Abstract
Lithium-associated hyperparathyroidism is the leading cause of hypercalcemia in lithium-treated patients. Lithium may lead to exacerbation of pre-existing primary hyperparathyroidism or cause an increased set-point of calcium for parathyroid hormone suppression, leading to parathyroid hyperplasia. Lithium may cause renal tubular concentration defects directly by the development of nephrogenic diabetes insipidus or indirectly by the effects of hypercalcemia. In this study, we present a female patient on long-term lithium treatment who was evaluated for hypercalcemia. Preoperative imaging studies indicated parathyroid adenoma and multinodular goiter. Parathyroidectomy and thyroidectomy were planned. During the postoperative course, prolonged intubation was necessary because of agitation and delirium. During this period, polyuria, severe dehydration, and hypernatremia developed, which responded to controlled hypotonic fluid infusions and was unresponsive to parenteral desmopressin. A diagnosis of nephrogenic diabetes insipidus was apparent. A parathyroid adenoma and multifocal papillary thyroid cancer were detected on histopathological examination. It was thought that nephrogenic diabetes insipidus was masked by hypercalcemia preoperatively. A patient on lithium treatment should be carefully followed up during or after surgery to prevent life-threatening complications of previously unrecognized nephrogenic diabetes insipidus, and the possibility of renal concentrating defects on long-term lithium use should be sought, particularly in patients with impaired consciousness.
Keywords: Lithium, nephrogenic diabetes insipidus, hyperparathyroidism
INTRODUCTION
Lithium salts are commonly used in the treatment and prophylaxis of unipolar, bipolar affective disorders, recurrent depression, and aggressive or self-mutilating behavior (1). A variety of side effects may develop during lithium treatment. Lithium-induced thyroid dysfunction is the most widely recognized disorder (2). Lesser known side effects involve calcium and water homeostasis: hypercalcemia and nephrogenic diabetes insipidus. Lithium-induced nephrogenic diabetes insipidus is reported to occur in up to 20% of patients (2).
The mechanisms leading to lithium-induced nephrogenic diabetes insipidus include inhibition of arginine vasopressin (AVP)-induced translocation of aquaporine-2 (AQP2) to the renal tubular apical membrane; inhibition of phosphorylation of AQP2, which results in inhibition of membrane transport and effectiveness; and inhibition of AQP2 gene expression during long term-use (3). Thereby, free water reabsorption is disturbed, leading to polyuria and hypo-osmolar urine. It is difficult to recognize this entity in the setting of hypercalcemia, because hypercalcemia itself is a well-known cause of renal tubular concentration defects, leading to polyuria (4). Parathyroid hyperplasia and solitary or multiple parathyroid adenomas have been reported in lithium-associated hyperparathyroidism (5).
In this study we present a 52-year-old hypercalcemic female on lithium therapy for 9 years with a diagnosis of bipolar affective disorder who developed lithium-induced nephrogenic diabetes insipidus, leading to severe hypernatremia after parathyroid and thyroid surgery.
CASE PRESENTATION
A 52-year-old female with a 25-year history of bipolar disorder presented with hypercalcemia. She had been taking lithium carbonate (900 mg daily for 9 years) and carbamazepine (600 mg daily for 3 years) for bipolar affective disorder. Her other medications included atorvastatin 20 mg/day for hypercholesterolemia for 2 years, alendronate 70 mg/week for osteoporosis diagnosed 2 years earlier, amlodipine 5 mg, and candesartan 16 mg/day for essential hypertension for 2 years. She has not been followed up on a regular basis for her psychiatric disorder. One month before admission, hypercalcemia was detected by a primary care physician. The patient was referred to our endocrinology department. On admission, she reported fatigue. The physical examination was normal, except for hypertension (160/100 mm-Hg).
The laboratory examination revealed the following findings: serum creatinine: 1.36 mg/dL (0.7–1.4 mg/dL), serum calcium: 12.2 mg/dL (8.5–10.5 mg/dL), serum albumin: 4.4 g/dL (3.2–5.5 g/dL), and parathyroid hormone (PTH): 577 pg/mL (10–65 pg/mL). Serum alkaline phosphatase and liver function tests were within normal limits. Serum electrolyte concentrations were as follows: sodium 142 mmol/L (135–146 mmol/L) and potassium: 4.2 mmol/L (3.5–5.1 mmol/L). Lithium-associated primary hyperparathyroidism was suspected. Her urinary calcium excretion was 101 mg/day (<350 mg/day). The urinalysis was within normal limits. The urinary microalbumin excretion was 7.6 mg/24 h (<30 mg/day). Renal ultrasonography revealed normal findings. The patient was treated with oral hydration and parenteral isotonic sodium chloride solution 2000 cc/day. Her daily urine output was not appropriately determined because of difficulty in collecting urine samples. The serum 25-hydroxyvitamin D concentration was 5.6 ng/mL (>30 ng/mL). Calcium and creatinine concentrations decreased to 11 mg/dL and 1.0 mg/dL, respectively, during parenteral and oral hydration. Although a diagnosis of primary hyperparathyroidism was apparent biochemically, the history of long-term lithium use required cautious localization studies because of the possibilities of double adenomas/parathyroid hyperplasia.
The neck ultrasonography showed a hypoechoic mass of 3.0 × 1.1 × 1.2 cm just below the posteroinferior aspect of the right lobe of the thyroid and multinodular goiter with multiple nodules up to 1 cm in diameter, with a dominant nodule dimensions measuring 8 × 17 mm in the left inferior thyroid lobe. Her thyroid function tests were as follows: thyroid-stimulating hormone (TSH): 0.33 µU/mL (0.27–4.2), free T4:12.1 pmol/L (12–22), and free T3: 4.8 pmol/L (3.1–6.8). Technetium-99-m sestamibi parathyroid scanning showed increased tracer uptake near the posterior margin of the right lobe of the thyroid, suggestive of right inferior parathyroid adenoma (Figure 1).
Figure 1.
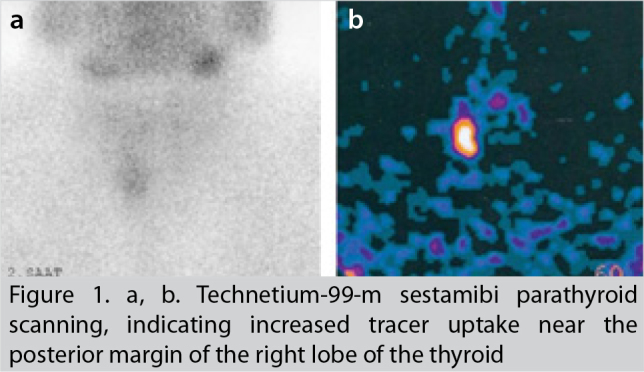
a, b. Technetium-99-m sestamibi parathyroid scanning, indicating increased tracer uptake near the posterior margin of the right lobe of the thyroid
Ultrasonography-guided fine-needle aspiration biopsy of the dominant nodule of the left thyroid lobe showed benign follicular cells. The serum calcitonin concentration was 2.6 pg/mL (<10 pg/mL). The patient decided to undergo both parathyroidectomy and thyroidectomy. A preoperative psychiatry consultation pointed to continued treatment with lithium with a dose of 1200 mg/day. The carbamazepine dose was increased to 800 mg/day. Olanzapine 15 mg/day was added to her psychiatric treatment regimen. The serum lithium concentration was within normal limits (0.9 mmol/L (0.5–1.0 mmol/L)) before the operation.
During the operation, right inferior parathyroidectomy and near-total thyroidectomy were performed (Figure 2). The excised parathyroid gland was 2.5 × 2.3 × 1.0 cm in size and 6 g in weight. Serum calcium and PTH concentrations decreased to normal levels immediately after the operation. However, during extubation, severe agitation developed. The patient was intubated, slept again, was kept in the post-anesthesia intensive care unit, and was closely observed for signs and symptoms of delirium. During this period, her sedative preparations included remifentanil and sevoflurane. Extubation was tried 5 times in the intensive care unit but was unsuccessful at every attempt because of agitation and delirium. Haloperidol and biperiden were added to the treatment regimen by the psychiatry consultation. Lithium was continued by nasogastric tube. During this time, while the patient was still unconscious, polyuria (daily urine output ≅ 9000 cc), rapidly increased serum sodium concentrations, and slightly increased creatinine concentrations were noted. Postoperative serum electrolyte concentrations are shown in Table 1.
Figure 2.
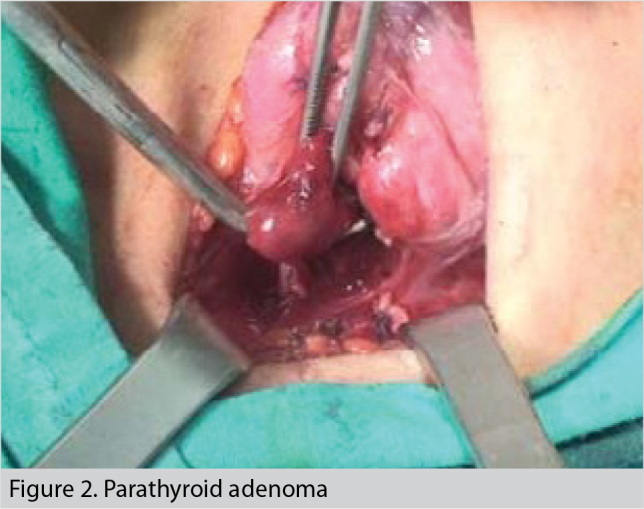
Parathyroid adenoma
Table 1.
Serial changes in serum electrolyte, creatinine concentrations and daily urine output during the postoperative period
| Serum sodium (n: 135–146 mmol/L) | Serum potassium (n: 3.5–5.1 mmol/L) | Serum creatinine (n: 0.7–1.4 mg/dL) | Daily urine output mL | |
|---|---|---|---|---|
| Day 1 | 144 | 4.2 | 1.45 | 4050 |
| Day 2 | 158 | 3.9 | 1.5 | 8020 |
| Day 3 | 166 | 3.8 | 1.48 | 9670 |
| Day 4 | 179 | 3.6 | 1.65 | 9030 |
| Day 5 | 166 | 3.9 | 1.6 | 8914 |
| Day 6 | 158 | 3.8 | 1.5 | 5680 |
| Day 7 | 148 | 4.1 | 1.45 | 4000 |
| Day 8 | 142 | 4.4 | 1.4 | 3880 |
Polyuria was unresponsive to parenterally administered desmopressin. A diagnosis of lithium-induced nephrogenic diabetes insipidus was made. Lithium was withdrawn. Pure water loss was determined, and 5% dextrose in water solution was infused to decrease the serum sodium concentration at a rate of 0.5 mmol/L/h. In addition, water was given through a nasogastric tube. It was necessary to perform a tracheostomy on day 5 because of prolonged intubation. Serum sodium concentrations steadily decreased to normal levels. Sedative medications were decreased. L-thyroxine replacement was already begun after the thyroidectomy. During this period, decreased serum calcium concentrations were treated with calcitriol (1 µg/day) and oral calcium (3 g/day). The patient was awake on day 8 without agitation. Oral fluid intake was begun. Serum electrolyte concentrations were within normal limits after day 8. During this period, her daily urine output was 3880 to 5680 mL. The tracheostomy tube was decannulated on day 15. The patient was discharged on day 20 with normal electrolyte and calcium concentrations. Her last medications were as follows: haloperidol 20 mg/day, biperiden 4 mg/day, L-thyroxine 100 µg/day, and oral calcium 1 g/day.
The pathological examination revealed a parathyroid adenoma consisting of chief and clear cells and multifocal papillary thyroid cancer (follicular variant without vascular or thyroid capsular invasion). Radioactive iodine treatment was planned.
DISCUSSION
Lithium is reported to be associated with transient/permanent renal injury during long-term use. Nephrogenic diabetes insipidus may be associated with medullary interstitial fibrosis (6). In our patient, increased creatinine concentrations on admission may reflect a decrease in glomerular filtration rate (GFR), although dehydration may play a role. Polyuria was not detected by the patient, and it was difficult to collect urine because of poor patient compliance. Therefore, polyuria was not noted as a prominent finding preoperatively. In addition, in the presence of hypercalcemia, polyuria is a well-described sign. It was thought that lithium-induced nephrogenic diabetes insipidus was present before surgery but did not draw attention because of concomitant hypercalcemia. Nephrogenic diabetes insipidus did not lead to a clinically significant problem when the patient was conscious and could easily reach water. However, during the postoperative period, prolonged intubation and sedation led to the inability to drink water, driven by patient’s thirst impulse. Severe hypernatremic dehydration developed. Hypernatremia has been reported previously in patients on lithium therapy during or after surgery (7, 8). Even in patients in whom lithium treatment was stopped days or months before the operation, this complication developed. Diabetes insipidus following cessation of lithium therapy, particularly in the setting of the intensive care unit, was reported previously (8).
Lithium therapy may lead to hypercalcemia in patients with normal parathyroid function, and increased PTH concentrations in association with normocalcemia may persist for as long as 5 months, even after termination of lithium treatment. Lithium therapy is considered an exacerbating factor in patients with primary hyperparathyroidism (9). The cause-and-effect relationship between chronic lithium use and development of a parathyroid adenoma is not clear. But, it is suggested that lithium treatment may lead both to exacerbation of preexisting hyperparathyroidism as well as to multiglandular disease by inhibition of calcium-sensing receptors on parathyroid cells (5). Our patient’s clinical presentation is in accordance with the first suggestion; it is thought that chronic lithium use led to higher calcium concentrations in our patient. A parathyroid adenoma was responsible for hyperparathyroidism in our case. Parathyroid gland hyperplasia was unlikely, because excision of one parathyroid gland led to rapid resolution of hypercalcemia in our patient. Afterwards, hypocalcemia, compatible with hungry bone syndrome, developed. It is worth pointing out the vitamin D deficiency in our patient. Vitamin D deficiency was identified as a factor for increased parathyroid adenoma weight and more severe clinical findings in a previous study at our institution (9).
In our patient, both lithium-associated hypercalcemia and nephrogenic diabetes insipidus developed. Previous cases with lithium-induced nephrogenic diabetes insipidus in association with hypercalcemia also were reported (10). In addition, papillary thyroid carcinoma was reported incidentally in our patient. Previous papillary carcinoma cases, together with parathyroid adenoma, in the setting of lithium use were found (5).
CONCLUSION
We conclude that chronic lithium treatment may uncover preexisting primary hyperparathyroidism. Polyuria of lithium-induced nephrogenic diabetes insipidus may be masked by concomitant hypercalcemia. Careful follow-up of those patients during or after surgery is necessary to prevent severe and life-threatening water depletion and hypernatremia, which may develop when the patients are compromised.
Footnotes
Informed Consent: The patients were informed about all surgical and invasive procedures before surgery and during follow up. They were also expected to sign a form of approval.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Y.E., C.E., N.A., B.Ö., F.A.; Design -Y.E., C.E., N.A., B.Ö., F.A.; Supervision - Y.E., C.E., N.A., B.Ö., F.A.; Funding - Y.E., C.E., B.Ö.; Materials - Y.E., C.E., N.A.; Data Collection and/or Processing - Y.E., C.E., N.A.; Analysis and/or Interpretation - Y.E., C.E., B.Ö., F.A.; Literature Review - Y.E., C.E., F.A., N.A.; / Writer - Y.E., C.E., F.A.; Critical Review - Y.E., C.E., N.A., B.Ö.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Vestergaard P, Licht RW. 50 years with lithium treatment in affective disorders. World J Biol Psychiatry. 2001;2:18–26. doi: 10.3109/15622970109039980. http://dx.doi.org/10.3109/15622970109039980. [DOI] [PubMed] [Google Scholar]
- 2.Lazarus JH. Lithium and thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:723–733. doi: 10.1016/j.beem.2009.06.002. http://dx.doi.org/10.1016/j.beem.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Bedford JJ, Weggery S, Ellis G, MacDonald FJ, Joyce PR, Leader JP, et al. Lithium induced nephrogenic diabetes insipidus: renal effects of amiloride. Clin J Am Soc Nephrol. 2008;3:1324–1331. doi: 10.2215/CJN.01640408. http://dx.doi.org/10.2215/CJN.01640408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Li C, Kwon TH, Knepper MA, Frokiaer J, Nielsen S. AQP3, p-AQP2 and AQP2 expression is reduced in polyuric rats with hypercalcemia. Prevention by cAMP-PDE inhibitors. Am J Physiol Renal Physiol. 2002;283:1313–1325. doi: 10.1152/ajprenal.00040.2002. http://dx.doi.org/10.1152/ajpcell.00553.2001. [DOI] [PubMed] [Google Scholar]
- 5.Szalat A, Mazeh H, Freund HR. Lithium-associated hyperparathyroidism: report of four cases. Eur J Endocrinol. 2009;160:317–323. doi: 10.1530/EJE-08-0620. http://dx.doi.org/10.1530/EJE-08-0620. [DOI] [PubMed] [Google Scholar]
- 6.Presne J, Fakhouri F, Noel LH, Stengel B, Even C, Kreis H, et al. Lithium induced nephrotoxicity: rate of progression and prognostic factors. Kidney Int. 2003;64:585–592. doi: 10.1046/j.1523-1755.2003.00096.x. http://dx.doi.org/10.1046/j.1523-1755.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 7.Sze L, Ulrich B, Brandle M. Severe hypernatremia due to nephrogenic diabetes insipidus- a life threatening side effect of chronic lithium therapy. Exp Clin Endocrinol Diab. 2006;114:596–598. doi: 10.1055/s-2006-924124. http://dx.doi.org/10.1055/s-2006-924124. [DOI] [PubMed] [Google Scholar]
- 8.Paw H, Slingo ME, Tinker M. Late onset nephrogenic diabetes insipidus following cessation of lithium therapy. Anaesth Intensive Care. 2007;35:278–280. doi: 10.1177/0310057X0703500220. [DOI] [PubMed] [Google Scholar]
- 9.Ozbey N, Erbil Y, Ademoglu E, Ozarmagan S, Barbaros U, Bozbora A. Correlations between vitamin D status and biochemical/clinical and pathological parameters in primary hyperparathyroidism. World J Surg. 2006;30:321–326. doi: 10.1007/s00268-005-0239-y. http://dx.doi.org/10.1007/s00268-005-0239-y. [DOI] [PubMed] [Google Scholar]
- 10.Kairallah W, Fawaz A, Brown EM, El-Hajj Fuleihan G. Hypercalcemia and diabetes insipidus in a patient treated with lithium. Nat Clin Pract Nephrol. 2007;3:397–404. doi: 10.1038/ncpneph0525. http://dx.doi.org/10.1038/ncpneph0525. [DOI] [PubMed] [Google Scholar]


