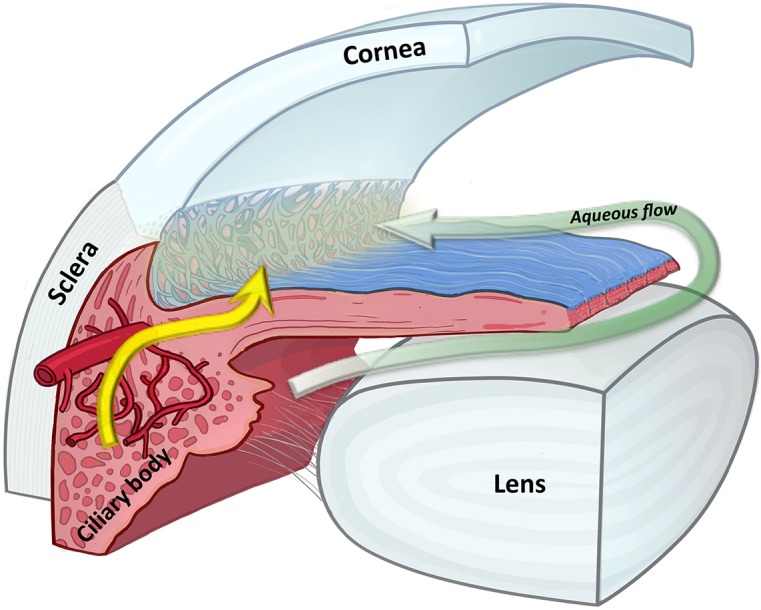
Our data demonstrated that pO2 measurements in the AC angle remain relatively low in all three groups and are independent of oxygen levels in the AC. We propose the presence of an alternative flow pathway in the AC angle as illustrated in this diagram. The sage arrow indicates the classic pathway of aqueous humor flowing from ciliary epithelium to the angle. The yellow arrow indicates the alternative pathway of plasma from the ciliary body vasculature. The light yellow “fog” around the trabecular meshwork indicates that plasma from the alternative pathway may have a critical role in maintaining a relatively stable oxygen level at the angle independent of the oxygen level in the aqueous humor.
In summary, our current study revealed a close relationship between subendothelial intraocular oxygen tension and the health of the corneal endothelium. To the best of our knowledge, this is the first report demonstrating the differences in subendothelial oxygen tension in vivo between human eyes with normal corneas and corneas with severe endothelial dysfunctions. It suggested that the pO2 near the corneal inner surface is regulated by oxygen diffusion from the air across the cornea as well as by corneal oxygen consumption and not directly regulated by the circulation in aqueous humor. We further demonstrated that pO2 in the AC angle is unaffected by the significant increase of in the subendothelial AC and mid-AC in patients with corneal decompensation, such as FECD or PBK. With an increasingly oxidized environment in the AC with FECD or PBK, oxidative stress may result in potential extracellular matrix overproduction in the Descemet's membrane of patients with FECD and reach a critical level of activating cellular apoptosis as reported previously by Jurkunas et al.48 Further study exploring the role of oxidative stress in the TM induced by corneal endothelial dysfunctions in the development of open angle glaucoma is indicated.
Acknowledgments
The authors thank Margaret Liu for the illustration of Figure 3.
Supported by the National Institutes of Health (NIH; Bethesda, MD, USA) Grants R01EY017609 (AJWH) and EY021515 (CS); the RPB Physician Scientist Award, and an unrestricted grant from Horncrest Foundation, Inc. (AJWH). Also supported in part by awards to the Department of Ophthalmology and Visual Sciences at Washington University from a Research to Prevent Blindness, Inc. unrestricted grant and NIH Vision Core Grant P30 EY02687. The authors alone are responsible for the content and writing of this paper.
Disclosure: A.J.W. Huang, None; Y.-B. Shui, None; Y.-P. Han, None; F. Bai, None; C.J. Siegfried, None; D.C. Beebe, None
References
- 1. Barbazetto IA,, Liang J,, Chang S,, et al. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004. ; 78: 917–924. [DOI] [PubMed] [Google Scholar]
- 2. Harocopos GJ,, Shui Y-B,, McKinnon M,, et al. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004; 45: 77–78. [DOI] [PubMed] [Google Scholar]
- 3. Holekamp NM,, Shui YB,, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005. ; 139: 302–310. [DOI] [PubMed] [Google Scholar]
- 4. Shui YB,, Holekamp NM,, Kramer BC,, et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009. ; 127: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McNulty R,, Wang H,, Mathias RT,, et al. Regulation of tissue oxygen levels in the mammalian lens. J Physiol (Lond). 2004. ; 559: 883–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmquist BM,, Philipson B,, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984. ; 68: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giblin FJ,, Padgaonkar VA,, Leverenz VR,, et al. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res. 1995. ; 60: 219–235. [DOI] [PubMed] [Google Scholar]
- 8. Truscott RJW. Age-related nuclear cataract--oxidation is the key. Exp Eye Res. 2004. ; 80: 709–725. [DOI] [PubMed] [Google Scholar]
- 9. Truscott RJ,, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977. ; 492: 43–52. [DOI] [PubMed] [Google Scholar]
- 10. Chang S. LXII Edward Jackson Lecture: open angle glaucoma after vitrectomy. Am J Ophthalmol. 2006; 141: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 11. Koreen L,, Yoshida N,, Escariao P,, et al. Incidence of, risk factors for, and combined mechanism of late-onset open-angle glaucoma after vitrectomy. Retina. 2012. ; 32: 160–167. [DOI] [PubMed] [Google Scholar]
- 12. Gabelt BT,, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005; 24: 612–637. [DOI] [PubMed] [Google Scholar]
- 13. Izzotti A,, Bagnis A,, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006. ; 612: 105–114. [DOI] [PubMed] [Google Scholar]
- 14. Izzotti A,, Sacca SC,, Cartiglia C,, et al. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003. ; 114: 638–646. [DOI] [PubMed] [Google Scholar]
- 15. Izzotti A,, Sacca SC,, Longobardi M,, et al. Sensitivity of ocular anterior-chamber tissues to oxidative damage and its relevance to glaucoma pathogenesis. Invest Ophthalmol Vis Sci. 2009. ; 50: 5251–5258. [DOI] [PubMed] [Google Scholar]
- 16. Kumar DM,, Agarwal N. Oxidative stress in glaucoma: a burden of evidence. J Glaucoma. 2007. ; 16: 334–343. [DOI] [PubMed] [Google Scholar]
- 17. Saccà SC,, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008; 173: 385–407. [DOI] [PubMed] [Google Scholar]
- 18. Sacca SC,, Pascotto A,, Camicione P,, et al. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005; 123: 458–463. [DOI] [PubMed] [Google Scholar]
- 19. Siegfried CJ, Shui YB, Holekamp NM. et al. Oxygen distribution in the human eye: relevance to the etiology of open angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010; 51: 5731–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siegfried CJ,, Shui YB,, Holekamp NM,, et al. Racial differences in ocular oxidative metabolism: implications for ocular disease. Arch Ophthalmol. 2011. ; 129: 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen A,, Shui YB,, Zhang Q,, et al. Aqueous humor oxygen measurements. J Glaucoma. 2013. ; 22: 23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siegfried CJ,, Shui YB,, Bai F,, et al. Central corneal thickness correlates with oxygen levels in the human anterior chamber angle. Am J Ophthalmol. 2015; 159: 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonanno JA. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog Retin Eye Res. 2003. ; 22: 69–94. [DOI] [PubMed] [Google Scholar]
- 24. Suh LH,, Emerson MV,, Jun AS. Fuchs endothelial dystrophy: pathogenesis and management. : Reinhard T,, Larkin F, Cornea and External Eye Disease. New York, NY: Springer; 2008: 1–13. [Google Scholar]
- 25. Adamis AP,, Filatov V,, Tripathi BJ,, et al. Fuchs' endothelial dystrophy of the cornea. Surv Ophthalmol. 1993. ; 38: 149–168. [DOI] [PubMed] [Google Scholar]
- 26. Serbecic N,, Beutelspacher SC. Anti-oxidative vitamins prevent lipid-peroxidation and apoptosis in corneal endothelial cells. Cell Tissue Res. 2005. ; 320: 465–475. [DOI] [PubMed] [Google Scholar]
- 27. Li QJ,, Ashraf MF,, Shen DF,, et al. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001. ; 119: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 28. Buddi R,, Lin B,, Atilano SR,, et al. Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 2002. ; 50: 341–351. [DOI] [PubMed] [Google Scholar]
- 29. Engler C,, Kelliher C,, Spitze AR,, et al. Unfolded protein response in Fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010; 149: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rao NA,, Romero JL,, Fernandez MA,, et al. Role of free radicals in uveitis. Surv Ophthalmol. 1987. ; 32: 209–213. [DOI] [PubMed] [Google Scholar]
- 31. Behndig A,, Karlsson K,, Johansson BO,, et al. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Invest Ophthalmol Vis Sci. 2001. ; 42: 2293–2296. [PubMed] [Google Scholar]
- 32. Lux-Neuwirth O,, Millar TJ. Lipid soluble antioxidants preserve rabbit corneal cell function. Curr Eye Res. 1990. ; 9: 103–109. [DOI] [PubMed] [Google Scholar]
- 33. Hull DS. Oxygen free radicals and corneal endothelium. Trans Am Ophthalmol Soc. 1990. ; 88: 463–511. [PMC free article] [PubMed] [Google Scholar]
- 34. Green K. Free radicals and aging of anterior segment tissues of the eye: a hypothesis. Ophthalmic Res. 1995; 27 (suppl 1): 143–149. [DOI] [PubMed] [Google Scholar]
- 35. Spector A,, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981. ; 33: 673–681. [DOI] [PubMed] [Google Scholar]
- 36. Bhuyan KC,, Bhuyan DK. Molecular mechanism of cataractogenesis. Toxic metabolites of oxygen as initiators of lipid peroxidation and cataract. Curr Eye Res. 1984. ; 3: 67–81. [DOI] [PubMed] [Google Scholar]
- 37. Halliwell B,, Gutteridge JMC. Protection against radical damage: systems with problems. : Free Radicals in Biology and Medicine, 2nd ed. Oxford, United Kingdom: Oxford University Press; 1989: 289–296. [Google Scholar]
- 38. Koh SW,, Waschek JA. Corneal endothelial cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthalmol Vis Sci. 2000; 41: 4085–4092. [PubMed] [Google Scholar]
- 39. Cho KS,, Lee EH,, Choi JS,, et al. Reactive oxygen species-induced apoptosis and necrosis in bovine corneal endothelial cells. Invest Ophthalmol Vis Sci. 1999. ; 40: 911–919. [PubMed] [Google Scholar]
- 40. Nagarsheth M,, Singh A,, Schmotzer B,, et al. Relationship between Fuchs endothelial corneal dystrophy severity and glaucoma and/or ocular hypertension. Arch Ophthalmol. 2012; 130: 1384–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shui Y-B,, Fu J-J,, Garcia C,, et al. Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006; 47: 1571–1580. [DOI] [PubMed] [Google Scholar]
- 42. Freddo TF,, Bartels SP,, Barsotti MF,, et al. The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci. 1990. ; 31: 125–137. [PubMed] [Google Scholar]
- 43. Freddo TF. Shifting the paradigm of the blood-aqueous barrier. Exp Eye Res. 2001; 73: 581–592. [DOI] [PubMed] [Google Scholar]
- 44. Bert RJ,, Caruthers SD,, Jara H,, et al. Demonstration of an anterior diffusional pathway for solutes in the normal human eye with high spatial resolution contrast-enhanced dynamic MR imaging. Invest Ophthalmol Vis Sci. 2006. ; 47: 5153–5162. [DOI] [PubMed] [Google Scholar]
- 45. Kleinstein RN,, Kwan M,, Fatt I,, et al. In vivo aqueous humor oxygen tension--as estimated from measurements on bare stroma. Invest Ophthalmol Vis Sci. 1981. ; 21: 415–421. [PubMed] [Google Scholar]
- 46. Bill A,, Tornquist P,, Alm A. Permeability of the intraocular blood vessels. Trans Oph thalmol Soc UK. 1980. ; 100: 332–336. [PubMed] [Google Scholar]
- 47. Kuchtey J,, Kunkel J., Goodwin Burgess L,, et al. Elevated transforming growth factor b1 in plasma of primary open-angle glaucoma patients. Invest Ophthalmol Vis Sci. 2014. ; 55: 5291–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jurkunas UV,, Bitar MS,, Funaki T,, et al. Evidence of oxidative stress in the pathogenesis of Fuchs endothelial corneal dystrophy. Am J Pathol. 2010. ; 177: 2278–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]


