Abstract
Purpose
Differences in the spectral composition of lighting between indoor and outdoor scenes may contribute to the higher prevalence of myopia in children who spend low amounts of time outdoors. Our goal was to determine whether environments dominated by long-wavelength light promote the development of myopia.
Methods
Beginning at 25 ± 2 days of age, infant monkeys were reared with long-wavelength-pass (red) filters in front of one (MRL, n = 6) or both eyes (BRL, n = 7). The filters were worn continuously until 146 ± 7 days of age. Refractive development, corneal power, and vitreous chamber depth were assessed by retinoscopy, keratometry, and ultrasonography, respectively. Control data were obtained from 6 monkeys reared with binocular neutral density (ND) filters and 33 normal monkeys reared with unrestricted vision under typical indoor lighting.
Results
At the end of the filter-rearing period, the median refractive error for the BRL monkeys (+4.25 diopters [D]) was significantly more hyperopic than that for the ND (+2.22 D; P = 0.003) and normal monkeys (+2.38 D; P = 0.0001). Similarly, the MRL monkeys exhibited hyperopic anisometropias that were larger than those in normal monkeys (+1.70 ± 1.55 vs. −0.013 ± 0.33 D, P < 0.0001). The relative hyperopia in the treated eyes was associated with shorter vitreous chambers. Following filter removal, the filter-reared monkeys recovered from the induced hyperopic errors.
Conclusions
The observed hyperopic shifts indicate that emmetropization does not necessarily target the focal plane that maximizes luminance contrast and that reducing potential chromatic cues can interfere with emmetropization. There was no evidence that environments dominated by long wavelengths necessarily promote myopia development.
Keywords: emmetropization, myopia, hyperopia, refractive error, longitudinal chromatic aberration
Restricting the ambient lighting for infant monkeys to relatively long wavelengths (>600 nm) produced axial hyperopia. The results demonstrate that environments dominated by long-wavelength light do not necessarily promote myopia and suggest that chromatic cues facilitate emmetropization.
Visual feedback associated with the eye's effective refractive state actively regulates refractive development and ocular growth, in particular vitreous chamber depth. In essence, error signals that encode the sign of optical defocus (i.e., whether the eye is myopic or hyperopic) can increase or decrease the rate of axial elongation in order to minimize the eye's refractive error.1,2 From an operational perspective, signals encoding the sign of defocus are ideal for driving the process of emmetropization; and although signals associated with defocus appear to dominate emmetropization, there is growing evidence that several aspects of ambient lighting can also influence the course of refractive development.
In laboratory animals, the intensity of ambient lighting has been shown to influence the normal course of emmetropization. For example, chickens reared with unrestricted vision under dim lighting develop myopic refractive errors and exhibit higher than normal intersubject variability in refractions.3 On the other hand, elevated lighting levels promote the development of low degrees of hyperopia. Elevated lighting levels have also been shown to reduce the eye's response to some myopiagenic stimuli. Specifically, elevated lighting levels significantly reduce the degree of axial myopia normally produced by form deprivation in chickens,4,5 tree shrews,6 and macaques.7 These results support the hypothesis that the protective effects that time outdoors has against myopia in children are due, at least in part, to the higher ambient light levels typically encountered outdoors.4,8,9 However, elevated lighting levels do not alter the final degree of myopia produced by optically imposed hyperopic defocus,6,10,11 which indicates that defocus growth signals can override the effects of elevated lighting levels.
The spectral composition of ambient lighting can also influence refractive development. As a consequence of longitudinal chromatic aberration (LCA), the total refracting power of the eye varies inversely with the wavelength of light so that the eye is relatively more hyperopic/less myopic for long- versus short-wavelength light. It is well established that signals associated with LCA provide directional cues for accommodation.12–14 The results from a recent series of experiments have demonstrated that the eye can also use chromatic signals from LCA in several different ways to encode the sign of defocus for the emmetropization process.15–19 In particular, Rucker and her colleagues15–19 have shown that color stimuli that mimic the color contrast differences produced by myopic and hyperopic defocus can produce predictable changes in refractive development. For example, with myopic defocus the relative contrast for long-wavelength image components is higher than that for short-wavelength components; and when chickens are reared viewing chromatic simulations of myopic defocus, the chicken eye exhibits alterations associated with reduced growth.16 However, the fact that the compensation to optically imposed defocus20,21 and the recovery from form deprivation22 can still take place when animals are reared in quasimonochromatic environments indicates that signals associated with luminance contrast signals can guide refractive development and that chromatic signals associated with LCA are not always essential for emmetropization.
Because both luminance and color signals can influence emmetropization, alterations in the spectral composition of ambient lighting could influence refractive development in multiple ways. For example, if the target for emmetropization is the focal plane that maximizes luminance contrast and chromatic cues are assumed not to be available, then manipulations of the wavelength composition of the ambient lighting should produce changes in the set point or target refractive error that are related to the magnitude of the eye's LCA. In this respect, the eyes of fish,23 chickens,14,19 and guinea pigs24–27 exposed to quasimonochromatic long-wavelength light develop longer ocular diameters and become more myopic/less hyperopic than eyes exposed to relatively shorter wavelengths. In some of these experiments, particularly those that involved relatively short treatment durations, the magnitude of the light-induced differences in ocular parameters matched the shift in the eye's focal length predicted on the basis of LCA.14,19 This pattern of results suggested that luminance-based cues alone were responsible for the changes in refractive development produced by exposure to quasimonochromatic light. However, with longer treatment durations, the magnitude of the ocular and refractive changes, at least in guinea pigs27 and chickens,28 continued to increase well beyond predictions based on LCA, indicating that luminance contrast cues are not always sufficient to control refractive development, and that either the absence of chromatic cues and/or the presence of anomalous sign-of-defocus cues produced by changes in the spectral composition of the ambient lighting interfered with emmetropization.
It is important to determine how and the extent to which the wavelength composition of ambient lighting influences emmetropization, especially in primates, because it may be possible to manipulate the spectral characteristics of ambient lighting in ways that could have therapeutic benefit. For example, based primarily on the wavelength-dependent shifts in the focal plane that has the maximum luminance contrast, it has been hypothesized that environments dominated by long-wavelength light, and the relative hyperopic defocus associated with long wavelengths, may promote the development of myopia. On the other hand, environments dominated by relatively short-wavelength light may be protective against myopia. In this respect, because outdoor scenes and artificially lighted indoor scenes tend to be dominated by relatively short- and long-wavelength light, respectively, it has been hypothesized that differences in the spectral composition of indoor and outdoor scenes may contribute to the protective effects that time outdoors has on myopia in children.28
To date we know relatively little about how variations in the wavelength composition of ambient lighting affect emmetropization in primates. Liu et al.29 reported that refractive development in macaques reared in relatively short-wavelength light (455 nm) was not different from that observed in monkeys housed in white light (5000 K). Similarly seven of the nine monkeys that they reared in quasimonochromatic red light (610 nm) exhibited refractive errors that were similar to those of control animals reared in white light. However, two of the monkeys exposed to the red light developed myopic errors, suggesting that there may be individual differences in the susceptibility to long-wavelength stimulation. The purpose of this study was to test the hypothesis that environments dominated by long-wavelength light promote the development of myopia in monkeys.
Methods
Subjects
Rhesus monkeys (Macaca mulatta) were employed as subjects in these experiments because there is a close correspondence, both qualitative and quantitative, between humans and macaques in terms of their spectral sensitivities and color vision.30,31 In particular, rhesus monkeys are ideal subjects for these experiments because the absorption spectra of the middle- and long-wavelength cone photopigments are virtually identical in humans and rhesus monkeys,32 the photopic luminosity functions for macaques and humans are similar in both relative and absolute terms,33 and the spectral sensitivity of the long-wavelength cone photoreceptor mechanisms of monkeys determined behaviorally via chromatic adaptation is very similar to that for Stiles' π5 mechanism obtained from human observers.30 In addition, the optical configuration of the monkey eye is qualitatively and quantitatively very comparable to that of human eyes, and the phenomenon of emmetropization proceeds in a similar manner in the two species.34–36
The spectral characteristics of the ambient lighting were manipulated by rearing infant monkeys with long-wavelength-pass filters (“red” filters) over one (n = 6) or both eyes (n = 7). The filters were held at an 11-mm vertex distance by goggles that provided monocular and binocular fields of view in the horizontal plane of 80° and 62°, respectively, and an 87° vertical field. Except for brief periods needed for routine cleaning and maintenance, the monkeys wore the helmets continuously from 25 ± 2 to 146 ± 7 days of age. The helmets were inspected at approximately 2-hour intervals throughout the day to ensure that they fit the subjects appropriately and that the spectacle lenses were clean and free of debris that might have interfered with the desired optical effects. To determine whether the treated animals would recover from any experimentally induced ametropias, the helmets were removed at the end of the filter-rearing period and the animals were housed under typical laboratory lighting levels (average = 580 human lux), and refractive development was monitored for an additional 6 months.
The red filters effectively absorbed wavelengths below approximately 570 nm (Fig. 1); transmission increased rapidly for longer wavelengths with at least 50% of the light transmitted for wavelengths longer than approximately 660 nm. The reduction in overall light levels produced by the red filters was determined by measuring the relative reduction in photopic brightness through the red filter and expressed in terms of human lux (LX-101 lux meter; Lutron Electronics Enterprise Co., Taipei, Taiwan), which, given the close similarities in the photopic luminosity functions between humans and monkeys, should accurately reflect photopic brightness in rhesus monkeys. Under our vivarium lighting, the red filters reduced the amount light reaching the treated eyes by slightly more than 1.0 log unit. Because dim ambient lighting levels have been reported to promote relative myopic shifts in chickens,3 control data for the binocularly treated monkeys were obtained from monkeys reared with either 1.0 (n = 3) or 1.3 log (n = 3) neutral density filters over both eyes.
Figure 1.
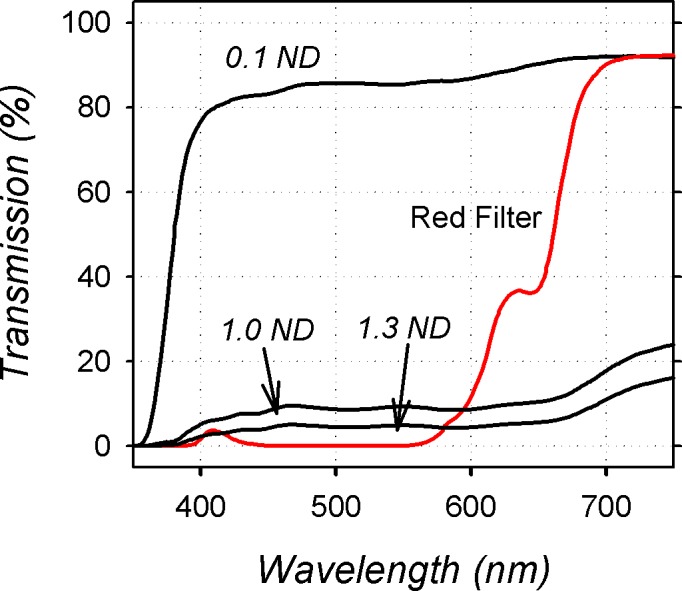
Percentage of light transmitted through the 0.1, 1.0, and 1.3 log unit neutral density filters and the red treatment filters plotted as a function of wavelength.
Based on the results that we obtained from the binocularly treated monkeys and the fact that elevated lighting levels can promote hyperopia,3 0.1 log neutral density filters were placed in front of the control eyes of the monkeys reared with monocular red filters in order to deliberately establish an interocular difference in light levels. Our goal was to ensure that the treated eyes did not receive higher lighting levels than the control eye. In addition, we wanted to increase the likelihood that the animal's accommodative posture was determined via the control eye, which would reduce the possibility that wavelength-dependent variations in accommodation14 would influence the end point for emmetropization.
Data on refractive development under typical indoor lighting conditions were also available from previous studies for 33 normal control animals.37–39 Twenty-nine of these control animals were reared with unrestricted vision, and 4 control monkeys were reared wearing helmets that held zero-powered spectacles in front of both eyes. All of the animals were obtained at 1 to 3 weeks of age and, following the initial biometry measurements at approximately 3 weeks of age, were assigned to their respective subject groups on a random basis. Although the different subject groups were studied at different times over a period of several years, the experimental methods were identical. The details of the nursery care for our infant monkeys have been described previously.37
The housing areas for all of the experimental monkeys were illuminated with fluorescent tubes (F32T8/TL735, correlated color temperature = 3500 K; Philips Lighting US, Somerset, NJ, USA) maintained on a 12-hour light/12-hour dark cycle. To ensure that the animals were exposed to a broad range of wavelengths, two 100-watt incandescent lamps were included in the housing area. The light levels provided by the combination of lights ranged from 305 (floors of lower cages) to 987 human lux (ceilings of upper cages), with an average of 580 ± 235 lux for all cages.
Ocular Biometry
The refractive status, corneal power, and axial dimensions were measured for each eye of each subject using methods described previously.37 The first measurements were obtained at ages corresponding to the start of filter wear; subsequently measurements were obtained at 2-week intervals throughout the treatment period and then at 2- to 4-week intervals following removal of the helmets. To make these measurements, the monkeys were anesthetized (intramuscular injection: ketamine hydrochloride, 15–20 mg/kg, and acepromazine maleate, 0.15–0.2 mg/kg; topical: 1–2 drops of 0.5% tetracaine hydrochloride) and cyclopleged (1% tropicamide). The refractive status of each eye was measured independently by two experienced investigators using a streak retinoscope and averaged.40 An eye's refractive error was defined as the spherical-equivalent, spectacle-plane refractive correction (95% limits of agreement = ±0.60 diopters [D])41; no corrections were made for the small-eye artifact associated with retinoscopy.42 The anterior radius of curvature of the cornea was measured by keratometry (Handheld Auto-keratometer; Alcon, Inc., Fort Worth, TX, USA), and central corneal power was calculated from the average of three readings using an assumed refractive index of 1.3375 (95% limits of agreement = +0.49 to −0.37 D for mean corneal power).43 The eyes' axial dimensions were measured by A-scan ultrasonography (Image 2000; Mentor, Norwell, MA, USA); 10 separate measurements were averaged (95% limits of agreement = ±0.05 mm).7
All of the rearing and experimental procedures were reviewed and approved by the University of Houston's Institutional Animal Care and Use Committee and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Statistical Methods
Mann-Whitney tests were used to compare the median refractive errors between subject groups. Paired Student's t-tests were employed to examine interocular differences within a given group. One-way ANOVAs and 2-sample t-tests were used to compare between-group differences in refractive error, anisometropia, corneal powers, and axial dimensions at the start and the end of the treatment period. Repeated-measure ANOVAs with Geisser-Greenhouse adjustments (G-G) and mixed-design, repeated-measure ANOVAs with G-G adjustments were used to compare within-group changes over the course of the treatment period and between-group differences in growth patterns, respectively. Linear regression and Pearson's correlation analyses were performed to characterize the relationship between refractive error and vitreous chamber depth. The above analyses were executed using Minitab software (Release 12.21; Minitab, Inc., State College, PA, USA) and SuperANOVA (Abacus Concepts, Inc., Berkeley, CA, USA).
Results
At ages corresponding to the start of the filter-rearing procedures (25 ± 2 days), the mean ametropia for all subject groups was a moderate degree of hyperopia (right eyes all subjects: +4.08 ± 1.81 D). There were no significant differences between the normal control animals and any of the experimental subject groups in either the median (normal control right eyes: +3.63 D versus treated eyes: +2.47 to +4.13 D, P = 0.19–0.67) or mean refractive errors (normal control right eye means ± SD: +4.02 ± 1.92 D versus treated eye means: +3.05 ± 1.45 to +4.30 ± 1.79 D, F = 0.57, P = 0.64). In addition, the mean corneal powers (normal control right eyes: 61.72 ± 2.03 D versus treated eyes: 60.61 ± 2.26–61.57 ± 2.02 D, F = 0.56, P = 0.65–0.86) and the axial dimension of the eyes of the experimental subjects were also comparable to those of the control monkeys (normal vitreous chambers right eyes: 8.63 ± 0.32 mm versus treated eyes: 8.53 ± 0.52–8.79 ± 0.39 mm, F = 0.70, P = 0.56).
Over the next 4 months, that is, the duration of the filter-rearing period, the control animals exhibited systematic reductions in the degree of hyperopia. Specifically, the average degree of hyperopia decreased from +4.02 ± 1.92 D at 25 ± 6 days to +2.42 ± 0.83 D at 142 ± 5 days of age (F = 10.63; G-G = 0.004), and there was a corresponding decrease in the variability of refractions across the group; that is, emmetropization occurred.
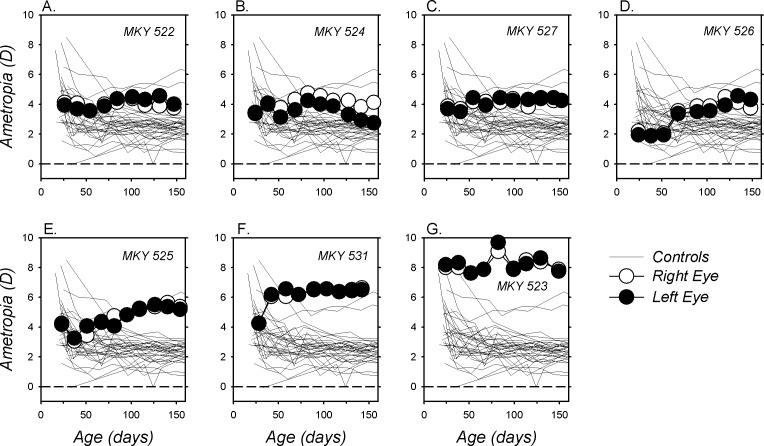
In contrast, as illustrated in Figure 2, which shows longitudinal refractive-error data for individual animals, the pattern of refractive development was very different in the monkeys reared with red filters over both eyes (BRL monkeys). Five of the seven binocularly treated monkeys exhibited more hyperopic refractive errors at the end versus the start of the treatment period. For the other two BRL monkeys (Figs, 2B, 2G), refractive error was essentially unchanged by the treatment regimen. There were no systematic interocular differences in refractive error (P = 0.77), corneal power (P = 0.51), or vitreous chamber depth (P = 0.15) in the BRL group; therefore, we chose to use the right eye data for analyses. Over the course of the treatment period, the average refractive error for the BRL monkeys increased in hyperopia from +4.30 ± 1.79 to +5.10 ± 1.65 D (F = 0.76; G-G = 0.03). At the end of the filter-rearing period, the median (+4.25 vs. +2.38 D, P = 0.0001) and mean refractive errors for the BRL monkeys (+5.10 ± 1.65 vs. +2.42 ± 0.83 D; t = 40.95, P < 0.0001) were significantly more hyperopic than those for the age-matched control monkeys.
Figure 2.
(A–G) Spherical-equivalent refractive corrections for the right (open symbols) and left eyes (filled symbols) of individual monkeys reared with the red filters in front of both eyes plotted as a function of age. The thin lines represent the data for the right eyes of the normal control monkeys. The first symbol in each plot denotes the onset of the filter-rearing period; the filters were worn continuously throughout the observation period.
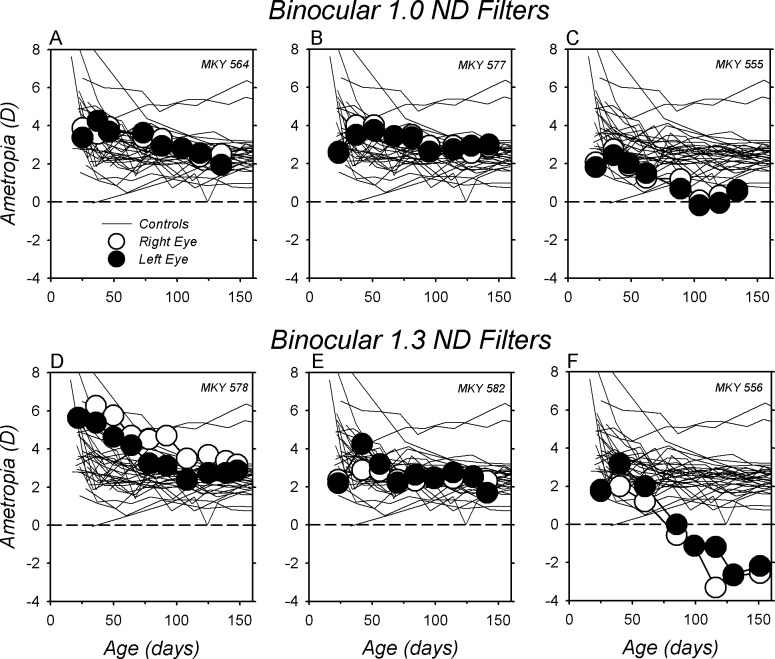
The relative hyperopic refractive errors observed in the BRL monkeys were not related to the overall reduction in light levels produced by the red filters. As shown in Figure 3, rearing infant monkeys with neutral density filters over both eyes (ND monkeys) that reduced the amount of light reaching the eyes to brightness levels equivalent to those produced by the red filters did not produce hyperopic shifts in refractions. Instead, during the filter-rearing period, the ND monkeys exhibited relative myopic shifts that either were equivalent to those observed in the normal monkeys (Figs. 3A, 3B, 3D, 3E) or resulted in relatively myopic errors outside the range for normal monkeys (Figs. 3C, 3F). At ages corresponding to the end of the filter-rearing period, the median right eye refraction for the six ND monkeys was comparable to that for normal monkeys (+2.22 vs. +2.38 D, P = 0.53), but significantly more myopic than that for the BRL monkeys (+2.22 vs. +4.25 D, P = 0.003). Similarly, the average refraction for the six ND monkeys was marginally less hyperopic than that for the normal monkeys (+1.40 ± 2.16 vs. +2.42 ± 0.83 D; t = 4.28, P = 0.05), but substantially less hyperopic than that for the BRL monkeys (+1.40 ± 2.16 vs. +5.10 ± 1.65 D; t = 12.28, P = 0.005).
Figure 3.
(A–F) Spherical-equivalent refractive corrections for the right (open symbols) and left eyes (filled symbols) of individual monkeys reared with either 1.0 (top row) or 1.3 log unit neutral density filters in front of both eyes (bottom row) plotted as a function of age. The thin lines represent the data for the right eyes of the normal control monkeys. The first symbol in each plot denotes the onset of the filter-rearing period; the filters were worn continuously throughout the observation period.
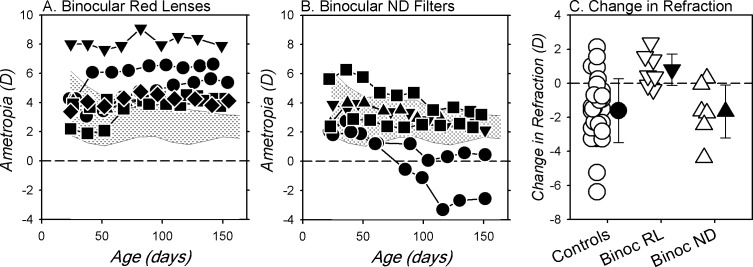
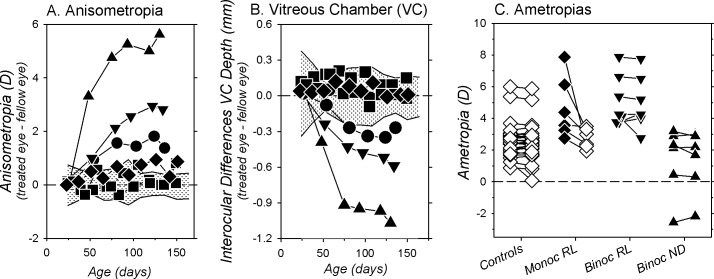
Figure 4 compares refractive development between the right eyes of the BRL, ND, and normal monkeys. At the start of the treatment period, the refractions for six of the seven BRL monkeys were within the 10th to 90th percentile range of refractive errors for the normal monkeys (shaded areas). However, by the end of the treatment period the refractions for all seven BRL monkeys were more hyperopic than for 90% of the normal monkeys. Even when the data from the one BRL monkey that exhibited relatively high hyperopic errors at the start of the rearing period (monkey [MKY] 523, Fig. 2G) were eliminated from the analysis, the median refraction for the BRL monkeys was still significantly more hyperopic than that for the normal monkeys (+4.03 vs. +2.38 D, P = 0.0002). More importantly, there was virtually no overlap between the end-of-treatment refractions for the BRL and ND monkeys. In contrast to the hyperopia found in the BRL monkeys, at the end of the filter-rearing period the refractive errors for the ND monkeys either were within the 10th to 90th percentile range for normal monkeys or shifted in a more myopic direction. There were also clear differences between the absolute changes in refractions that took place during the treatment period between the BRL and the normal monkeys (Fig. 4C; F = 7.18, G-G = 0.003) and between the BRL and the ND monkeys (F = 11.24, G-G = 0.001). In particular, the average change for the BRL monkeys was in the hyperopic direction (final refraction − initial refraction: +0.81 ± 1.00 D) and significantly different from the myopic shifts observed in the normal (−1.61 ± 1.88 D; t = 10.71, P < 0.0001) and the ND monkeys (−1.66 ± 1.70 D; t = 10.56, P = 0.008).
Figure 4.
Longitudinal spherical-equivalent refractive corrections for the right eyes of the BRL (A) and ND monkeys (B). The shaded areas in each plot show the 10th to 90th percentile range of ametropias for the 33 normal control monkeys; (C) shows the changes in refractive error that took place in individual animals (open symbols) during the filter-rearing period for the monkeys reared with binocular red (inverted triangles) and binocular ND filters (upright triangles) and age-matched normal control monkeys (circles). The filled symbols represent the group means (±SD).
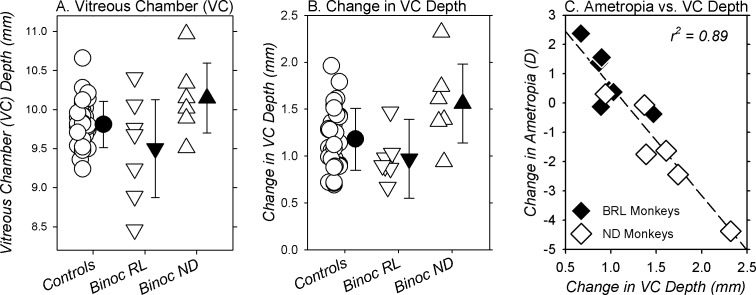
The relative hyperopic ametropias appeared to be axial in nature. At the end of the rearing period, there were no between-group differences in corneal power (right eyes, BRL = 55.49 ± 1.78 D; normals = 55.77 ± 1.59 D; ND = 55.73 ± 0.96 D, F = 0.09, P = 0.91). However, the between-group differences in refractive errors were associated with alterations in vitreous chamber depth (Fig. 5). At the end of the treatment period, there was a trend for the average vitreous chamber depths of the BRL monkeys (9.50 ± 0.68 mm, Fig. 5A) to be shorter than those for the normal (9.81 ± 0.30 mm; t = 3.69, P = 0.06) and ND monkeys (9.50 ± 0.68 vs. 10.15 ± 0.49 mm; t = 3.75, P = 0.08). As shown in Figure 5B, the changes in vitreous chamber depth that took place in the BRL monkeys during the treatment period (0.97 ± 0.025 mm) were significantly smaller than those in the ND monkeys (1.56 ± 0.46 mm; F = 7.93, G-G = 0.01) and marginally smaller than those in the normal monkeys (1.18 ± 0.33 mm; F = 3.04, G-G = 0.06). As illustrated in Figure 5C, the changes in the ametropias of the BRL and ND monkeys that took place during the treatment period were highly correlated with the changes in vitreous chamber depth (r2 = 0.89; P < 0.0001).
Figure 5.
Vitreous chamber (VC) depth obtained at ages corresponding to the end of the filter-rearing period (A) and the changes in vitreous chamber (VC) depth that took place during the treatment period (B) for individual normal controls (circles) and binocularly treated monkeys (red filters, inverted triangles; ND filters, upright triangles). The filled symbols represent the group means (±SD). In (C), the changes in refractive errors for the right eyes of the BRL (filled symbols) and ND monkeys (open symbols) are plotted as a function of the changes in vitreous chamber (VC) depth that took place over the course of the filter-rearing period. The dashed line represents the regression function for all of the filter-reared monkeys.
Based on previous findings in chickens,3 the interocular differences in lighting levels that were established in the monkeys reared with the red filters in front of one eye (MRL monkeys) should have promoted the development of relative myopia in the treated eyes. However, as illustrated in Figure 6A, the treated eyes of the MRL monkeys became more hyperopic than their fellow control eyes that were viewing through the 0.1 log neutral density filters (+4.46 ± 1.61 vs. +2.76 ± 0.61 D, t = 2.69, P = 0.04). The increase in hyperopic anisometropia that took place during the treatment period in the MRL monkeys was significantly greater than that observed in normal monkeys (+1.39 ± 1.58 vs. +0.01 ± 1.59 D; F = 15.03, G-G = 0.001). Consequently, at the end of the filter-rearing period, five of the six MRL subjects exhibited hyperopic anisometropias that were outside the 95% confidence limits for anisometropias in normal monkeys (shaded area), and the average degree of anisometropia was significantly larger in the MRL versus the normal monkeys (+1.70 ± 1.55 vs. −0.013 ± 0.33 D; t = 35.72, P < 0.0001). Comparisons between subject groups (Fig. 6C) revealed that the median and mean end-of-treatment refractive errors for the treated eyes of the MRL monkeys (median = +3.97 D; mean = +4.46 ± 1.61 D) were significantly more hyperopic than the ametropias in the normal (median = +2.38 D, P = 0.001; mean = +2.41 ± 0.83, t = 22.25, P < 0.0001) and ND monkeys (median = +2.22 D, P = 0.01; mean = +1.40 ± 2.16 D, t = 7.75, P = 0.02), but similar to the hyperopic refractive errors in the BRL monkeys (+4.25 D, P = 0.45; mean = +5.10 ± 1.65 D, t = 0.50, P = 0.50). The anisometropias exhibited by the MRL monkeys appeared to be axial in nature. In the three MRL monkeys that exhibited the higher degrees of anisometropia, the treated eyes clearly had shorter vitreous chambers, and the interocular differences in vitreous chamber depth for these animals were outside the 95% confidence limits for normal monkeys. For the group of MRL monkeys, the interocular differences in vitreous chamber depth were not significant (10.25 ± 0.78 vs. 9.95 ± 0.46 mm, t = −1.53, P = 0.19). However, the interocular differences in vitreous chamber depth were significantly different from those observed in the normal monkeys (F = 7.08; G-G = 0.0006)
Figure 6.
Interocular differences (treated eye − control eye) in spherical-equivalent refractive corrections (A) and vitreous chamber (VC) depth (B) for individual monkeys reared with red filters over one eye plotted as a function of age. The shaded areas in each plot represent the means ± 2 SDs for the normal control monkeys; (C) shows the refractive corrections for the right and left eyes of individual animals in each treatment group obtained at ages corresponding to the end of the filter-rearing period. For the monkeys reared with monocular red filters, the treated and control eyes are represented by the filled and open symbols, respectively.
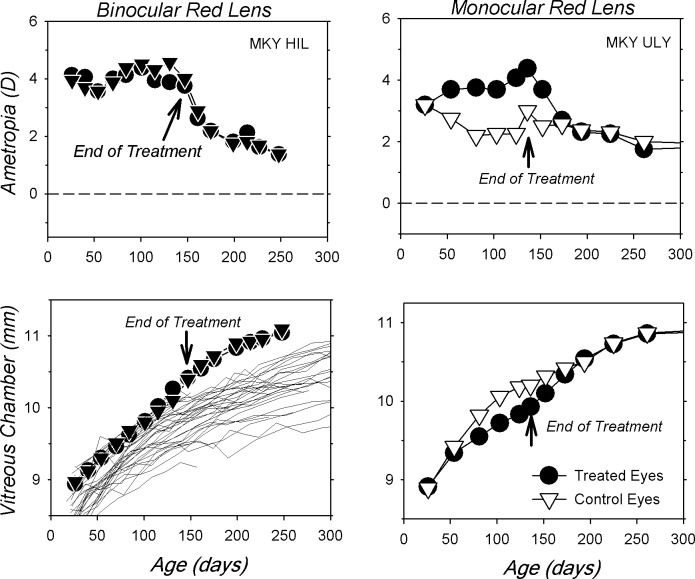
Another indication that the red filters altered refractive development was noted during the observation period following filter removal. Figure 7 shows longitudinal refractive error and vitreous chamber data for representative BRL and MRL monkeys. Following filter removal, the animals were housed with unrestricted vision under typical vivarium lighting conditions (average luminance = 580 lux). In the case of the BRL monkey (left), removing the red filters resulted in an increase in vitreous chamber growth rate and a concomitant binocular reduction in hyperopia down to levels similar to those in normal age-matched monkeys. In the MRL monkey (right), the onset of unrestricted vision resulted in an increase in the vitreous chamber growth rate of the treated eye relative to the control eye and a reduction in the treated eye's relative hyperopia. Once a balance in refractive errors was achieved, the subsequent growth rates in the two eyes of the MRL monkey were virtually identical and the refractive errors remained matched.
Figure 7.
Longitudinal refractive corrections (top row) and vitreous chamber depths (bottom row) plotted as a function of age for both eyes of representative monkeys reared with red filters over one (right) or both eyes (left). For the monocularly treated monkey, the filled and open symbols represent the treated and control eyes, respectively. The thin lines in the vitreous chamber plot for the binocularly filter-reared monkeys represent the data for the right eyes of the normal control monkeys.
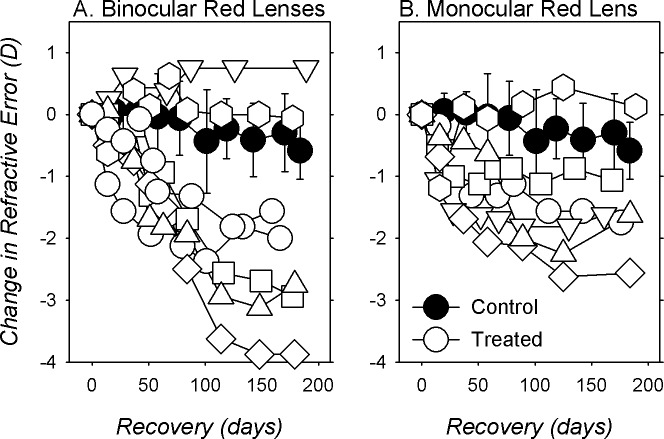
Figure 8 compares the changes in refractive error (normalized to the end-of-treatment refractive errors) for the BRL (open symbols left) and MRL monkeys (open symbols right) during the recovery period to those of age-matched normal monkeys (filled symbols). Over the 6-month period following filter removal, the average change in refraction during the recovery for the BRL monkeys was −1.78 ± 1.64 D (versus −0.68 ± 0.65 D for normal; t = 4.63, P = 0.05), and five of the seven BRL monkeys showed relative myopic changes that were more outside the 95% confidence limits for the normal monkeys. Interestingly, the BRL monkey that had the highest degree of hyperopia at the end of the filter-rearing period (MKY 523, Fig. 2G; inverted triangles in Fig. 8A) showed no signs of recovery. Similar to the majority of BRL monkeys, five of the six MRL monkeys showed larger myopic shifts in their treated eyes during the recovery period than the average normal monkey (Fig. 8B), and the average change in refractive error was twice as large as that observed in normal monkeys (−1.42 ± 0.90 vs. −0.68 ± 0.65 D; t = 4.15, P = 0.06).
Figure 8.
Changes in spherical-equivalent refractive corrections for the right or treated eyes (open symbols) of individual binocularly (A) and monocularly filter-reared monkeys (B) obtained following the removal of the treatment filters. The first symbol in each plot represents the end of the treatment period. The filled symbols represent the mean (±SD) changes in refraction obtained from age-matched normal control monkeys.
Discussion
In both the BRL and MRL subject groups, the eyes viewing through the red filters developed relative hyperopic refractions. The consistency of the results is noteworthy. For example, in the MRL monkeys, the treated eyes of all six monkeys were more hyperopic than their fellow control eyes (Fig. 6C), and all seven of the BRL monkeys developed refractive errors that were more hyperopic than in 90% of the normal monkeys (Fig. 4A). Similar to the experimental refractive errors produced in infant monkeys either by form deprivation44–47 or by optically induced defocus,37,48,49 the refractive errors produced by the red filter regimen were associated with alterations in vitreous chamber depth; specifically, the hyperopic treated eyes exhibited relatively shorter vitreous chambers.
The observed hyperopic refractive errors were very different from predictions based on LCA and the wavelength-dependent variations in the eye's total refracting power. The degree of LCA is slightly larger in macaques than in humans.50 Assuming that the emmetropization process normally targets wavelengths near the peak of the photopic luminosity function, the red filters that were employed in this study should have produced relative myopic shifts in refraction. The magnitude of the predicted shift would depend on which wavelength was targeted when viewing through the red filters. For instance, if the eyes of infant monkeys targeted a wavelength of 660 nm, the halfway point on the rising edge of the transmission function for the red filters (Fig. 1), then it would be expected that the eyes should develop slightly more than 0.5 D of relative myopia, that is, the approximate difference in refracting power between 555 and 660 nm. The fact that virtually all of the red filter–reared eyes exhibited hyperopic shifts suggests that the emmetropization process in the experimental monkeys was not simply targeting the wavelengths that would maximize luminance contrast.
The results of this study differ in a significant way from the findings of previous studies in other species, specifically in fish,23 chickens,14,28 and guinea pigs.26,27 In contrast to the hyperopic refractions found in our monkeys reared with red filters, these previous studies found that restricting ambient lighting to relatively long wavelengths produced myopic shifts in refractive error in comparison to broadband white light or short-wavelength light. There are, however, some similarities between our results and those of these previous studies. Although some previous studies have reported a close correspondence between the induced changes in refractive error and the predicted power changes produced by LCA,14 other studies, particularly those with longer rearing periods, found exaggerated refractive-error changes that were much larger than those required to compensate for LCA.27,28 This pattern of results and the results from our monkeys suggest that reducing potential chromatic cues by restricting the spectral composition of ambient light interferes with emmetropization and supports the hypothesis that chromatic cues may normally contribute to the regulation of refractive development.
It seems unlikely that species differences could have contributed to the differences noted above between our study and those studies using fish, chickens, and guinea pigs. First, a recent investigation in tree shrews51 reported that chronic exposure to long-wavelength light produced hyperopic shifts, as observed in our monkeys. Moreover, our results also differed significantly from the Liu et al.29 findings in rhesus monkeys. In the only previous study on the effects of the spectral composition of ambient lighting on refractive development in monkeys, Liu et al.29 found that emmetropization was essentially unaffected by rearing animals in quasimonochromatic environments. Although two of their nine subjects reared under long-wavelength light (610 nm) exhibited somewhat exaggerated myopic refractive changes, the rest of the animals reared under red light and all seven of those reared under 455 nm light exhibited essentially normal emmetropization. This overall pattern of results suggests that chromatic cues are not essential for normal emmetropization, in agreement with previous observations that rearing animals under quasimonochromatic conditions does not prevent recovery from form deprivation myopia22 or refractive compensation for optically imposed defocus.20,21
Why are the patterns of results obtained in rhesus monkeys in the Liu et al.29 study and in this study so different? There were substantial methodologic differences between the two studies. Whereas our infants were housed in regular cages that allowed distance viewing and our rearing period started at 3 weeks of age, viewing distance was restricted in the Liu et al.29 study, and the altered lighting regimen was initiated at approximately 8 weeks of age. We employed broad, long-wavelength-pass filters to alter the spectral composition of ambient lighting, whereas Liu et al.29 used narrow-band light-emitting diodes (LEDs; 610 nm; half bandwidth = 20 nm). Moreover, the peak of the emission spectra for the red LEDs employed by Liu et al.29 was at a shorter wavelength than the plateau of the transmission spectrum for our red filters. However, in this respect, the long-wavelength lighting employed by Liu et al.29 and that used in this study were within the wavelengths encompassed by the long-wavelength component of the increment threshold spectral sensitivity functions of rhesus monkeys.30 We speculate that the most critical methodologic difference between the two studies was the absolute lighting levels. The red LEDs employed by Liu et al.29 resulted in light levels near 200 lux. On the other hand, the filters that we employed reduced the light levels reaching the eye by a little more than 1 log unit, resulting in an average illuminance level of approximately 50 lux. This may have been a critical difference because Rucker and Wallman17 have demonstrated that at dim luminance levels, lens compensation is generally more effective in white light than under monochromatic illumination, suggesting that reducing light levels can reduce the ability of the emmetropization process to utilize luminance cues to guide ocular growth—in essence, increasing the likelihood that chromatic cues could influence refractive development. The effects of chromatic cues also become more obvious when luminance contrast cues are degraded by astigmatic blur.15 In the study by Liu et al.,29 the light levels were apparently high enough to support normal or near-normal emmetropization even under monochromatic lighting. It is possible that at the lower light levels associated with our filter-rearing regimen there were insufficient luminance cues and/or that chromatic cues dominated emmetropization. In any case, neither our study nor the Liu et al.29 study provided compelling evidence that the primate eye can predictably alter its growth to compensate for changes in the focal plane associated with LCA.
Why did exposure to relatively long-wavelength light promote relative hyperopic shifts in our infant monkeys? Because it is likely that the MRL monkeys fixated with the fellow control eyes, the similarities in treated-eye refractive errors in the MRL and BRL monkeys suggest that potential wavelength-dependent variations in accommodation were not responsible for the hyperopic shifts. Moreover, the fact that the BRL monkeys were consistently and significantly more hyperopic than our control animals reared with neutral density filters over both eyes indicates that the hyperopic shifts were not due simply to a reduction in lighting levels. On the contrary, our ND monkeys, like chickens exposed to comparable white lighting levels (e.g., 50 lux),3 exhibited a tendency to develop relative myopic refractions. The hyperopic refractions observed in our BRL and MRL animals were more likely related to the chromaticity of the ambient lighting. It is possible that the eye can compare or weigh the strengths of the signals for long versus short wavelengths that result from LCA and thus decode the sign of defocus. If that is the case, then a potential reason our animals became hyperopic is that when they were wearing the red filters, the strength of retinal excitation signals for long wavelengths was much stronger than that associated with short wavelengths. The mechanisms regulating eye growth could have interpreted this imbalance between the strengths of long- and short-wavelength signals as chronic myopic defocus and, in the absence of strong luminance cues, effectively slowed eye growth, producing inappropriate hyperopic shifts in refractive error. However, sign of defocus signals derived from comparisons of excitation levels between short (S-cone) and middle- (M-cone) and long-wavelength cone (L-cone) mechanisms would potentially be problematic because variations in the spectral composition of ambient lighting that normally occur outdoors during the course of the day and variations in the color of indoor scenes could produce aberrant error signals.
In this respect, experiments from the Rucker laboratory15–18 have identified several strategies involving LCA that the emmetropization could use to guide eye growth. These strategies are based on color contrast signals and are potentially more robust than strategies based on simple comparisons of relative cone excitation levels. In particular, theoretical analyses by Rucker and Wallman18 have demonstrated that changes in the focus of the eye over time produce different changes in the pattern of luminance and color contrasts. Specifically, they showed that when the degree of hyperopic defocus increases over time (as would occur for an approaching object), luminance contrast decreases in conjunction with decreases in the contrast in the M- and L-cone mechanisms. However, any decreases in S-cone contrast would be smaller and, depending on the level of defocus, the contrast signals in the S-cones could increase (i.e., hyperopic defocus produces changes in luminance contrast and in the balance of color contrast for the S-cone versus the M- and/or L-cone contrast mechanisms). In the case of increasing myopic defocus over time, the L- and M-cone luminance contrast signals decrease, but now the reductions in contrast for the S-cones and M- and L-cones are similar; that is, the balance of color contrast between S-cone and M- and/or L-cone components does not change over time. Most importantly, Rucker and Wallman18 demonstrated that flickering stimuli that simulate these two different scenarios produce predictable changes in ocular growth in young chickens. In our experiments, when the monkeys were viewing through the red filters, the eye would experience changes in luminance contrast with a change in object distance, but a relatively stable balance between the S-cone mechanism versus the M- and/or L-cone color mechanisms, which the emmetropization process could interpret as chronic myopic defocus and thus potentially initiate hyperopic compensating changes in eye growth.
Regardless of the exact mechanism that was responsible for the hyperopic shifts found in the BRL and MRL monkeys, the main findings in this study do not support the hypothesis that environments dominated by long-wavelength lighting are necessarily myopiagenic. On the contrary, the results suggest that exposure to long-wavelength lighting may, at least under certain circumstances, be beneficial in efforts to reduce myopia progression. The results also support the emerging view that the eye can utilize chromatic cues associated with LCA to regulate ocular growth and facilitate emmetropization.15 However, the disparity of results between this and previous studies clearly emphasizes that our understanding of how chromatic cues can influence eye growth is not complete.
Acknowledgments
Supported by National Institutes of Health Grants EY-03611, EY-07551, and EY-021242 and funds from the Vision Cooperative Research Centre and the University of Houston Foundation.
Disclosure: E.L. Smith III, P; L.-F. Hung, None; B. Arumugam, None; B.A. Holden, P; M. Neitz, P; J. Neitz, P
References
- 1. Smith III EL. Charles F. Prentice Award Lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011; 88: 1029–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallman J,, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004; 43: 447–468. [DOI] [PubMed] [Google Scholar]
- 3. Cohen Y,, Belkin M,, Yehezkel O,, Solomon AS,, Polat U. Dependency between light intensity and refractive development under light-dark cycles. Exp Eye Res. 2011; 92: 40–46. [DOI] [PubMed] [Google Scholar]
- 4. Ashby R,, Ohlendorf A,, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009; 50: 5348–5354. [DOI] [PubMed] [Google Scholar]
- 5. Karouta C,, Ashby RS. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci. 2014; 56: 299–309. [DOI] [PubMed] [Google Scholar]
- 6. Norton TT,, Siegwart JT, Jr. Light levels, refractive development, and myopia - a speculative review. Exp Eye Res. 2013; 114: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith III EL, Hung L-F, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012; 53: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rose KA,, Morgan IG,, Smith W,, Burlutsky G,, Mitchell P,, Saw SM. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008; 126: 527–530. [DOI] [PubMed] [Google Scholar]
- 9. Mutti DO,, Marks AR. Blood levels of vitamin D in teens and young adults with myopia. Optom Vis Sci. 2011; 88: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashby RS,, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010; 51: 5247–5253. [DOI] [PubMed] [Google Scholar]
- 11. Smith EL, III, Hung L-F,, Arumugam B,, Huang J. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci. 2013; 54: 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kruger PB,, Mathews S,, Aggarwala KR,, Sanchez N. Chromatic aberration and ocular focus: Fincham revisited. Vision Res. 1993; 33: 1397–1411. [DOI] [PubMed] [Google Scholar]
- 13. Kruger PB,, Mathews S,, Aggarwala KR,, Yager D,, Kruger ES. Accommodation responds to changing contrast of long middle and short spectral-waveband components of the retinal image. Vision Res. 1995; 35: 2415–2429. [DOI] [PubMed] [Google Scholar]
- 14. Seidemann A,, Schaeffel F. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002; 42: 2409–2417. [DOI] [PubMed] [Google Scholar]
- 15. Rucker FJ. The role of luminance and chromatic cues in emmetropization. Ophthalmic Physiol Opt. 2013; 33: 196–214. [DOI] [PubMed] [Google Scholar]
- 16. Rucker FJ,, Wallman J. Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Res. 2009; 49: 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rucker FJ,, Wallman J. Cone signals for spectacle-lens compensation: differential responses to short and long wavelengths. Vision Res. 2008; 48: 1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rucker FJ,, Wallman J. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis. 2012; 12 (6): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rucker FJ,, Osorio D. The effects of longitudinal chromatic aberration and a shift in the peak of the middle-wavelength sensitive cone fundamental on cone contrast. Vision Res. 2008; 48: 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaeffel F,, Howland H. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991; 31: 717–734. [DOI] [PubMed] [Google Scholar]
- 21. Rohrer B,, Schaeffel F,, Zrenner E. Longitudinal chromatic aberration and emmetropization: results from the chicken eye. J Physiol. 1992; 449: 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wildsoet CF,, Howland HC,, Falconer S,, Dick K. Chromatic aberration and accommodation: their role in emmetropization in the chick. Vision Res. 1993; 33: 1593–1603. [DOI] [PubMed] [Google Scholar]
- 23. Kröger RHH,, Fernald R. Regulation of eye growth in the African cichlid fish Haplochromis burtoni. Vision Res. 1994; 34: 1807–1814. [DOI] [PubMed] [Google Scholar]
- 24. Jiang L,, Zhang S,, Schaeffel F,, et al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Res. 2014; 94: 24–32. [DOI] [PubMed] [Google Scholar]
- 25. Qian YF,, Liu R,, Dai JH,, Chen MJ,, Zhou XT,, Chu RY. Transfer from blue light or green light to white light partially reverses changes in ocular refraction and anatomy of developing guinea pigs. J Vis. 2013; 13 (11): 1–9. [DOI] [PubMed] [Google Scholar]
- 26. Long Q,, Chen DH,, Chu RY. Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutan Ocul Toxicol. 2009; 28: 176–180. [DOI] [PubMed] [Google Scholar]
- 27. Liu R,, Qian YF,, He JC,, Hu M,, Zhou XT,, Dai JH. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011; 92: 447–453. [DOI] [PubMed] [Google Scholar]
- 28. Foulds WS,, Barathi VA,, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci. 2013; 54: 8004–8012. [DOI] [PubMed] [Google Scholar]
- 29. Liu R,, Hu M,, He JC,, et al. The effects of monochromatic illumination on early eye development in rhesus monkeys. Invest Ophthalmol Vis Sci. 2014; 55: 1901–1909. [DOI] [PubMed] [Google Scholar]
- 30. Harwerth RS,, Smith EL, III. Rhesus monkey as a model for normal vision of humans. Am J Optom Physiol Opt. 1985; 62: 633–641. [DOI] [PubMed] [Google Scholar]
- 31. Kalloniatis M,, Harwerth RS. Spectral sensitivity and adaptation characteristics of cone mechanisms under white-light adaptation. J Opt Soc Am A. 1990; 7: 1912–1928. [DOI] [PubMed] [Google Scholar]
- 32. Bowmaker JK,, Dartnall HJA,, Lythgoe JN,, Mollon JD. The visual pigments of rods and cones in the rhesus monkey, Macaca mulatta. J Physiol. 1978; 274: 329–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeValois RL,, Morgan MC,, Polson MC,, Mead WR,, Hull EM. Psychophysical studies of monkey vision. 1. Macaque luminosity and color vision tests. Vision Res. 1974; 14: 53–67. [DOI] [PubMed] [Google Scholar]
- 34. Qiao-Grider Y,, Hung L-F,, Kee C-S,, Ramamirtham R,, Smith E, III. Normal ocular development in young rhesus monkeys (Macaca mulatta). Vision Res. 2007; 47: 1424–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bradley DV,, Fernandes A,, Lynn M,, Tigges M,, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999; 40: 214–229. [PubMed] [Google Scholar]
- 36. Kiely PM,, Crewther SG,, Nathan J,, Brennan NA,, Efron N,, Madigan M. A comparison of ocular development of the cynomolgus monkey and man. Clin Vision Sci. 1987; 1: 269–280. [Google Scholar]
- 37. Smith EL, III, Hung L-F. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999; 39: 1415–1435. [DOI] [PubMed] [Google Scholar]
- 38. Huang J,, Hung L-F,, Ramamirtham R,, et al. Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci. 2009; 50: 4033–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hung L-F,, Ramamirtham R,, Huang J,, Qiao-Grider Y,, Smith EL, III. Peripheral refraction in normal infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2008; 49: 3747–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris WF. Algebra of sphero-cylinders and refractive errors and their means, variance, and standard deviation. Am J Optom Physiol Opt. 1988; 65: 794–802. [DOI] [PubMed] [Google Scholar]
- 41. Kee C-S,, Hung L-F,, Qiao-Grider Y,, Roorda A,, Smith EL, III. Effects of optically imposed astigmatism on emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2004; 45: 1647–1659. [DOI] [PubMed] [Google Scholar]
- 42. Hung L-F,, Ramamirtham R,, Wensveen JM,, Harwerth RS,, Smith EL. III. Objective and subjective refractive error measurements in monkeys. Optom Vis Sci. 2012; 89: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kee C-S,, Hung L-F,, Qiao Y,, Smith EL, III. Astigmatism in infant monkeys reared with cylindrical lenses. Vision Res. 2003; 43: 2721–2739. [DOI] [PubMed] [Google Scholar]
- 44. Smith EL, III, Harwerth RS,, Crawford MLJ,, von Noorden GK. Observations on the effects of form deprivation on the refractive status of the monkey. Invest Ophthalmol Vis Sci. 1987; 28: 1236–1245. [PubMed] [Google Scholar]
- 45. Tigges M,, Tigges J,, Fernandes A,, Eggers HM,, Gammon JA. Postnatal axial eye elongation in normal and visually deprived rhesus monkeys. Invest Ophthalmol Vis Sci. 1990; 31: 1035–1046. [PubMed] [Google Scholar]
- 46. Wiesel TN,, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkyes. Nature. 1977; 266: 66–68. [DOI] [PubMed] [Google Scholar]
- 47. Troilo D,, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vision Res. 1993; 33: 1311–1324. [DOI] [PubMed] [Google Scholar]
- 48. Hung L-F,, Crawford MLJ,, Smith EL, III. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995; 1: 761–765. [DOI] [PubMed] [Google Scholar]
- 49. Judge SJ,, Graham B. Differential ocular growth of infant marmoset (Callithrix jacchus jacchus) induced by optical anisometropia combined with alternating occlusion. J Physiol (Lond). 1995; 485: 27. [Google Scholar]
- 50. Hughes A. A useful table of reduced schematic eyes for vertebrates which includes computed longitudinal chromatic aberrations. Vision Res. 1979; 19: 1273–1275. [DOI] [PubMed] [Google Scholar]
- 51. Gawne TJ,, Siegwart JT,, Ward AH,, Norton TT. Temporally modulated long-wavelength light radically slows eye growth in young tree shrews (E-Abstract 59.09/Z4). Soc Neurosci. 2014.