Abstract
Temozolomide (TMZ) as a concomitant and adjuvant chemotherapy to radiotherapy following maximal surgical resection is the established standard therapy for patients with newly diagnosed high-grade glioma. However, detailed analysis of chemotherapy-induced nausea and vomiting (CINV) associated with concomitant TMZ has not been sufficiently described. We prospectively analyzed the profile of CINV associated with concomitant TMZ. Eighteen consecutive patients with newly diagnosed high-grade glioma treated with concomitant chemoradiotherapy including TMZ were enrolled. CINV was recorded using a daily diary including nausea assessment, emetic episodes, degree of appetite suppression, and antiemetic medication use. The observed incidence rates of all grade nausea, moderate/severe (CTC grade 2, 3) nausea, emetic episodes, and appetite suppression for the overall period were 89%, 39%, 39%, and 83%, respectively. Moderate/severe nausea and severe (CTC grade 3) appetite suppression were frequently observed during the delayed phase of the treatment. Emetic episodes and moderate/severe nausea were significantly correlated with female gender. Moderate/severe nausea and severe appetite suppression were significantly correlated with low lymphocyte counts before chemoradiotherapy. For CINV associated with concomitant TMZ, enhanced antiemetic therapy focused on the delayed phase of the treatment will likely be beneficial, especially in female patients with a low lymphocyte count before chemoradiotherapy.
Keywords: temozolomide, chemotherapy-induced nausea and vomiting, concomitant, delayed phase
Introduction
Concomitant and adjuvant temozolomide (TMZ, Temodal, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, New Jersey, USA), an oral alkylating agent, and radiotherapy following maximal surgical resection have been established as the worldwide standard therapy for patients with newly diagnosed malignant gliomas.1) One of the most distressing side effects associated with TMZ is chemotherapy-induced nausea and vomiting (CINV). Prophylactic antiemetic therapy including 5-hydroxytriptamine-3 (5-HT3) antagonists and corticosteroids is recommended for CINV associated with TMZ, which is classified as a moderate emetogenic oral agent in several antiemetic guidelines regardless of a concomitant or adjuvant regimen.2–4) However, concomitant TMZ is a unique regimen with multiple-day, long-term administration. Some doubts exist about whether standard antiemetic therapy is applicable for this kind of chemotherapy with such a unique regimen. Although a few reports have been published regarding CINV associated with a 5-day regimen of adjuvant TMZ,5–7) detailed analysis of CINV associated with a long-term regimen of concomitant TMZ has not been sufficiently described. Determination of optimal antiemetic therapy will depend on an accurate understanding of the profile of CINV. Accordingly, we prospectively analyzed the profile of CINV associated with concomitant TMZ and prophylactic antiemetic therapy consisting of addition of aprepitant to the standard antiemetic therapy.
Materials and Methods
We investigated 18 consecutive patients with newly diagnosed supratentorial high-grade glioma (grade III–IV) who were treated with concomitant chemoradiotherapy including TMZ at Tsukuba University Hospital from July 2011 to September 2012 during the registration period of 2 years. Patients were eligible if they were adults (> 18 years old) and had a Karnofsky performance status (KPS) of 60 or more. Patients were not eligible for participation in the study if they could not record notes in a self-reported diary due to neurological deficits such as consciousness disturbances or aphasia, if they experienced vomiting during the 24 h before the first administration of TMZ, or if they had any of the following abnormal laboratory values: absolute neutrocyte count < 1,000/μl, platelet count < 100,000/μl, aspartate aminotransferase > 2.5 × the upper limit of normal, alanine aminotransferase > 2.5 × the upper limit of normal, bilirubin > 1.5 × the upper limit of normal, or creatinine > 1.5 × the upper limit of normal. The radiation schedule for patients with high-grade glioma treated at our facilities consisted of two protocols. As the standard radiotherapy, daily conventional fractionated photon radiotherapy (CRT) of 2 Gy was administered five times per week, amounting to a total dose of 60 Gy. For selected patients, proton therapy (PT) for a total dose of 96.6 GyE in 56 fractions was administered.8) CRT was delivered in 30 fractions (30 days), and PT in 56 fractions (28 days). Concomitant chemotherapy consisted of TMZ at a daily dose of 75 mg/m2 from the first until the last day of radiotherapy. Accordingly, TMZ administration varied from 42 days to 48 days depending on radiation modalities used and radiotherapy non-operating days. Discontinuation of TMZ was decided according to a slightly modified standard protocol (absolute neutrocyte count < 1,500/μl, platelet count < 100,000/μl, and prolonged lymphopenia < 200/μl).9)
All patients in the study received oral ramosetron 0.1 mg and oral dexamethasone 4 mg before TMZ administration on Day 1. All patients also received oral aprepitant 125 mg before TMZ administration on Day 1, followed by oral aprepitant 80 mg daily on Days 2–5. Patients completed a daily diary in which the degree of nausea, number of emetic episodes, and degree of appetite suppression were recorded based on Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. In this study, the degree of CINV was reported as mild, moderate, or severe, corresponding to CTC grades 1, 2, and 3, respectively. Patients also recorded all uses of rescue antiemetic medication. The daily diary was recorded until the last day of chemoradiotherapy.
To determine the predictive factors associated with CINV, several factors including age, sex, KPS, World Health Organization (WHO) grading of tumors, extent of removal, modality of radiotherapy, white blood cell (WBC) count before chemoradiotherapy (pre-WBC), neutrocyte count before chemoradiotherapy (pre-neutro value), and lymphocyte count before chemoradiotherapy (pre-lymph value) were analyzed, and each cell count cut-off line was determined to be 6,000/μl, 3,000/μl, and 1,200/μl, respectively, based on our previous study.9)
The study was approved by the institutional ethics committees. Written and signed informed consent was obtained from all patients before study entry.
Statistical analyses were performed using SPSS software (version 21; SPSS, Inc., Chicago, Illinois, USA). The Fisher’s exact test was used to evaluate the difference in categorical variables. The Mann-Whitney U test was used to evaluate the difference between group median values. The paired Student’s t-test was used to evaluate the change in body weight. A value of p < 0.05 was considered to be statistically significant in all analyses.
Results
The characteristics of the 18 patients we studied are summarized in Table 1. Included in the analysis were 11 males and 7 females aged 18–75 years (mean, 49.7 years). Three (16.7%) patients had a KPS of 100, seven (38.9%) had a KPS of 90, four (22.2%) had a KPS of 80, two (11.1%) had a KPS of 70, and two (11.1%) had a KPS of 60. According to the 2007 WHO classification, two patients had grade III glioma and 16 had grade IV glioma. Surgical resection resulted in gross total resection of the tumor in eight patients (44.4%), subtotal resection in two (11.1%), partial resection in seven (38.9%), and biopsy in one (5.6%). Eleven patients (61.1%) received CRT, and seven (38.9%) received PT. The mean pre-WBC, pre-neutro values, and pre-lymph values were 5,522.2 ± 1,811.3; 3,620.6 ± 1,421.1; and 1,412.7 ± 623.6, respectively.
Table 1.
Patient characteristics
| Characteristics | No. of patients | % |
|---|---|---|
| Age (yrs) | ||
| Mean ± SD | 49.7 ± 17 | |
| Range | 18–75 | |
| Gender | ||
| Male | 11 | 61.1 |
| Female | 7 | 38.9 |
| KPS | ||
| 100 | 3 | 16.7 |
| 90 | 7 | 38.9 |
| 80 | 4 | 22.2 |
| 70 | 2 | 11.1 |
| 60 | 2 | 11.1 |
| Pathology | ||
| WHO grade 4 glioma | 16 | 88.9 |
| WHO grade 3 glioma | 2 | 11.1 |
| Extent of resection | ||
| GTR | 8 | 44.4 |
| STR | 2 | 11.1 |
| PR | 7 | 38.9 |
| B | 1 | 5.6 |
| Radiotherapy | ||
| CRT | 11 | 61.1 |
| PT | 7 | 38.9 |
B: biopsy, CRT: conventional radiotherapy, GTR: gross total resection, KPS: Karnofsky performance status, PR: partial resection, PT: proton therapy, SD: standard deviation, STR: subtotal resection, WHO: World Health Organization.
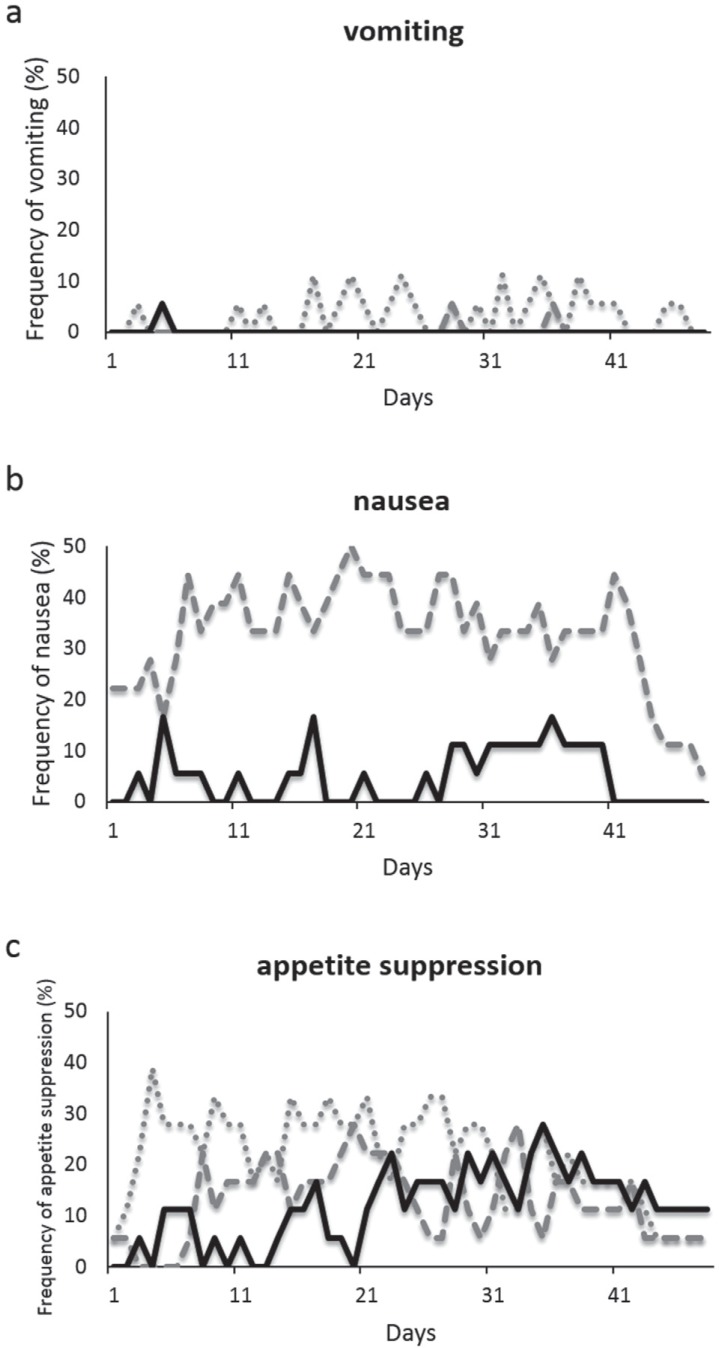
Despite the prophylactic antiemetic therapy, 39% of the patients experienced moderate/severe nausea (CTC grade 2, 3) during the overall study period. Surprisingly, 89% of the patients experienced nausea when including mild CTC grade 1. The daily incidence and severity of nausea are shown in Fig. 1b. Moderate/severe nausea was observed relatively frequently during the latter half of the treatment period, i.e., after Day 21 (delayed phase), whereas mild nausea was observed at an almost constant rate throughout the overall period. As for the use of rescue antiemetic medication, 61% of the patients required additional use of ramosetron, especially during the delayed phase of the treatment. Thirty-nine percent of patients reported emesis during the overall study period. The daily incidence of emesis and its prevalence are shown in Fig. 1a. The prevalence of emetic episodes was similar throughout the overall period. Moderate/severe emetic episodes (CTC grade 2, 3) were only observed occasionally. Eighty-three percent of patients experienced a varied degree of appetite suppression during the overall study period. The daily incidence and severity of appetite suppression are shown in Fig. 1c. Severe appetite suppression (CTC grade 3) was observed in 67% of the patients and prominently observed during the delayed phase of the treatment. Because the severity of appetite suppression was analyzed according to daily self-reported diary, the cases that did not require intravenous infusion or tube feeding in spite of inadequate oral caloric and fluid intake were classified as the incidence of severe appetite suppression. In fact, 33% of the patients underwent intravenous infusion and 72% of the patients had their body weight decreased. The mean change in body weight after treatment in all patients was −2.8 ± 2.9 kg (p < 0.001).
Fig. 1.
a: Frequency of vomiting during concomitant chemoradiotherapy including TMZ. Dotted line: CTC grade 1; dashed line: CTC grade 2; solid line: CTC grade 3. b: Frequency of nausea during concomitant chemoradiotherapy including TMZ. Dashed line: CTC grade 1; solid line: CTC grade 2/3. c: Frequency of appetite suppression during concomitant chemoradiotherapy including TMZ. Dotted line: CTC grade 1; dashed line: CTC grade 2; solid line: CTC grade 3. The degree of CINV was evaluated based on CTCAE version 4.0. CINV: chemotherapy-induced nausea and vomiting, CTCAE: Common Terminology Criteria for Adverse Events.
The results of statistical analysis of the association between CINV and several exploratory factors are shown in Table 2. Female gender was significantly correlated with a high incidence of moderate/severe nausea and emetic episodes (Fisher’s exact test). Low pre-lymph values (less than 1,200/μl) were significantly correlated with a high incidence of moderate/severe nausea and severe appetite suppression (Fisher’s exact test). The median pre-lymph value (1,073.6/μl) in patients with moderate/severe nausea was significantly lower than that (1,531.2/μl) in patients without moderate/severe nausea (Mann-Whitney U test). Similarly, the median pre-lymph value (1,131.9/μl) in patients with severe appetite suppression was lower than that (1,470.2/μl) in patients without severe appetite suppression, although this difference was not statistically significant (Mann-Whitney U test). No significant correlation was observed between sex and lymphocyte count (Mann-Whitney U test). Age, KPS, WHO grading of tumors, extent of removal, modality of radiotherapy, pre-WBC count, and pre-neutro values were not associated with the incidence of CINV.
Table 2.
Associations between several factors and chemotherapy-induced nausea and vomiting
| Factor | Moderate/severe nausea (CTC grade 2, 3) | Vomiting | Severe appetite suppression (CTC grade 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p value (Fisher’s exact test) | Yes | No | p value (Fisher’s exact test) | Yes | No | p value (Fisher’s exact test) | |
| Age | |||||||||
| < 59 | 4 | 7 | 1.000 | 3 | 8 | 0.332 | 7 | 4 | 1.000 |
| 60 ≤ | 3 | 4 | 4 | 3 | 5 | 2 | |||
| Gender | |||||||||
| Male | 2 | 9 | 0.049 | 2 | 9 | 0.049 | 6 | 5 | 0.316 |
| Female | 5 | 2 | 5 | 2 | 6 | 1 | |||
| KPS | |||||||||
| 60–70 | 2 | 2 | 1.000 | 2 | 2 | 1.000 | 2 | 2 | 0.569 |
| 80–100 | 5 | 9 | 5 | 9 | 10 | 4 | |||
| Pathology | |||||||||
| WHO grade 4 glioma | 6 | 10 | 1.000 | 6 | 10 | 1.000 | 11 | 5 | 1.000 |
| WHO grade 3 glioma | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Extent of resection | |||||||||
| GTR, STR | 5 | 5 | 0.367 | 4 | 6 | 1.000 | 8 | 2 | 0.321 |
| PR, B | 2 | 6 | 3 | 5 | 4 | 4 | |||
| Radiotherapy | |||||||||
| CRT | 4 | 7 | 1.000 | 4 | 7 | 1.000 | 6 | 5 | 0.316 |
| PT | 3 | 4 | 3 | 4 | 6 | 1 | |||
| pre-WBC | |||||||||
| < 6,000 | 6 | 6 | 0.316 | 4 | 8 | 0.627 | 8 | 4 | 1.000 |
| 6,000 ≤ | 1 | 5 | 3 | 3 | 4 | 2 | |||
| pre-neutro | |||||||||
| < 3,000 | 5 | 4 | 0.335 | 3 | 6 | 1.000 | 6 | 3 | 1.000 |
| 3,000 ≤ | 2 | 7 | 4 | 5 | 6 | 3 | |||
| Pre-lymph | |||||||||
| < 1,200 | 5 | 2 | 0.049 | 3 | 4 | 1.000 | 7 | 0 | 0.038 |
| 1,200 ≤ | 2 | 9 | 4 | 7 | 5 | 6 | |||
B: biopsy, CRT: conventional radiotherapy, GTR: gross total resection, KPS: Karnofsky performance status, PR: partial resection, pre-lymph: lymphocyte counts before chemoradiotherapy, pre-neutro: neutrocyte counts before chemoradiotherapy, pre-WBC: white blood cell counts before chemoradiotherapy, PT: proton therapy, STR: subtotal resection, WHO: World Health Organization.
Discussion
In the present study, the observed incidence rates of all grade nausea, moderate/severe nausea, emetic episodes, and appetite suppression associated with concomitant use of TMZ were 89%, 39%, 39%, and 83%, respectively.
In a previous study that focused on the treatment efficacy of concomitant TMZ, the incidence rates of moderate nausea/vomiting (CTC grade 2) and severe nausea/vomiting (CTC grade 3, 4) were reported to be 13% and < 1%, respectively.1) No description was included in this previous report of how to evaluate CINV events. Furthermore, patients did not receive established prophylactic antiemetic therapy, and the timing of CINV and the incidence rate of mild CINV (CTC grade 1) were not described. Other previous reports concerning CINV associated with TMZ have only described cases with a 5-day regimen of adjuvant use of TMZ.5–7) Thus, this is the first report of detailed analysis of CINV associated with concomitant TMZ. The current results show that even with appropriate prophylactic antiemetic therapy, approximately 90% of patients treated with concomitant chemoradiotherapy including TMZ still suffer from varied degrees of nausea (CTC grades 1–3). Regarding the timing of CINV, moderate/severe nausea and particularly severe appetite suppression tended to develop during the delayed phase of the treatment. These results indicate the need for further improvement in antiemetic therapy that is particularly focused on the delayed phase for a long-term chemotherapeutic regimen of concomitant TMZ. CINV associated with TMZ has been thought to be readily controlled with standard antiemetics, but this idea may need to be reconsidered.10)
TMZ is classified as a moderate emetogenic agent by several antiemetic guidelines such as the Multinational Association of Supportive Care in Cancer (MASCC) guidelines, the American Society of Clinical Oncologists (ASCO) guidelines, and the National Comprehensive Cancer Network (NCCN) guidelines.2–4) Prophylactic antiemetic therapy in the present study was determined based on these guidelines. In addition to 5-HT3 antagonists and corticosteroids that are generally recommended for moderate emetic treatment by these guidelines, aprepitant, a potent and selective oral nonpeptide antagonist of the neurokinin-1 receptor that is known to protect against delayed emesis, was employed in the present study.11) Nevertheless, in this study, severe appetite suppression and moderate/severe nausea, especially during the delayed phase of the treatment, were not sufficiently controlled. Reports have been published concerning the difficulty in preventing CINV in patients receiving multiple-day chemotherapy compared to preventing CINV in those receiving single-day chemotherapy.12) The concomitant regimen of TMZ consists of multiple-day, long-term chemotherapy. Thus, daily, continuous emetogenic stimuli result in an overlap of acute and delayed CINV, particularly during the delayed phase of the treatment, leading to difficulty in determining the optimal strategy. Current updated antiemetic guidelines published after the antiemetic regimen of our study had been determined to recommend the use of palonosetron, a second-generation 5-HT3 receptor antagonist with a prolonged half-life that is reported to be more effective than first-generation 5-HT3 receptor antagonists for preventing acute and delayed CINV.13–15) The efficacy of palonosetron and aprepitant for preventing CINV during multiple-day chemotherapy has been reported.12,16) However, in the present study, addition of single-cycle administration of aprepitant to standard antiemetic therapy poorly prevented CINV, especially during the delayed phase of the treatment. Multiple-dose administration of palonosetron and multiple-cycle administration of aprepitant during the concomitant regimen of TMZ may prevent CINV during the delayed phase of multiple-day, long-term chemotherapy.
Interestingly, as a result of exploratory factor analysis, low pre-lymph values (less than 1,200/μl) as well as female gender were identified as predictive factors for CINV associated with concomitant TMZ. In many previous reports, female gender has been well recognized as a significant risk factor for CINV, although the exact mechanism underlying the increased risk for female patients remains unclear.17,18) In a few previous reports, the low lymphocyte count before treatment has been reported as a potential biomarker to predict the risk of chemotherapy toxicity, although the particular relationship between the low lymphocyte count and chemotherapy toxicity is unclear.19,20) In a previous study, we identified low pre-lymph values (less than 1,200/μl) as a predictive factor for severe (CTC grade 4) lymphopenia associated with concomitant TMZ.9) Furthermore, we have also demonstrated that severe lymphopenia during concomitant TMZ is associated with the occurrence of other severe adverse effects including severe infections and CINV.9) Thus, low pre-lymph values might become a predictive factor for CINV that is associated with severe lymphopenia during concomitant TMZ, even though the exact mechanism is still unclear. Although some type of confounding influence mediating the correlation between low pre-lymph values and CINV may exist, pre-lymph values will be important for readily predicting the occurrence of CINV before beginning concomitant TMZ.
The major limitation of the present study is the small number of patients included, and this may limit the impact of our conclusions. Nevertheless, the present study has profound importance because we focused specifically on the analysis of the incidence rates of CINV associated with concomitant TMZ, which currently appears to be underestimated. In general, studies designed to specifically analyze adverse events are considered more likely to find an elevated rate of such events compared to studies focused on treatment efficacy such as phase III trials.21) No study that focuses on the incidence rates of CINV associated with concomitant TMZ has been reported to date, and the present study revealed unexpected high rates of CINV for the first time. Given the unexpected high rates of CINV, we report the urgent need for further improvement in antiemetic therapy for concomitant chemoradiotherapy including TMZ. We have therefore recently begun a new prospective study using improved antiemetic therapy.
In conclusion, for CINV associated with concomitant TMZ, enhanced antiemetic therapy focused on the delayed phase of the treatment will likely be beneficial, especially for female patients with a low lymphocyte count before beginning chemoradiotherapy.
References
- 1). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 2). Roila F, Hesketh PJ, Herrstedt J, Antiemetic Subcommitte of the Multinational Association of Supportive Care in Cancer : Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 17: 20– 28, 2006. [DOI] [PubMed] [Google Scholar]
- 3). American Society of Clinical Oncology. Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM: American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24: 2932– 2947, 2006. [DOI] [PubMed] [Google Scholar]
- 4). National Comprehensive Cancer Network : Antiemesis. NCCN Clinical Practice Guidelines in Oncology v.1.2010. http://nccn.org. [DOI] [PubMed]
- 5). Bower M, Newlands ES, Bleehen NM, Brada M, Begent RJ, Calvert H, Colquhoun I, Lewis P, Brampton MH: Multicentre CRC phase II trial of temozolomide in recurrent or progressive high-grade glioma. Cancer Chemother Pharmacol 40: 484– 488, 1997. [DOI] [PubMed] [Google Scholar]
- 6). Nishikawa R, Shibui S, Maruno M, Sugiyama K, Sato S, Fujimaki T, Takahashi H, Wakabayashi T, Takahashi J, Kochi M, Nakamura H, Sawamura Y, Ikeda J, Hori T, Aoki T, Matsutani M: [Efficacy and safety of monotherapy with temozolomide in patients with anaplastic astrocytoma at first relapse—a phase II clinical study]. Gan To Kagaku Ryoho 33: 1279– 1285, 2006. [PubMed] [Google Scholar]
- 7). Rozzi A, Nardoni C, Corona M, Restuccia MR, Fabi A, Bria E, Minniti G, Lanzetta G: Palonosetron for the prevention of chemotherapy-induced nausea and vomiting in glioblastoma patients treated with temozolomide: a phase II study. Support Care Cancer 19: 697– 701, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Matsuda M, Yamamoto T, Ishikawa E, Nakai K, Zaboronok A, Takano S, Matsumura A: Prognostic factors in glioblastoma multiforme patients receiving high-dose particle radiotherapy or conventional radiotherapy. Br J Radiol 84 (Spec No 1): S54– S60, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Ishikawa E, Yamamoto T, Sakamoto N, Nakai K, Akutsu H, Tsuboi K, Takano S, Matsumura A: Low peripheral lymphocyte count before focal radiotherapy plus concomitant temozolomide predicts severe lymphopenia during malignant glioma treatment. Neurol Med Chir (Tokyo) 50: 638–644, 2010. [DOI] [PubMed] [Google Scholar]
- 10). Trinh VA, Patel SP, Hwu WJ: The safety of temozolomide in the treatment of malignancies. Expert Opin Drug Saf 8: 493– 499, 2009. [DOI] [PubMed] [Google Scholar]
- 11). Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F, Aprepitant Protocol 054 Study Group : Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97: 3090– 3098, 2003. [DOI] [PubMed] [Google Scholar]
- 12). Navari RM: Prevention of emesis from multiple-day and high-dose chemotherapy regimens. J Natl Compr Canc Netw 5: 51– 59, 2007. [DOI] [PubMed] [Google Scholar]
- 13). Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH, American Society of Clinical Oncology : Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29: 4189– 4198, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D, ESMO/MASCC Guidelines Working Group : Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21 (Suppl 5): v232– v243, 2010. [DOI] [PubMed] [Google Scholar]
- 15). Botrel TE, Clark OA, Clark L, Paladini L, Faleiros E, Pegoretti B: Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer 19: 823– 832, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Jordan K, Kinitz I, Voigt W, Behlendorf T, Wolf HH, Schmoll HJ: Safety and efficacy of a triple antiemetic combination with the NK-1 antagonist aprepitant in highly and moderately emetogenic multiple-day chemotherapy. Eur J Cancer 45: 1184– 1187, 2009. [DOI] [PubMed] [Google Scholar]
- 17). Hesketh PJ: Treatment of chemotherapy-induced emesis in the 1990s: impact of the 5-HT3 receptor antagonists. Support Care Cancer 2: 286– 292, 1994. [DOI] [PubMed] [Google Scholar]
- 18). Hesketh PJ, Grunberg SM, Herrstedt J, de Wit R, Gralla RJ, Carides AD, Taylor A, Evans JK, Horgan KJ: Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer 14: 354– 360, 2006. [DOI] [PubMed] [Google Scholar]
- 19). Arrieta O, Michel Ortega RM, Villanueva-Rodríguez G, Serna-Thomé MG, Flores-Estrada D, Diaz-Romero C, Rodríguez CM, Martínez L, Sánchez-Lara K: Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 10: 50, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Arrieta Ó, Núñez-Valencia C, Reynoso-Erazo L, Alvarado S, Flores-Estrada D, Angulo LP, Oñate-Ocaña LF: Health-related quality of life in patients with lung cancer: validation of the Mexican-Spanish version and association with prognosis of the EORTC QLQ-LC13 questionnaire. Lung Cancer 77: 205– 211, 2012. [DOI] [PubMed] [Google Scholar]
- 21). Bock HC, Puchner MJ, Lohmann F, Schütze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A: First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev 33: 441– 449, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]