Abstract
Objectives. We sought to estimate the return on investment of a streamlined version of an evidence-based community health worker (CHW) asthma home visit program.
Methods. We used a randomized parallel group trial of home visits by CHWs to Medicaid-enrolled children with uncontrolled asthma versus usual care.
Results. A total of 373 participants enrolled in the study (182 in the intervention group and 191 in the control group, of whom 154 and 179, respectively, completed the study). The intervention group had greater improvements in asthma symptom–free days (2.10 days more over 2 weeks; 95% CI = 1.17, 3.05; P < .001) and caretakers’ quality of life (0.43 units more; 95% CI = 0.20, 0.66; P < .001) and a larger reduction in urgent health care utilization events (1.31 events fewer over 12 months; 95% CI = −2.10, −0.52; P = .001). The intervention arm compared with the control arm saved $1340.92 for the $707.04 additional costs invested for the average participant. The return on investment was 1.90.
Conclusions. A streamlined CHW asthma home visit program for children with uncontrolled asthma improved health outcomes and yielded a return on investment of 1.90.
The burden of asthma in the United States remains high—nearly 7 million children currently have asthma (9.5% of all children).1,2 Most have asthma that is not well controlled.3
Asthma self-management support is effective in improving asthma control.4–8 However, many children with asthma and their caretakers do not effectively practice self-management.9,10 Barriers to self-management include lack of knowledge and skills, difficulties in accessing asthma education, and lack of resources to reduce exposure to triggers and implement other self-management tasks. One effective approach to address these barriers is the provision of self-management support in the home.11,12
Despite compelling evidence of effectiveness, home-based asthma self-management support is not widely available. A major obstacle to wider implementation is the lack of reimbursement by health care payers, who seek additional evidence of the cost-effectiveness and return on investment (ROI) of home visits.
The King County Asthma Program in Seattle, Washington, developed a community health worker (CHW) home visit program (Healthy Homes) and demonstrated its effectiveness.12,13 We designed a streamlined version of the program that was simpler and cost less to implement to facilitate broad dissemination and adoption. Here we reported on the effectiveness, cost-effectiveness, and ROI of a streamlined Healthy Homes program.
METHODS
We used a randomized parallel group design to test the hypotheses that a simplified version of our Healthy Homes program would reduce urgent medical care, improve asthma-related quality of life and symptom-free days, generate a positive ROI relative to usual care, and be cost-effective.
Participants
Eligibility criteria for the study were as follows: children aged 3 to 17 years who (1) lived in King County with provider-diagnosed asthma that was either not well controlled or very poorly controlled, (2) were enrolled in 1 of 2 Medicaid plans (that together enroll 71% of all Medicaid-covered children in King County), and (3) had a caretaker conversant in either English or Spanish. Not well-controlled or very poorly controlled asthma was defined by the National Asthma Education and Prevention Program’s (NAEPP’s) “expert panel report 3”6 impairment and risk criteria (except spirometry).
With a population of 1.9 million, King County is the 13th largest county in population in the United States; 12.5% live in households with incomes less than 200% of the federal poverty level, and nearly half of all children are non-White. The childhood prevalence for current asthma, averaged from 2009 to 2013, was 7%. The prevalence rates for African Americans and Asians were higher than the rate for Whites.14
We excluded potential participants if they planned to move out of King County within the next year or lacked permanent housing, were enrolled in another asthma study within the past 3 years, had another serious medical condition (e.g., sickle cell disease or cystic fibrosis), or were participating in another asthma study; if the caretaker had a mental or physical disability that made participation impossible; or if the home appeared to be unsafe for CHW visitation.
We recruited participants using lists provided by the Medicaid health plans (1378) or from provider referrals (184). Assent of the child and informed consent of 1 parent or legal guardian were obtained.
Data Collection
Baseline data were collected on a rolling basis in the participant’s home before randomization between May 2010 and October 2011. A CHW other than the one who provided the intervention collected exit data 1 year later upon study completion (between May 2011 and October 2012). Participants received a $10 incentive for completing baseline data collection and $35 for completing exit data collection.
Intervention
We implemented a streamlined version of the home visit program developed through our previous home visit research studies.12,13 Modifications included reducing the number of visits from 5–7 to 4, allowing for up to 2 telephone contacts in lieu of home visits, compressing visits into a shorter time frame, reducing the duration of visits, tailoring the intervention to the topics most relevant to each participant using motivational interviewing methods,15 using exposure alone rather than exposure and allergic sensitization to tailor trigger reduction activities (because of low participation in allergy testing in prior projects), and simplifying coordination with health care providers.
For each household in the intervention group, a CHW provided education, support, and service coordination. The CHW made an initial visit to assess the participant’s knowledge of asthma, asthma control level, challenges with controlling asthma, self-management practices, and exposure to asthma triggers, followed by additional visits occurring 0.5, 1.5, and 3.5 months later. CHWs also provided as-needed support via telephone, e-mail, or additional home visits. Participants received a low-emission vacuum cleaner, cleaning supplies, roach abatement supplies (if roaches were present), and allergen-impermeable bedding covers. The CHWs included children in the home visit activities. Depending on the child’s age, CHWs assessed asthma control based on the child’s self-report, included the child in asthma education, and offered coaching on the correct use of asthma devices.
The CHWs followed protocols that specified educational content, participant skill development, and participant and CHW actions. Protocols, questionnaires, forms, and client educational materials are available at http://www.kingcounty.gov/healthservices/health/chronic/asthma/resources/tools.aspx. Control group participants received asthma education and asthma control resources after their exit interviews.
Outcome Measures
Primary prespecified outcomes were asthma symptom–free days (self-reported number of 24-hour periods during the prior 2 weeks without wheeze, tightness in chest, cough, or shortness of breath; a decrease in usual activities because of asthma; or nighttime awakening because of asthma), Pediatric Asthma Caregiver Quality of Life Scale score (range = 1–7, with higher scores indicating better quality of life, and a minimum clinically important difference of 0.5 points), and self-reported asthma-related urgent health services use during the last 12 months (emergency department, hospital, or unscheduled clinic visits). Secondary prespecified outcomes included asthma attack frequency (defined as a time when asthma symptoms were worse, limiting activity more than usual or making you seek medical care for your child), days with rescue medication use, activity limited by asthma, nights with symptoms, and asthma control levels (as defined by NAEPP6).
Sample Size
A group size of 154 participants (the number of intervention group participants completing the study) had 80% power, or 80% probability of rejecting the null hypothesis when the null hypothesis is false, to detect differences of 1.38 symptom-free days, 0.36 points in the quality of life score, and 1.14 episodes of urgent health services use between groups, with α set at .05, or 5% probability of rejecting the null hypothesis when the null is true.
Randomization and Blinding
We randomly assigned participants to groups using a permuted block design with varying block size. We stratified randomization into 4 groups based on age (3–11 years and 12–17 years) and asthma-control level (not well controlled or very poorly controlled), respectively.
Sequence numbers and group allocation were concealed in sealed, opaque, numbered envelopes that were centrally prepared and sequentially provided to the CHWs, who assigned participants to study groups. Blinding was not possible because of the nature of the intervention.
Statistical Methods for Analysis of Outcomes
We examined baseline differences across groups with the t or χ2 test and used paired t and McNemar tests for assessing within-group baseline-to-exit changes. For evaluating intervention effects, we used multivariable linear regression and logistic regression for continuous and binary outcome variables, respectively, with the robust option for estimating standard errors using the Huber–White sandwich estimators.16 The regression models controlled for the baseline value of the outcome variable and prespecified demographic covariates: age, gender, race/ethnicity (White, Black, Hispanic, and Other), and education level. Because the distributions of the 2-week outcome variables (e.g., symptom days) were not normal (they clustered at 0 and 14), we used negative binomial regression to confirm the results of linear models. We used linktest in Stata version 13 (StataCorp LP, College Station, TX) to examine the specifications of each model. We tested for the presence of modification of the intervention effect by age, gender, education, and race/ethnicity using separate regression models that included an interaction term and considered an interaction significant if the P value of the interaction term was < .05.
Analyses were performed on an intention to treat basis. We performed a complete case and multiple imputation analysis for each outcome.17 The complete case analysis included only participants for whom baseline and exit data were available. We used multiple imputation to generate missing exit values of outcome variables for the 40 participants who did not complete the study. The imputation models included baseline outcome variable, age, gender, and race/ethnicity. All analyses were performed using Stata version 13 and were 2-tailed.
Economic Analysis
We estimated intervention costs by using a simple cost allocation method that sums fixed and variable costs for program implementation while excluding costs of research and development. Wage rates were based on actual salaries and benefits. Other costs (e.g., administrative, logistical, participant resources, and training) were valued at actual cost. The estimated cost for each home visit was $205.20. Both study arms had baseline and exit interview home visits, whereas the intervention arm included 3 additional home visits. Both study arms received $210 worth of asthma control supplies. Health care costs were computed for each participant using unit costs and utilization. The average unit costs for hospital stays (mean = $8030), emergency department visits (mean = $330), and physician visits (mean = $151) were derived from a large medical and pharmacy claims database of children on Medicaid aged 3 to 17 years (PharMetrics Integrated Outcomes Database). Medication unit costs were estimated from Costco’s pharmacy prices.18 Utilization was based on self-reported health care and medication use with 12 months of recall. Return on investment was estimated as the costs saved associated with the intervention divided by the costs of the intervention itself.
Sensitivity analyses were conducted to test the impact of influential unit costs and average resource utilization estimates. Under scenarios where the intervention cost more than the control but also was shown to be more effective, a cost-effectiveness ratio was estimated to display additional costs per additional symptom-free day. In this case, because symptom-free days were measured over the course of 14 days but costs were measured over the entire 12-month study duration, we prorated costs to a 14-day time period. Bootstrap resampling with replacement methods were used to estimate the likelihood that the intervention reduced costs. All unit costs were in 2012 US dollars.
RESULTS
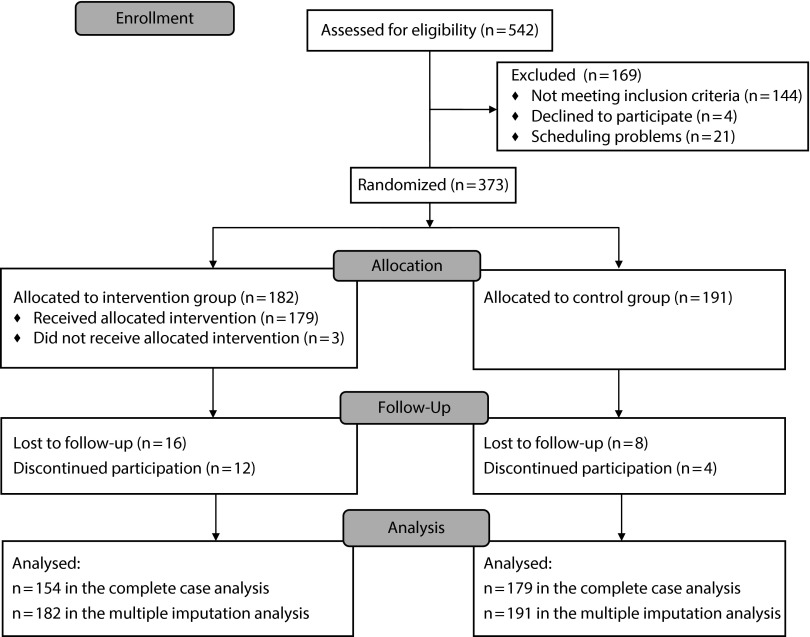
Of 1234 potential participants we attempted to contact, 542 were reached and assessed for eligibility, 488 were not reachable (disconnected phone, no answer, busy, or wrong number), and 204 were reached but refused phone screening. Of the 542 who were assessed for eligibility, 373 were eligible and enrolled (Figure 1).
FIGURE 1—
Consort flow diagram.
Of the 373 enrolled participants, 182 were randomized to the intervention group and 191 to the control group, and 154 (84.6%) and 179 (93.7%), respectively, completed the study (Figure 1). Of the 40 who did not complete the study, 24 were lost to follow-up, 10 moved out of the county, 4 refused to continue, and 2 had other reasons. Those who did not complete the study were similar to those who did with respect to the baseline characteristics listed in Table 1 with the following exceptions: the noncompleters were more likely to be renters (97.5% vs 79.5%), had lower scores on the caretaker’s quality of life (4.31 vs 4.99), and had more emergency department visits (2.75 vs 1.72).
TABLE 1—
Baseline Comparison Between Groups
| Baseline Demographics and Clinical Characteristics | Control | CHW | Total | P |
| No. | 191 | 182 | 373 | |
| Child’s age, y, mean | 8.4 | 8.2 | 8.3 | .628 |
| Child’s age, y, % | .852 | |||
| 3–5 | 27.7 | 28.6 | 28.2 | |
| 6–9 | 35.6 | 33.0 | 34.3 | |
| 10–12 | 22.0 | 25.3 | 23.6 | |
| 13–17 | 14.7 | 13.2 | 13.9 | |
| Child’s gender, % female | 42.4 | 39.0 | 40.8 | .504 |
| Child’s race/ethnicity, % | .612 | |||
| Non-Hispanic White | 10.5 | 7.1 | 8.8 | |
| Non-Hispanic Black | 16.2 | 14.3 | 15.3 | |
| Non-Hispanic Asian/PI | 5.2 | 4.4 | 4.8 | |
| Non-Hispanic multiracial | 6.3 | 4.4 | 5.4 | |
| Non-Hispanic other | 2.6 | 4.4 | 3.5 | |
| Hispanic, any race | 59.2 | 65.4 | 62.2 | |
| Caretaker’s language, % | .183 | |||
| English | 49.7 | 42.9 | 46.4 | |
| Spanish | 50.3 | 57.1 | 53.6 | |
| Caretaker rents home (vs owns), % | 78.4 | 84.6 | 81.5 | .124 |
| Caretaker’s education, % | .422 | |||
| < high school | 38.9 | 45.9 | 42.3 | |
| High school graduate | 23.7 | 23.2 | 23.5 | |
| Some college | 26.3 | 23.8 | 25.1 | |
| College graduate | 11.1 | 7.2 | 9.2 | |
| Asthma control level, % | .391 | |||
| Well controlled | 6.3 | 3.3 | 4.8 | |
| Not well controlled | 47.1 | 47.3 | 47.2 | |
| Very poorly controlled | 46.6 | 49.5 | 48.0 | |
| Main outcome variables, mean | ||||
| Asthma symptom-free days per 2 wk | 6.7 | 6.4 | 6.6 | .432 |
| Asthma-related quality-of-life score | 5.0 | 4.8 | 4.9 | .163 |
| Health care utilization during past year, mean | ||||
| Hospitalization | 0.58 | 0.65 | 0.61 | .674 |
| Emergency department | 1.65 | 1.90 | 1.77 | .687 |
| Urgent clinic visit | 2.52 | 2.97 | 2.73 | .354 |
Note. CHW = community health worker intervention arm; PI = Pacific Islander. P values were generated from the t test for differences in means and the χ2 test for differences in categorical variables. For asthma symptom–free days, asthma-related quality-of-life score, and the health care utilization variables, we also performed a nonparametric Kruskal–Wallis rank test. The P values were similar to those obtained from the t test and are not statistically significant.
Of the 182 participants in the intervention group, 3 did not receive any CHW home visits after the initial visit (1.6%), 5 received 1 visit (2.7%), 3 (1.6%) received 2 visits, 159 (87.4%) received 3 visits, and 12 (6.6%) received 4 visits.
The study groups were well balanced (Table 1). Participants were mostly younger than 13 years (86.1%) and predominantly male (59.2%) and Hispanic (62.2%). The burden of asthma was high, with nearly half (48.0%) having very poorly controlled asthma and only 6.6 days free of symptoms over 2 weeks. Urgent health care utilization was common, with an average of 0.61 hospitalizations, 1.77 emergency department visits, and 2.73 urgent clinic visits per 12 months. Among the participants’ caretakers, 65.8% had a high school or lower level of education, 53.6% used Spanish as their primary language, and 81.5% were renters.
Primary Clinical Outcomes
Outcomes improved in both groups (Table 2). In the completed case analysis, the intervention group had significantly greater improvements in asthma symptom–free days (2.1 days more over 2 weeks) and caretakers’ quality-of-life scores (0.4 units more) compared with the control group. The intervention group also experienced a reduction in urgent health care utilization events (1.3 visits fewer over 12 months). Multiple imputation analysis yielded similar results.
TABLE 2—
Intervention Effect for the Main Outcome Variables by Study Group
| Intervention Group (n = 154/182) |
Control Group (n = 179/191) |
Intervention Effect (Adjusted Model)a |
||||||||
| Main Outcomes | Base | Exitb | ∆ (95% CI) | P | Base | Exit | ∆ (95% CI) | P | Coefficient (95% CI) | P |
| Symptom-free days in prior 2 wk | ||||||||||
| Completers only | 6.25 | 11.15 | 4.90 (3.95, 5.84) | < .001 | 6.88 | 9.08 | 2.20 (1.33, 3.07) | < .001 | 2.10 (1.17, 3.05) | < .001 |
| Multiple imputation | 6.37 | 10.93 | 4.56 (3.99, 5.12) | < .001 | 6.74 | 9.10 | 2.36 (1.77, 2.94) | < .001 | 1.80 (0.84, 2.76) | < .001 |
| Quality of Life scorec | ||||||||||
| Completers only | 4.83 | 6.10 | 1.27 (1.03, 1.51) | < .001 | 5.12 | 5.77 | 0.66 (0.45, 0.87) | < .001 | 0.43 (0.20, 0.66) | < .001 |
| Multiple imputation | 4.80 | 6.06 | 1.26 (1.11, 1.42) | < .001 | 5.03 | 5.75 | 0.72 (0.58, 0.87) | < .001 | 0.38 (0.15, 0.61) | .001 |
| Health care utilization in past yeard | ||||||||||
| Completers only | 5.20 | 1.79 | −3.41 (−4.45, −2.37) | < .001 | 4.94 | 3.07 | −1.87 (−2.81, −0.93) | < .001 | −1.31 (−2.10, −0.52) | .001 |
| Multiple imputation | 5.49 | 1.86 | −3.64 (−4.49, −2.79) | < .001 | 4.94 | 3.04 | −1.90 (−2.62, −1.18) | < .001 | −1.24 (−2.03, −0.45) | .002 |
Note. CI = confidence interval.
Regression-adjusted before and after differences between intervention and control groups controlling for age, gender, race/ethnicity (as White, Black, Hispanic, and other), and education level.
Time at which study completion data were collected (approximately 1 year after baseline).
Pediatric Asthma Caretaker Quality of Life score.
Sum of hospitalizations + emergency department visits (not leading to admission) + urgent clinic visits.
The intervention was significantly more effective among caretakers with less than a high school education compared with those with a college degree. For quality of life, the difference across study groups was 0.9 points greater (P = .034) among those with less education; for symptom-free days it was 3.7 days greater (P = .039). No other interactions were significant.
Secondary Clinical Outcomes
Secondary outcomes generally improved in both groups (Table 3), with the exception of the number of asthma episodes per 3 months which did not decrease in the control group. Most outcomes improved significantly more in the intervention group relative to the control group: nights with symptoms per 2 weeks (decreased 1.1 nights more), days with activity limitation per 2 weeks (decreased 0.6 days more), days using rescue medications per 2 weeks (decreased 1.7 days more), and proportion with well-controlled asthma (increased 3.3-fold more).
TABLE 3—
Intervention Effect for the Secondary Outcome Variables by Study Group
| Intervention Group (n = 154/182) |
Control Group (n = 179/191) |
Intervention Effect (Adjusted Model)a |
||||||||
| Secondary Outcomes | Base | Exitb | ∆, 95% CI | P | Base | Exit | ∆, 95% CI | P | Coefficient (95% CI) | P |
| Symptom nights per 2 wk | ||||||||||
| Completers only | 3.19 | 1.08 | −2.11 (−2.76, −1.46) | < .001 | 2.85 | 2.12 | −0.74 (−1.28, −0.19) | .009 | −1.09 (−1.70, −0.48) | < .001 |
| Multiple imputation | 3.24 | 1.17 | −2.07 (−2.47, −1.67) | < .001 | 2.99 | 2.14 | −0.86 (−1.25, −0.46) | < .001 | −0.97 (−1.59, −0.35) | .002 |
| Activity limitation days per 2 wk | ||||||||||
| Completers only | 4.16 | 1.23 | −2.93 (−3.70, −2.16) | < .001 | 3.39 | 1.80 | −1.59 (−2.21, −0.97) | < .001 | −0.64 (−1.27, −0.02) | .044 |
| Multiple imputation | 4.09 | 1.21 | −2.89 (−3.43, −2.35) | < .001 | 3.47 | 1.81 | −1.66 (−2.14, −1.19) | < .001 | −0.63 (−1.25, −0.06) | .048 |
| Rescue medication use days per 2 wk | ||||||||||
| Completers only | 5.83 | 2.18 | −3.65 (−4.64, −2.65) | < .001 | 4.78 | 3.73 | −1.05 (−1.83, −0.27) | .008 | −1.70 (−2.59, −0.81) | < .001 |
| Multiple imputation | 5.67 | 2.31 | −3.36 (3.99, −2.74) | < .001 | 4.96 | 3.73 | −1.26 (−1.84, −0.68) | < .001 | −1.48 (−2.36, −0.60) | .001 |
| Asthma episodes per 3 mo | ||||||||||
| Completers only | 5.00 | 2.50 | −2.50 (−4.71, −0.29) | .027 | 3.96 | 3.50 | −0.46 (−1.73, 0.80) | .471 | −0.99 (−2.67, 0.68) | .244 |
| Multiple imputation | 4.88 | 2.58 | −2.31 (−3.81, −0.81) | .003 | 4.55 | 3.50 | −1.06 (−2.28, 0.16) | .089 | −0.84 (−2.41, 0.73) | .293 |
| Well-controlled asthma (%) | ||||||||||
| Completers only | 3.25 | 48.05 | 44.81 (36.10, 53.51) | < .001 | 6.70 | 22.91 | 16.20 (8.87, 23.54) | < .001 | 3.33c (2.05, 5.40) | < .001 |
| Multiple imputation | 3.30 | 46.03 | 42.74 (40.54, 44.94) | < .001 | 6.28 | 23.47 | 17.19 (14.57, 19.81) | < .001 | 2.90c (1.78, 4.73) | < .001 |
| Very poorly controlled (%) | ||||||||||
| Completers only | 50.00 | 17.53 | −32.47 (−42.96, −21.98) | < .001 | 45.25 | 24.02 | −21.23 (−30.26, −12.19) | < .001 | 0.65c (0.37, 1.14) | .135 |
| Multiple imputation | 49.45 | 17.90 | −31.55 (−38.20, −24.91) | < .001 | 46.60 | 23.94 | −22.66 (−29.03, −16.30) | < .001 | 0.69c (0.39, 1.21) | .194 |
Note. CI = confidence interval.
Regression-adjusted before and after differences between intervention and control groups controlling for age, gender, race/ethnicity (as White, Black, Hispanic, and Other), and education level.
Time at which study completion data were collected (approximately 1 year after baseline).
Odds ratios from a logistic regression model controlling for the same covariates.
Economic Analysis
Given annual cost savings (mainly from larger reductions in hospitalizations within the intervention arm), the intervention was expected to save $1340.92 for the $707.04 additional costs invested in the average patient (Table 4). Thus, the intervention over 1 year was estimated to cost $633.88 less per participant than the control group from the payer perspective. The return on investment was 1.90 (or 190%). Bootstrap resampling methods showed that the likelihood that the intervention was cost saving was 68.2% (95% CI = 65.3%, 71.1%). Because return on investment was greater than 1, the cost-effectiveness for this scenario was not computed.
TABLE 4—
Net Costs and Savings of Intervention Compared With Control
| Cost Category | Intervention (Study Period—Baseline Period), $ | Control (Study Period—Baseline Period), $ | Net Intervention Costs (Intervention – Control) > $0, $ | Net Intervention Savings (Intervention – Control) < $0, $ |
| Hospitalization stays | −4484.46 | −3409.52 | −1074.94 | |
| Emergency department visits | −306.69 | −282.31 | −24.38 | |
| Provider visits (total) | −290.06 | −90.21 | −199.85 | |
| Oral steroid bursts | −1.69 | −2.39 | 0.70 | |
| Controller medications | 166.20 | 122.91 | 43.29 | |
| Reliever medications | −17.68 | 24.07 | −41.75 | |
| Allergy medications | 1.74 | 0.29 | 1.45 | |
| CHW visit costs | 1072 + 210 (supplies) | 410.40 + 210 (supplies) | 661.60 | |
| Subtotal | −3650.62 | −3016.75 | 707.04 | −1340.92 |
| Total savings per participant | −633.88 | |||
Note. CHW = community health worker.
Notable sensitivity analyses on the costs were as follows: (1) when no costs were attributed to the control arm, the intervention average total costs were $13.47 less than the direct medical costs of the control group, and (2) when 2 outliers who had more than 10 hospitalizations in the past year were removed from the cost analysis, the intervention average total costs were $1113.37 more than that of the control group. When the 2 outliers were removed, the expected costs were $42.66 higher for each 14-day period for the intervention group with an estimated cost per symptom-free day of $20.31.
DISCUSSION
A streamlined community health worker asthma home visit program for children with uncontrolled asthma enrolled in Medicaid increased symptom-free days and caretaker asthma-related quality of life and reduced urgent health care utilization and costs. The intervention economically dominated usual care (i.e., less costly and more effective), and the program yielded a return on investment of 1.90.
Home visits are an increasingly common method of providing asthma self-management support. Traditionally, such asthma education has been provided in clinical settings by asthma educators.4,5,19 However, many potential participants lack the time, resources, or interest to attend classes or participate in preventive asthma care visits where education is provided. Home visits present an attractive alternative.
Our previous studies used more intensive home visit protocols in which CHWs made a mean of 5.5 to 7 visits12,13 compared with 5 visits in this study (including baseline and exit interview visits). The initial enrollment visit took 3 to 4 hours in prior studies versus 1 to 2 hours in this study. The outcomes of this study compared favorably to those observed in the prior studies, suggesting that the reduction in visit frequency and duration did not lessen program effectiveness.
The outcomes achieved in this study were comparable to those seen in other studies that had a wide range of intervention intensities.11 A meta-analysis of 20 studies reported that home visits decreased asthma symptom days by 0.8 days per 2 weeks (range = 0.6–2.3 days). The overall median improvement in the Juniper quality-of-life score in the 6 studies that employed this measure was 0.4 points (range = 0.02–1.41 points). The number of urgent asthma care visits decreased by 0.57 visits per year (interquartile interval = 0.33–1.71). The frequency of home visits was quite varied, ranging from 1 (3 studies), to 2 to 7 (15 studies), to 8 or more (5 studies). The extent of environmental remediation was also heterogeneous, but most were of moderate intensity and similar to that used in this study. Therefore, the benefits observed in this study were equal to or greater than those reported in the meta-analysis, despite this study being on the low end of the intervention intensity spectrum.
The economic findings are also equal to or more favorable than those reported in other types of asthma educational interventions.20–22 A Boston, Massachhusetts, study found that an educational intervention yielded a return on investment of 1.33 that resulted from savings from emergency department visits and hospitalizations.18 A recent study on mobile health care operations in underserved Californian children with asthma found a higher return on investment of 6.73 per dollar spent.4 However, most of these cost savings resulted from the valuing of quality-of-life improvements and therefore were considered outside of the health care payer perspective. A review of the cost-effectiveness of asthma educational interventions found some that were cost saving (with a return on investment > 1.0), whereas most cost more than their comparators.5
Strengths of this study included a randomized controlled trial design, adequate sample size, use of clinically meaningful and patient-centered outcomes, high level of participant retention, use of multiple imputation methods to account for attrition, relatively low-intensity intervention that should prove easy to replicate, and implementation in a population affected by asthma health inequities.
Our conclusions were subject to several limitations. We could not blind participants to group assignment given the nature of the intervention. Loss to follow-up could have biased results, but 89% of participants completed the study, and we used multiple imputation methods in our clinical analyses.
Resources were not sufficient to permit following up with participants after the completion of the study to assess the durability of intervention effects. Other studies have shown that benefits from CHW home visit programs continue after participation in the program ends.13,23
Data on outcomes came from participant self-reports. The use of self-reported symptoms is a well-accepted measure and is recommended by the National Heart, Blood, and Lung Institute and Agency for Health Care Research and Quality Asthma Outcomes Workshop as a core asthma outcome measure in clinical research.24
The cost findings were sensitive to reported hospital utilization. When the outliers were removed from the cost analysis, the intervention no longer reduced costs. We recommend that during the replication of this CHW intervention, administrative claims data be analyzed to further reduce the uncertainty in the cost findings.
The findings should be generalizable to low-income, predominantly minority children with significant asthma enrolled in Medicaid health plans. Only 4 of 398 eligible caretakers reached declined participation. However, it was difficult to reach all potentially eligible families, suggesting the need for other approaches to connect with harder-to-reach households.
Home visits by CHWs that provide self-management support to children with asthma and their caretakers are effective and provide good value for their cost. They therefore contribute to the triple aim of health care reform—improving the experience of care for individuals, improving the health of populations, and lowering per capita costs.25 Policymakers, health care delivery systems, and health care payers implementing health reform should make asthma home visits more widely available.
Acknowledgments
This study was funded by the Centers for Disease Control and Prevention, National Center for Environmental Health grant 5R18EH000537.
We thank the following individuals from Public Health – Seattle & King County: Michelle DeMicio, Marguerita Mendoza, Maria Rodriguez, Meg Tremblay, and Jesse Gritton (project community health workers who delivered the implementation, collected data, and provided field-based feedback on project protocols); Karen Artz (project nurse who provided clinical supervision to CHWs and was responsible for quality control); Penelope Brewer (health educator who provided motivational interviewing training and support for CHWs); and Nathan Drain (project manager who oversaw operations and managed data). All were salaried employees of this project.
Human Participant Protection
This study was approved by the University of Washington institutional review board.
References
- 1.Schiller JS, Lucas JW, Peregoy JA. Summary health statistics for US adults: national health interview survey, 2011. Vital Health Stat. 2012;256:1–218. [PubMed] [Google Scholar]
- 2.Bloom B, Cohen RA, Freeman G. Summary health statistics for US children: national health interview survey, 2010. Vital Health Stat. 2011;250:1–80. [PubMed] [Google Scholar]
- 3.Colice GL, Ostrom NK, Geller DE et al. The CHOICE survey: high rates of persistent and uncontrolled asthma in the United States. Ann Allergy Asthma Immunol. 2012;108(3):157–162. doi: 10.1016/j.anai.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Wolf FM, Guevara JP, Grum CM, Clark NM, Cates CJ. Educational interventions for asthma in children. Cochrane Database Syst Rev. 2003;1 doi: 10.1002/14651858.CD000326. CD000326. [DOI] [PubMed] [Google Scholar]
- 5.Coffman JM, Cabana MD, Halpin H, Yelin E. Effects of asthma education on emergency department visits and hospitalizations for children with asthma: a meta-analysis. Pediatrics. 2008;121:575–586. doi: 10.1542/peds.2007-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Asthma Education and Prevention Program. Expert panel report 3 (EPR3): guidelines for the diagnosis and management of asthma. NIH Publication no. 07-4051. 2007. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf. Accessed March 1, 2013.
- 7.British Thoracic Society. Asthma guidelines. Available at: http://www.brit-thoracic.org.uk/guidelines/asthma-guidelines.aspx. Accessed March 26, 2013.
- 8.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2012. Available at: http://www.ginasthma.org. Accessed March 26, 2014.
- 9.Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):547–552. [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. 2012 national healthcare disparities report. 2013. Available at: http://www.ahrq.gov/research/findings/nhqrdr/nhdr12/2012nhdr.pdf. Accessed April 1, 2013. [DOI] [PubMed]
- 11.Crocker DD, Kinyota S, Dumitru GG et al. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: a community guide systematic review. Am J Prev Med. 2011;41(2, suppl 1):S5–S32. doi: 10.1016/j.amepre.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Krieger J, Takaro TK, Song L, Beaudet N, Edwards K. A randomized controlled trial of asthma self-management support comparing clinic-based nurses and in-home community health workers: the Seattle–King County Healthy Homes II project. Arch Pediatr Adolesc Med. 2009;163(2):141–149. doi: 10.1001/archpediatrics.2008.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle–King County Healthy Homes project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659. doi: 10.2105/AJPH.2004.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King County Government. King County’s changing demographics: a view of our increasing diversity. Available at: http://www.kingcounty.gov/∼/media/exec/PSB/documents/AGR/KingCountyDemographics2012.ashx?la=en. Accessed March 20, 2015.
- 15.Rollnick S, Miller WR, Butler C. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008. [Google Scholar]
- 16.UCLA Institute for Digital Research and Education. Regression with Stata: chapter 4–beyond OLS. Available at: http://www.ats.ucla.edu/stat/stata/webbooks/reg/chapter4/statareg4.htm. Accessed May 5, 2013.
- 17.Little RJ, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 18. Costco Pharmacy. FLOVENT. Available at: http://www.costco.com/Pharmacy/drug-results-details-price?storeId=10301&catalogId=10701&langId=-1&searchKeyword=flovent&drugId=263&drugName=FLOVENT&drugSearch=headerDrugSearch&isPharmacy=true&encodedDrugName=FLOVENT. Accessed December 20, 2013.
- 19.Clark NM, Griffiths C, Keteyian SR, Partridge MR. Educational and behavioral interventions for asthma: who achieves which outcomes? A systematic review. J Asthma Allergy. 2010;3:187–197. doi: 10.2147/JAA.S14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhaumik U, Norris K, Charron G et al. A cost analysis for a community-based case management intervention program for pediatric asthma. J Asthma. 2013;50(3):310–317. doi: 10.3109/02770903.2013.765447. [DOI] [PubMed] [Google Scholar]
- 21.Morphew T, Scott L, Li M et al. Mobile health care operations and return on investment in predominantly underserved children with asthma: the Breathmobile Program. Popul Health Manag. 2013;16(4):261–269. doi: 10.1089/pop.2012.0060. [DOI] [PubMed] [Google Scholar]
- 22.Campbell JD, Spackman DE, Sullivan SD. Health economics of asthma: assessing the value of asthma interventions. Allergy. 2008;63(12):1581–1592. doi: 10.1111/j.1398-9995.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 23.Morgan WJ, Crain EF, Gruchalla RS et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan JA, Lemanske RF, Canino GJ et al. Asthma outcomes: symptoms. J Allergy Clin Immunol. 2012;129(3, suppl):S124–S135. doi: 10.1016/j.jaci.2011.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27(3):759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]