Abstract
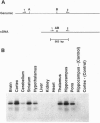
Sequential polymerase chain reaction experiments were performed to amplify a unique sequence representing a guanine nucleotide-binding protein (G-protein)-coupled receptor from rat hypothalamic cDNA. Degenerate oligonucleotides corresponding to conserved amino acids from transmembrane domains III, V, and VI of known receptors [5-HT1A, 5-HT1C, and 5-HT2; 5-HT is serotonin (5-hydroxytryptamine)] were used as primers for the sequential reactions. The resulting product was subcloned and used to screen a rat genomic library to identify a full-length clone (MR77) containing an intronless open reading frame encoding a 366-amino acid seven-transmembrane domain protein. The human homolog was isolated, and its encoded protein had 93% overall amino acid identity with the rat sequence. Within the conserved transmembrane domains, the sequences exhibit approximately 52%, 59%, 65%, and 68% amino acid identity with the known rat 5-HT1A, rat 5-HT1B, rat 5-HT1D, and human 5-HT1E receptors, respectively. MR77 was subcloned into a eukaryotic expression vector system and expressed in CosM6 cells. Studies on broken cell preparations indicate that the expressed receptor exhibits 125I-labeled d-lysergic acid diethylamide (LSD) binding that can be displaced by serotonin but not by other biogenic amines. The specific binding is displaced by the selective 5-HT1D agonist sumatriptan but not by the mixed 5-HT1A/1D agonist 5-carboxyamidotryptamine. 125I-labeled LSD binding was competitively antagonized by the ergot alkaloids methysergide and ergotamine. HeLa cells transfected with the MR77 gene exhibited inhibition of adenylate cyclase in response to serotonin. MR77 is expressed at low levels throughout the brain, with the greatest expression in the cortex, hippocampus, and striatum. MR77 thus represents a 5-HT receptor of the 5-HT1 class, and we propose that, based on the pharmacological characterization, MR77 represents an additional 5-HT1E-like receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert P. R., Zhou Q. Y., Van Tol H. H., Bunzow J. R., Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990 Apr 5;265(10):5825–5832. [PubMed] [Google Scholar]
- Amlaiky N., Ramboz S., Boschert U., Plassat J. L., Hen R. Isolation of a mouse "5HT1E-like" serotonin receptor expressed predominantly in hippocampus. J Biol Chem. 1992 Oct 5;267(28):19761–19764. [PubMed] [Google Scholar]
- Baron B. M., Siegel B. W. Alpha 2-adrenergic and muscarinic cholinergic receptors have opposing actions on cyclic AMP levels in SK-N-SH human neuroblastoma cells. J Neurochem. 1989 Aug;53(2):602–609. doi: 10.1111/j.1471-4159.1989.tb07376.x. [DOI] [PubMed] [Google Scholar]
- Baron B. M., Siegel B. W. p-[125I]iodoclonidine, a novel radiolabeled agonist for studying central alpha 2-adrenergic receptors. Mol Pharmacol. 1990 Sep;38(3):348–356. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dechant K. L., Clissold S. P. Sumatriptan. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the acute treatment of migraine and cluster headache. Drugs. 1992 May;43(5):776–798. doi: 10.2165/00003495-199243050-00010. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A., Maayani S., Wolfe B. B. Subtypes of receptors for serotonin. Annu Rev Pharmacol Toxicol. 1990;30:307–348. doi: 10.1146/annurev.pa.30.040190.001515. [DOI] [PubMed] [Google Scholar]
- Hamblin M. W., Metcalf M. A. Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor. Mol Pharmacol. 1991 Aug;40(2):143–148. [PubMed] [Google Scholar]
- Hartig P. R., Branchek T. A., Weinshank R. L. A subfamily of 5-HT1D receptor genes. Trends Pharmacol Sci. 1992 Apr;13(4):152–159. doi: 10.1016/0165-6147(92)90053-9. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L., Azmitia E. C. Structure and function of the brain serotonin system. Physiol Rev. 1992 Jan;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Dixon R. A., Frielle T., Dohlman H. G., Bolanowski M. A., Sigal I. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B. K., Frielle T., Collins S., Yang-Feng T., Kobilka T. S., Francke U., Lefkowitz R. J., Caron M. G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987 Sep 3;329(6134):75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leonhardt S., Herrick-Davis K., Titeler M. Detection of a novel serotonin receptor subtype (5-HT1E) in human brain: interaction with a GTP-binding protein. J Neurochem. 1989 Aug;53(2):465–471. doi: 10.1111/j.1471-4159.1989.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Levy F. O., Gudermann T., Birnbaumer M., Kaumann A. J., Birnbaumer L. Molecular cloning of a human gene (S31) encoding a novel serotonin receptor mediating inhibition of adenylyl cyclase. FEBS Lett. 1992 Jan 20;296(2):201–206. doi: 10.1016/0014-5793(92)80379-u. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Hoffman B. J., Snutch T. P., van Dyke T., Levine A. J., Hartig P. R., Lester H. A., Davidson N. cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4332–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister G., Charlesworth A., Snodin C., Beer M. S., Noble A. J., Middlemiss D. N., Iversen L. L., Whiting P. Molecular cloning of a serotonin receptor from human brain (5HT1E): a fifth 5HT1-like subtype. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5517–5521. doi: 10.1073/pnas.89.12.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Teitler M. Quantitative autoradiography of 5-CT-sensitive (5-HT1D) and 5-CT-insensitive (5-HT1E) serotonin receptors in human brain. Neurosci Lett. 1992 Mar 2;136(2):223–226. doi: 10.1016/0304-3940(92)90054-b. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Bach A. W., Wozny M., Taleb O., Dal Toso R., Shih J. C., Seeburg P. H. Structure and functional expression of cloned rat serotonin 5HT-2 receptor. EMBO J. 1988 Dec 20;7(13):4135–4140. doi: 10.1002/j.1460-2075.1988.tb03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands E., Candelore M. R., Cheung A. H., Hill W. S., Strader C. D., Dixon R. A. Mutational analysis of beta-adrenergic receptor glycosylation. J Biol Chem. 1990 Jun 25;265(18):10759–10764. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese T. M., Fraser C. M. In vitro mutagenesis and the search for structure-function relationships among G protein-coupled receptors. Biochem J. 1992 Apr 1;283(Pt 1):1–19. doi: 10.1042/bj2830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M. M., Laurie D. J., Seeburg P. H., Bach A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J. 1991 Dec;10(13):4017–4023. doi: 10.1002/j.1460-2075.1991.tb04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]