Abstract
Objectives. We examined the prevalence and correlates of human papillomavirus (HPV) vaccine initiation among adolescents in low-income, urban areas.
Methods. The study consisted of electronic health record data on HPV vaccination for 3180 adolescents (aged 10–20 years) at a multisite community health center in 2011.
Results. Only 27% initiated the HPV vaccine. The adjusted odds ratio (AOR) of HPV vaccination was lower among older adolescents (AOR = 0.552; 95% confidence interval [CI] = 0.424, 0.718) and those seen by nonpediatric health care providers (HCPs; AOR = 0.311; 95% CI = 0.222, 0.435), and higher among non-English speakers (AOR = 1.409; 95% CI = 1.134, 1.751) and those seen at 2 site locations (AOR = 1.890; 95% CI = 1.547, 2.311). Insurance status was significant only among female and Hispanic adolescents. Language was not a predictor among Hispanic adolescents. Across all analyses, the interaction of age and HCP specialty was associated with HPV vaccination. Dramatically lower HPV vaccination rates were found among older adolescents seen by nonpediatric HCPs (3%–5%) than among other adolescents (23%–45%).
Conclusions. Improving HPV vaccination initiation in low-income urban areas is critical to reducing disparities in cervical and other HPV-related cancer, especially among Black, Hispanic, and low-income populations.
Human papillomavirus (HPV) infection is a known risk factor for the development of several cancers. Between 2004 and 2008, there was a national average of 33 369 HPV-associated cancers annually, including cervical, vulvar, vaginal, penile, anal, and oropharyngeal cancers.1 The Centers for Disease Control and Prevention estimates 26 000 new HPV-associated cancers each year, 18 000 for women and 8000 for men,1 which could be prevented through the HPV vaccine.
According to the US Cancer Statistics Working Group,2 there are pervasive disparities in national morbidity and mortality rates of HPV-related cancers for Black and Hispanic individuals. Cervical cancer is more common among Black and Hispanic women and results in disproportionately higher mortality for Black women. In 2009, the national age-adjusted cervical cancer incidence rates (per 100 000) for Hispanic and Black women (10.9 and 10.0, respectively) were higher than the rate for White women (7.6).2 The national age-adjusted cervical cancer mortality rate (per 100 000) for Black women (4.2) is considerably higher than the rates for White and Hispanic women (2.1 and 2.9, respectively).2 Also, Black women have higher morbidity and mortality rates of vaginal cancer. Morbidity and mortality rates of penile cancers are significantly higher among Black and Hispanic men. Black men have higher morbidity and mortality rates of anal cancer.2 In addition to race/ethnicity, incidence rates of penile, cervical, and vaginal cancers increase with higher poverty rates.3 Factors that contribute to cancer disparities among Black, Hispanic, and low-income populations include higher exposure to risk factors such as smoking, physical inactivity, and HPV infection as well as lack of access to early detection and treatment services.4
New Jersey had the 10th highest morbidity rate for cervical cancer nationally for 2006 through 2010.5 According to the New Jersey State Cancer Registry, cervical cancer morbidity from 2005 to 2009 was significantly higher in the Greater Newark area (relative risk = 1.86; the study target area) than other areas in the state, as well as among women who are Black, Hispanic, foreign-born, non–English-speaking, uninsured, with lower income and education, unmarried, unemployed, and living in a rented residence.6 According to a community health needs assessment for the City of Newark in 2013,7 52.4% of the residents are Black, 33.8% are Hispanic, and 30% are foreign-born, compared with 13%, 18%, and 20%, respectively, in the state. Also, 28.4% of the residents are below the federal poverty level compared with 9.4% statewide, and 28% are uninsured compared with 8.4% statewide. A significant proportion of the residents has less than a high-school education (30%) and a low level of English proficiency (25%).7
Transmission of HPV can be reduced through limiting the number of sexual partners, delaying the initiation of sexual activity, practicing safe sex, and getting vaccinated.8 Two vaccines have been approved by the Food and Drug Administration for protection against HPV: the quadrivalent vaccine (Gardasil, Merck, Kenilworth, NJ) for female and male individuals aged 9 to 26 years,9 and the bivalent vaccine (Cervarix, GlaxoSmithKline, Middlesex, England) for female individuals aged 10 to 25 years.10 The HPV vaccine requires a series of 3 injections within 6 months. Markowitz et al.11 examined the rates of HPV infection among female individuals before and after the vaccine was introduced in 2006, by using data from the National Health and Nutrition Examination Surveys for 2003 through 2010. They found that for female adolescents aged 14 to 19 years, there was a 55.7% reduction in vaccine-type HPV infection rate (HPV types 6, 11, 16, and 18) and a 50% reduction in high-risk vaccine-type HPV infection rate (HPV types 16 and 18). There was also an 88% decrease among the sexually active women in their rate of vaccine-type HPV infection when they compared those who were vaccinated to those who were not vaccinated.11 Niccolai et al.12 also found significant decline in the rates of high-grade cervical lesions from 2008 to 2011 among women aged 21 to 24 years in Connecticut. Unfortunately, this trend was attenuated in urban areas as well as areas with higher concentrations of Black, Hispanic, and low-income populations.12
According to the National Immunization Survey—Teen (NIS-Teen),13 HPV vaccine initiation rates for female adolescents were 44.3% in 2009, 48.7% in 2010, 53.0% in 2011, and 53.8% in 2012. This reflects minimal improvement in 2011, no improvement in 2012, and reaching a plateau for female vaccination at a level dramatically lower than the goal of 80% completion rate for girls aged 13 to 15 years set by Healthy People 2020. In site-based studies, HPV vaccine initiation among female adolescents ranged between 9.4% and 62.9%.14–21 Also, initiation for female adolescents was lower for Spanish speakers,22 those who were uninsured,23–25 those with shorter duration of enrollment in health insurance,26 in nonpediatric settings,21,24 among those who have not had a preventive visit in the past 12 months,21,24,27–30 and with mothers’ lack of knowledge about HPV infection or vaccine.18,27,28,31,32 Some studies reported lower initiation among younger female adolescents,15,18,21,24,29,30 whereas others reported the opposite.21,26 Several studies have shown the importance of health care providers’ (HCPs’) recommendations for HPV vaccine initiation among female adolescents.16,28,30,31,33
According to NIS-Teen,13 HPV vaccine initiation rates for male adolescents were 1.4% in 2010, 8.3% in 2011, and 20.8% in 2012. This reflects low but steady improvement in HPV vaccination rates among male adolescents. In site-based studies, HPV vaccine initiation among male adolescents ranged between 1.1% and 30%.14,34–37 Literature is lacking on factors associated with HPV vaccine initiation among male adolescents. One study reported lower levels of knowledge among Black and Hispanic parents about the use of HPV vaccine for male adolescents.35 A few studies indicated the importance of HCPs’ recommendation for HPV vaccine initiation among male adolescents.14,35,36,38
Pervasive disparities exist in HPV vaccination among Black, Hispanic, and low-income groups, and more specifically in the study target area. Even though the NIS-Teen data for 2011 and 2012 show slightly higher HPV vaccination among Black and low-income groups,39,40 several studies have demonstrated a significant and continuing trend of lower HPV vaccination among Black and Hispanic adolescents,14,15,17,24,26,41,42 as well as in low-income and urban areas.22,33,41,43 Vaccination disparities in urban areas (compared with suburban or rural areas) may be attributed to residential segregation, differential distribution of health clinics and health professionals, and unequal access to a broad range of services.44–46 As urban areas, particularly the Greater Newark area, have high proportions of immigrants who may be hesitant to seek health care services because of cultural or language barriers or concerns about immigration status,7 a study of adolescents’ adherence to public health recommendations in underserved, inner-city areas is warranted and important.
Literature is lacking information on correlates of HPV vaccination among Black and Hispanic adolescents in low-income urban areas, who represent populations with the greatest disparities in cervical cancer and other HPV-related cancers compared with White and higher-income groups. Therefore, the purpose of this study was to examine the correlates of HPV vaccine initiation in a sample of predominantly Black and Hispanic adolescents at inner-city community health centers. The study addresses gaps in knowledge about the correlates of HPV vaccination among both male and female adolescents as well as a low-income predominantly minority population with pervasive disparities in cervical cancer morbidity and mortality.1–3,5,6
METHODS
This is a descriptive correlational study of factors associated with HPV vaccine initiation. We obtained study data from electronic health records from the Newark Community Health Centers (NCHC), Newark, New Jersey. The NCHC is a federally qualified health care organization composed of 7 health centers in 4 communities of the Greater Newark area. Four of the centers are located in Newark, 1 in Irvington, 1 in East Orange, and 1 in Orange. Annually, NCHC serves approximately 19 000 ethnically diverse patients, most of whom are from low-income and uninsured families. Five of their health centers were included in this study; the 2 excluded centers do not provide pediatric services.
The study sample included 3180 adolescents who were seen at NCHC in 2011. The inclusion criteria were being aged 10 to 20 years and having had at least 1 pediatric, obstetrics/gynecology, internal medicine, or nurse visit in 2011. The exclusion criterion was being pregnant. Table 1 provides a summary of the study sample characteristics. In the study sample, 61% were female, 85% were Black or Hispanic, 25% were non-English speakers, 33% were uninsured, and 67% were seen by a pediatric HCP. The outcome variable was HPV vaccine initiation by comparing adolescents who had received at least 1 dose of the HPV vaccine with those who did not receive any HPV vaccination. The study included adolescents who had a visit at NCHC in 2011; however, HPV vaccination may have taken place in 2011 or 2010. The majority of adolescents in this study who received the vaccine (86%) had it in 2011. The predictor variables were gender, race/ethnicity, age, insurance status, language, specialty of HCP, and site of service. Characteristics of the study sample are presented in Table 1. The rates and correlates for completion of the HPV vaccine 3-dose series as well as adherence with recommended dosing intervals are reported elsewhere.47
TABLE 1—
Characteristics of Study Sample: Electronic Health Record Data From Adolescents Served at the Newark Community Health Centers; Greater Newark Area, NJ; 2011
| Study Variable | All (n = 3810), No. (%) | Black (n = 1713), No. (%) | Hispanic (n = 1000), No. (%) |
| Gender | |||
| Female | 1940 (61.0) | 1038 (60.6) | 631 (63.1) |
| Male | 1240 (39.0) | 675 (39.4) | 369 (36.9) |
| Race/ethnicity | |||
| Hispanic | 1000 (31.4) | . . . | . . . |
| Non-Hispanic Black | 1713 (53.9) | . . . | . . . |
| Non-Hispanic White | 75 (2.4) | . . . | . . . |
| Non-Hispanic other | 223 (7.0) | . . . | . . . |
| Age, y (mean = 15.68; SD = 3.18) | |||
| 10–12 | 701 (22.0) | 384 (22.4) | 204 (20.4) |
| 13–15 | 760 (23.9) | 414 (24.2) | 242 (24.2) |
| 16–18 | 916 (28.8) | 479 (28.0) | 299 (29.9) |
| 19–20 | 803 (25.3) | 436 (25.5) | 255 (25.5) |
| Language | |||
| English | 2033 (75.0) | 1359 (89.2) | 410 (45.7) |
| Spanish | 506 (18.7) | 5 (0.3) | 487 (54.3) |
| Haitian Creole | 173 (6.4) | 160 (10.5) | |
| Insurance | |||
| Private | 1635 (51.5) | 1009 (58.9) | 386 (38.6) |
| Medicaid | 500 (15.7) | 289 (16.9) | 145 (14.5) |
| Uninsured or self-pay | 1042 (32.8) | 414 (24.2) | 468 (46.8) |
| Department | |||
| Pediatrics | 2118 (66.6) | 1162 (67.8) | 652 (65.2) |
| OB/GYN | 404 (12.7) | 206 (12.0) | 143 (14.3) |
| Internal medicine | 286 (9.0) | 183 (10.7) | 69 (6.9) |
| Nurse visit (RN or NP) | 372 (11.7) | 162 (9.5) | 136 (13.6) |
| Site | |||
| Newark-North | 777 (25.3) | 156 (9.1) | 406 (43.8) |
| Newark-South | 361 (11.7) | 98 (5.7) | 211 (22.8) |
| East Orange | 785 (25.5) | 657 (38.5) | 79 (8.5) |
| Irvington | 773 (25.1) | 579 (33.9) | 112 (12.1) |
| Orange | 381 (12.4) | 217 (12.7) | 119 (12.8) |
| HPV vaccine initiationa | |||
| No | 2308 (72.6) | 1206 (70.4) | 746 (74.6) |
| Yes | 872 (27.4) | 507 (29.6) | 254 (25.4) |
Note. HPV = human papillomavirus; NP = nurse practitioner; OB/GYN = obstetrics/gynecology; RN = registered nurse. Percentages may not add to 100 because of rounding.
Received at least 1 dose of the HPV vaccine.
For the data analysis, we dichotomized the study predictors. We dichotomized age into younger (aged 10–15 years) versus older (aged 16–20 years). Age, as a continuous predictor of HPV vaccine initiation, had a quadratic distribution, which prohibited its use as a continuous measure. Dichotomization of age was based on a sharp decrease in HPV vaccine initiation that takes place at around age 16 years for both female and male adolescents. The sample was not heavily skewed toward either younger or older participants. We examined the initiation rates by age as a continuous measure in a simple linear regression. The relation between initiation rates and age follows a quadratic function (F2, 10 = 59.22; P < .001; adjusted R2 = 0.92).
Examination of the association by gender shows the same quadratic associations as well as lower rates for female relative to male participants by age 15 years. Because the rates fell after age 15 years, we recoded the age factor into 2 groups, 10 to 15 years and 16 to 20 years, and examined the first-order interactions such as provider to identify factors associated with the lower rates of initiation. Examination of initiation rates over age by provider shows higher rates among the pediatrician providers. Therefore, we conducted dichotomization of age because of the quadratic distribution of HPV vaccine initiation by age as a continuous measure and the sharp decrease in HPV vaccine initiation that takes place at around age 16 for both female and male adolescents.
We dichotomized insurance status into uninsured versus insured (including private insurance and Medicaid). We dichotomized preferred language into English versus non-English (including Spanish or Creole). We dichotomized HCP specialty into pediatric versus nonpediatric (including obstetrics/gynecology, internal medicine, or nurse visit). We dichotomized site of service for HPV vaccine into the 2 centers with highest initiation rates (site locations 1: Irvington and Orange) versus the remaining 3 centers (site locations 2: Newark-North, Newark-South, and East Orange). The HPV vaccine initiation rates by site of services varied significantly by geographic location. The rates of HPV vaccine initiation were significantly higher for Irvington and Orange (37.5% and 36.5%, respectively) compared with Newark-North, Newark-South, and East Orange (19.9%, 28.8%, and 23.4%, respectively; Pearson χ2 = 80.754; P < .001). Therefore, the rationale for this dichotomization for site of service was to examine whether the difference in HPV vaccine initiation by site survives in the multivariate analysis, independent of the other study predictors.
We imported the electronic health record data into SPSS statistical software, version 21 (SPSS Inc, Chicago, IL) for analysis. We conducted bivariate and multivariate analyses to examine the associations between the study predictors and HPV vaccine initiation. We conducted the study analyses for the whole study sample as well as through subset analyses for female, male, Black, and Hispanic adolescents, which is consistent with national reports on HPV vaccination39,40 and similar studies.14,18,35 In our study, however, the numbers of White adolescents (n = 75; 2.4%) as well as those of other racial/ethnic backgrounds (n = 233; 7%) were too small to compare against Black and Hispanic adolescents. Therefore, White adolescents and those of other racial/ethnic backgrounds were included in the analysis for the whole study sample as well as in subset analyses for female and male adolescents to provide representation of the larger population served in a similar setting. However, the subset analyses for Black and Hispanic adolescents did not represent any comparisons against White adolescents and those of other racial/ethnic backgrounds.
For the bivariate analysis of HPV vaccine initiation, we conducted the χ2 test, as shown in Table 2. Multivariate analysis included logistic regression for predictors of HPV vaccine initiation and calculation of adjusted odds ratios (AORs) and 95% confidence intervals (CIs). We have checked for possible collinearity among the study predictors. In that process, we centered the study predictors and conducted a logistic regression with backward Wald tests (with probability to enter predictor = .05; probability to remove predictor = .001; and cutoff between classifying the case as success of initiation or failure = 0.5) and tests of collinearity. Tests of collinearity for study predictors were within acceptable parameters,48 with the variance inflation factor (VIF) values below 2. Findings of the regression analyses (Table 3) show only the predictors that were significantly associated with HPV vaccine initiation. In other words, predictors that were not found significant are not listed in Table 3. We also examined in the regression analysis the effect of the following interactions among study predictors on HPV vaccination: gender*race/ethnicity (only for the whole study sample analysis); age*HCP specialty; insurance*language; insurance*site; HCP specialty*site; and language*site. We conducted posthoc analyses to further explore the interactions that were significant in the regression analysis.
TABLE 2—
Bivariate Analysis of Human Papillomavirus Vaccination Initiation by Gender and Race/Ethnicity: Electronic Health Record Data From Adolescents Served at the Newark Community Health Centers; Greater Newark Area, NJ; 2011
| Initiationa Rate | All (27.4%), % | Females, % | Males, % | Black, % | Hispanic, % |
| Gender | |||||
| Female | 24.8*** | . . . | . . . | 27.6* | 21.7*** |
| Male | 31.5 | . . . | . . . | 32.7 | 31.7 |
| Race/ethnicity | |||||
| Black | 29.6* | 27.6** | 32.7 | . . . | . . . |
| Hispanic | 25.4 | 21.7 | 31.7 | . . . | . . . |
| Age, y | |||||
| 10–15 | 36.6*** | 38.8*** | 34.0* | 39.0*** | 35.0*** |
| 16–20 | 19.7 | 15.6 | 28.3 | 21.4 | 17.7 |
| Language | |||||
| English | 18.5*** | 15.8*** | 22.8 | 20.0*** | 13.9*** |
| Non-English | 25.8 | 25.5 | 26.2 | 30.9 | 23.4 |
| Insurance status | |||||
| Insured | 28.9** | 25.7 | 33.8* | 31.6** | 26.5 |
| Uninsured | 24.5 | 23.0 | 26.7 | 23.4 | 24.5 |
| Department | |||||
| Pediatrics | 38.1*** | 38.4*** | 37.9*** | 40.8*** | 36.8*** |
| Nonpediatric | 6.0 | 5.9 | 6.3 | 6.0 | 4.0 |
| Site—recodedb | |||||
| Site locations 1 | 37.2*** | 37.0*** | 37.4*** | 36.6*** | 41.1*** |
| Site locations 2 | 23.0 | 19.6 | 28.0 | 23.7 | 22.8 |
Received at least 1 dose of the human papillomavirus vaccine.
Site locations 1 were Irvington and Orange (the 2 locations with highest rates of human papillomavirus vaccine initiation) and site locations 2 were Newark-North, Newark-South, and East Orange.
*P < .05; **P < .01; ***P < .001; P values determined by χ2 test.
TABLE 3—
Multivariate Logistic Regression Analysis for Human Papillomavirus Vaccination Initiation With Backward Wald Test: Electronic Health Record Data From Adolescents Served at the Newark Community Health Centers; Greater Newark Area, NJ; 2011
| Significant Predictors of Initiationa | B (SE) | Wald | P | Exp(B) (95% CI) |
| All adolescents | ||||
| Older vs younger adolescents | −0.594 (0.134) | 19.610 | < .001 | 0.552 (0.424, 0.718) |
| Non-English vs English speakers | 0.343 (0.111) | 9.579 | .002 | 1.409 (1.134, 1.751) |
| Nonpediatric vs pediatric | −1.169 (0.171) | 46.491 | < .001 | 0.311 (0.222, 0.435) |
| Site locations 1 vs 2b | 0.637 (0.102) | 38.671 | < .001 | 1.890 (1.547, 2.311) |
| Age * department | −2.236 (0.354) | 39.881 | < .001 | 0.107 (0.053, 0.214) |
| Constant | −1.287 (0.066) | 376.951 | < .001 | 0.276 |
| Female adolescents | ||||
| Older vs younger adolescents | −0.934 (0.203) | 21.122 | < .001 | 0.393 (0.264, 0.585) |
| Non-English vs English speakers | 0.457 (0.150) | 9.224 | .002 | 1.579 (1.176, 2.121) |
| Insured vs uninsured | 0.360 (0.153) | 5.553 | .018 | 1.433 (1.062, 1.933) |
| Nonpediatric vs pediatric | −1.188 (0.209) | 32.265 | < .001 | 0.305 (0.202, 0.459) |
| Site locations 1 vs 2b | 0.799 (0.137) | 33.794 | < .001 | 2.223 (1.698, 2.911) |
| Age * department | −1.901 (0.459) | 17.174 | < .001 | 0.149 (0.061, 0.367) |
| Constant | −1.489 (0.095) | 245.140 | < .001 | 0.226 |
| Male adolescents | ||||
| Nonpediatric vs pediatric | −1.256 (0.308) | 16.626 | < .001 | 0.285 (0.156, 0.521) |
| Site locations 1 vs 2b | 0.410 (0.157) | 6.828 | .009 | 1.507 (1.108, 2.050) |
| Age * department | −2.767 (0.550) | 25.273 | < .001 | 0.063 (0.021, 0.185) |
| Constant | −1.083 (0.088) | 149.692 | < .001 | 0.339 |
| Black adolescents | ||||
| Older vs younger adolescents | −0.536 (0.178) | 9.087 | .003 | 0.585 (0.413, 0.829) |
| Non-English vs English speakers | 0.498 (0.193) | 6.692 | .01 | 1.646 (1.128, 2.401) |
| Nonpediatric vs pediatric | −1.385 (0.237) | 34.299 | < .001 | 0.250 (0.157, 0.398) |
| Site locations 1 vs 2b | 0.498 (0.134) | 13.807 | < .001 | 1.646 (1.266, 2.141) |
| Age * department | −2.332 (0.482) | 23.408 | < .001 | 0.097 (0.038, 0.250) |
| Constant | −1.288 (0.090) | 205.292 | < .001 | 0.276 |
| Hispanic adolescents | ||||
| Older vs younger adolescents | −0.792 (0.261) | 9.217 | .002 | 0.453 (0.271, 0.755) |
| Insured vs uninsured | 0.408 (0.184) | 4.939 | .026 | 1.504 (1.049, 2.155) |
| Nonpediatric vs pediatric | −1.479 (0.332) | 19.849 | < .001 | 0.228 (0.119, 0.437) |
| Site locations 1 vs 2b | 0.781 (0.195) | 16.027 | < .001 | 2.183 (1.490, 3.200) |
| Age * department | −2.740 (0.678) | 16.337 | < .001 | 0.065 (0.017, 0.244) |
| Constant | −1.465 (0.129) | 128.874 | < .001 | 0.231 |
Note. CI = confidence interval. Predictors presented in this table include only those that were significantly associated with human papillomavirus vaccine initiation.
Received at least 1 dose of the human papillomavirus vaccine.
Site locations 1 were Irvington and Orange (the 2 locations with highest rates of HPV vaccine initiation) and site locations 2 were Newark-North, Newark-South, and East Orange.
RESULTS
As shown in Table 1, only 27.4% of the adolescents in the study sample initiated HPV vaccination. The bivariate analysis shown in Table 2 revealed that HPV vaccine initiation was slightly higher among male versus female adolescents (31.5% vs 24.8%) and among Black versus Hispanic adolescents (29.6% vs 25.4%). In addition, HPV vaccine initiation was significantly associated with all of the study predictors for the whole sample as well as among female, male, Black, and Hispanic adolescents.
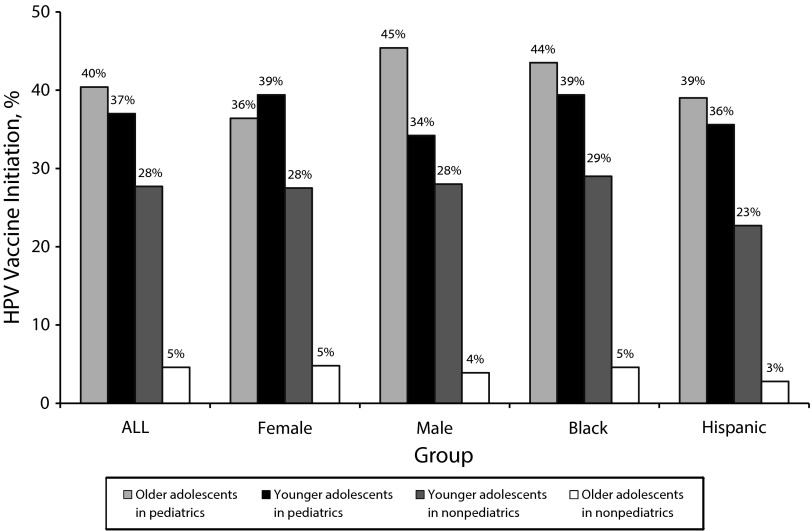
Predictors of HPV vaccine initiation with multivariate analysis are shown in Table 3 for the whole study sample as well as for female, male, Black, and Hispanic adolescents. For the whole study sample, predictors of HPV vaccine initiation included age, language, HCP specialty, site, and the interaction of age and HCP specialty. For the whole sample, the odds of HPV vaccine initiation were 44.8% lower for older adolescents (AOR = 0.552; 95% CI = 0.424, 0.718) and 68.9% lower for adolescents seen by nonpediatric HCPs (AOR = 0.311; 95% CI = 0.222, 0.435). Also, the odds of HPV vaccine initiation were 40.9% higher for non-English speakers (AOR = 1.409; 95% CI = 1.134, 1.751) and 89% higher for adolescents seen at site locations 1 (AOR = 1.890; 95% CI = 1.547, 2.311). The interaction of age and HCP specialty revealed that older adolescents seen by nonpediatric HCPs had 89.3% lower odds of HPV vaccine initiation than other adolescents (AOR = 0.107; 95% CI = 0.053, 0.214). Figure 1 presents a post hoc analysis of the interaction of age and HCP specialty, in which older adolescents seen by nonpediatric HCPs have a dramatically lower rate of HPV vaccine initiation (4.6%) compared with other subsets of adolescents (ranging between 27.7% and 40.4%).
FIGURE 1—
Interaction effect of age and specialty of health care provider on human papillomavirus vaccine initiation: electronic health record data from adolescents served at the Newark Community Health Centers, Greater Newark area, NJ, 2011.
Note. HPV = human papillomavirus.
Among female adolescents, predictors of HPV vaccine initiation in the multivariate analysis included age, language, insurance status, HCP specialty, site, and the interaction of age and HCP specialty. The odds of HPV vaccine initiation were 60.7% lower among older female adolescents (AOR = 0.393; 95% CI = 0.264, 0.585) and 69.5% lower for female adolescents seen by nonpediatric HCPs (AOR = 0.305; 95% CI = 0.202, 0.459). Also, the odds of HPV vaccine initiation were 57.9% higher for non-English speakers (AOR = 1.579; 95% CI = 1.176, 2.121), 43.3% higher for insured female adolescents (AOR = 1.433; 95% CI = 1.062, 1.933), and 2.2 times higher for female adolescents seen at site locations 1 (AOR = 2.223; 95% CI = 1.698, 2.911). The interaction of age and HCP specialty revealed that older female adolescents seen by nonpediatric HCPs had 85.1% lower odds of HPV vaccine initiation than other female adolescents (AOR = 0.149; 95% CI = 0.061, 0.367). As shown in Figure 1, analysis of the interaction of age and HCP specialty showed that older female adolescents seen by nonpediatric HCPs have a dramatically lower rate of HPV vaccine initiation (4.8%) than other subsets of female adolescents (ranging between 27.5% and 39.4%).
Among male adolescents, predictors of HPV vaccine initiation included HCP specialty, site, and the interaction of age and HCP specialty. The odds of HPV vaccine initiation were 71.5% lower among male adolescents seen by nonpediatric HCPs (AOR = 0.285; 95% CI = 0.156, 0.521) and 50.7% higher among male adolescents seen at site locations 1 (AOR = 1.507; 95% CI = 1.108, 2.050). The interaction of age and HCP specialty revealed that older male adolescents seen by nonpediatric HCPs had 93.7% lower odds of HPV vaccine initiation than other male adolescents (AOR = 0.063; 95% CI = 0.021, 0.185). As shown in Figure 1, analysis of the interaction of age and HCP specialty showed that older male adolescents seen by nonpediatric HCPs have a dramatically lower rate of HPV vaccine initiation (3.9%) than other subsets of male adolescents (ranging between 28% and 45.4%).
Among Black adolescents, predictors of HPV vaccine initiation in the multivariate analysis included age, language, HCP specialty, site, and the interaction of age and HCP specialty. The odds of HPV vaccine initiation were 41.5% lower for older Black adolescents (AOR = 0.585; 95% CI = 0.413, 0.829) and 75% lower for Black adolescents seen by nonpediatric HCPs (AOR = 0.250; 95% CI = 0.157, 0.398). The odds of HPV vaccine initiation were 64.6% higher for both Black non-English speakers (AOR = 1.646; 95% CI = 1.128, 2.401) and Black adolescents seen at site locations 1 (AOR = 1.646; 95% CI = 1.266, 2.141). The interaction of age and HCP specialty revealed that Black adolescents seen by nonpediatric HCPs had 90.3% lower odds of HPV vaccine initiation than other Black adolescents (AOR = 0.097; 95% CI = 0.038, 0.250). As shown in Figure 1, analysis of the interaction of age and HCP specialty showed that older Black adolescents seen by nonpediatric HCPs have a dramatically lower rate of HPV vaccine initiation (4.6%) than other subsets of Black adolescents (ranging between 29.0% and 35.5%).
Among Hispanic adolescents, predictors of HPV vaccine initiation included age, insurance, HCP specialty, site, and the interaction of age and HCP specialty. The odds of HPV vaccine initiation were 54.7% lower for older Hispanic adolescents (AOR = 0.453; 95% CI = 0.271, 0.755) and 77.2% for Hispanic adolescents seen by nonpediatric HCPs (AOR = 0.228; 95% CI = 0.119, 0.437). The odds of HPV vaccine initiation were 50.4% higher for insured Hispanic adolescents (AOR = 1.504; 95% CI = 1.049, 2.155) and 2.2 times higher for Hispanic adolescents seen at site locations 1 (AOR = 2.183; 95% CI = 1.490, 3.200). The interaction of age and HCP specialty revealed that Hispanic adolescents seen by nonpediatric HCPs had 93.5% lower odds of HPV vaccine initiation than other Hispanic adolescents (AOR = 0.065; 95% CI = 0.017, 0.244). As shown in Figure 1, analysis of the interaction of age and HCP specialty showed that Hispanic adolescents seen by nonpediatric HCPs have a dramatically lower rate of HPV vaccine initiation (2.8%) than other subsets of Hispanic adolescents (ranging between 22.7% and 39.0%).
DISCUSSION
The study findings provide insight on correlates of HPV vaccine initiation in a population with the greatest disparities in HPV-related cancers, particularly cervical cancer. The study addresses several gaps in the literature about HPV vaccination, including vaccination of male adolescents, underserved populations, and inner-city, Black, and Hispanic adolescents. The rate of HPV vaccine initiation among male adolescents in this study (32%) is higher than rates reported in NIS-Teen data13,39,40 as well as in site-based studies.34,36,49,50 However, the rate of HPV vaccine initiation among female adolescents (25%) is dramatically lower than the rates reported in NIS-Teen data for the same year (2011) in cities with relatively similar demographic composition (e.g., 56.8% in New York City, NY; 75.9% in Philadelphia, PA; and 47% in Chicago, IL).13,39,40 The rates of HPV vaccine initiation for the whole sample and among female, Black, and Hispanic adolescents are closer to those reported in studies in underserved and low-income areas.15,17,22,24,26,41 Nevertheless, the differences in HPV vaccination between our findings and NIS-Teen data are concerning. This could be attributed to challenges that are unique in underserved populations such as lack of information and education as well as access to services among parents in these communities.16,51 Several factors may present barriers for HPV vaccination in underserved areas such as cost, insurance coverage, or scheduling and transportation issues.
Age was a significant predictor in our study in which older adolescents had lower HPV initiation in the whole sample and among female, Black, and Hispanic adolescents. This finding is consistent with 2 other studies.21,26 However, the majority of studies reported the opposite.15,18,21,24,29,30 Furthermore, the HPV vaccine for male adolescents was relatively new in 2011, which may explain why age was not a predictor for male adolescents in our study. This suggests that we need to examine more recent data on HPV vaccination, particularly among male adolescents. Nevertheless, the effect of age could be explained by the HCP specialty. The study showed that adolescents seen by a pediatric HCP consistently had higher HPV vaccination than adolescents who had an obstetrics/gynecology, internal medicine, or nurse visit, which is consistent with findings from other studies.21,24 More important is the intertwining of age and HCP specialty, which showed a dire situation for older adolescents who receive care from nonpediatric HCPs, in which HPV vaccination rates drop to low levels of 3% to 5%. Older female adolescents may seek obstetrics/gynecology services to obtain birth control or for sexually transmitted infection prevention and treatment. Older male adolescents may seek internal medicine services for various health issues.
These are missed opportunities to educate parents about the HPV vaccine and improve the vaccine uptake. Several studies have shown the importance of HCPs’ recommendation (pediatric and nonpediatric HCPs) in improving HPV vaccination for both female16,28,30,31,33 and male adolescents and young adults.14,35,36,38 Qualitative studies have shown the importance of HCPs’ recommendation for mothers in the decision-making process for HPV vaccination.32,38,52,53 This indicates not only the importance of pediatric HCPs in improving HPV vaccination but also the critical need to educate and involve nonpediatric HCPs in efforts to promote HPV vaccination.
Language was a significant predictor in our study in which English speakers had lower HPV initiation in the whole sample and among female and Black adolescents. This may be attributable to cultural norms among non-English speakers to comply with doctors’ recommendations and not question medical authority. English-speaking Black mothers (who are most likely African American) may be more inclined to question the doctor’s recommendation for HPV vaccination and ask for information about the indications and side effects, and non–English-speaking Black mothers (who are most likely Haitian) may feel obligated to respect the doctor’s authority and comply with the recommendation for HPV vaccination. Studies have shown higher levels of distrust with the health care system and skepticism about the HPV vaccine among African American mothers than among mothers of other Black ethnicities.32,54,55 Among Hispanic adolescents, there was no difference in HPV vaccination between parents who spoke English and parents who spoke Spanish in our study. Some studies found lower HPV vaccine knowledge and uptake among Spanish speakers22,56,57 whereas others reported no difference by language.22,58 The issue of language among Hispanics may or may not be tied to acculturation. Certainly, studies are needed to further examine HPV vaccination within Hispanic subgroups.
Insurance in our study was a predictor of HPV vaccination among only female and Hispanic adolescents. The impact of insurance status has been reported in other studies,23–25 more specifically regarding the duration of enrollment in health insurance,26 which we did not examine in our study. Even though the HPV vaccine is available for free at the study site regardless of insurance status, uninsured mothers are less likely to have ever heard of the HPV vaccine,25 are unaware of its availability,32 and have perceived concerns about costs associated with the vaccine.53 Furthermore, uninsured mothers may have hardships that would prevent them from seeking preventive services, such as transportation and childcare issues.
The site at which the services were obtained was a significant predictor for HPV vaccine initiation in our study. Furthermore, the site had no significant interaction effect on initiation with any of the other study predictors. This indicates that the impact of site on HPV vaccine initiation is independent of age, insurance status, and HCP specialty. Possible explanations for this finding are the resources available at specific sites that may allow for flexible scheduling during evening hours and on weekends, use of reminders for appointments, staff willingness to answer questions and address parents’ concerns, etc. This could also be attributed to staff’s cultural competence with populations served at these sites. In light of the significant differences in HPV vaccine initiation by site, fidelity assessment is needed to investigate more closely potential explanations for these differences. Anecdotal data from key informants at the study site indicate several resources that could explain higher initiation at the Irvington and Orange centers, such as having more proactive pediatricians, late-night appointments, and employees who reside in the surrounding area and of similar ethnic backgrounds as the served community. More work is needed to examine endogeneity issues and the features of the different sites that may have an impact on HPV vaccine initiation.
The study findings provide several implications for practice, policy, and research. Public health practitioners and HCPs should discuss HPV vaccination initiation and completion with mothers and address any concerns they may have about the vaccine efficacy and safety, vaccination recommendations, cost, and insurance coverage. Pediatric HCPs have taken on the overwhelming portion of the responsibility in the uptake of HPV vaccination. There is a critical need to educate nonpediatric providers who provide services to adolescents and young adults about the HPV vaccine. This includes HCPs and health care workers in women’s health as well as family and internal medicine.
The findings also emphasize the need to increase efforts to improve HPV vaccination among mothers of uninsured adolescents. Uptake of HPV vaccination may be impacted by mothers’ perception of cost issues or lack of insurance coverage for the vaccine. Mothers may not be aware that the vaccine is available for free regardless of insurance status or may worry about additional cost related to return visits for the second and third HPV vaccine doses.
With regard to policy, the vaccine manufacturers and the Centers for Disease Control and Prevention have used media and advertising campaigns to raise awareness about HPV vaccination. However, these campaigns may have not adequately reached inner-city Black and Hispanic mothers.25 Also, our findings indicate the need for policy and funding to support the use of culturally competent outreach strategies to improve HPV vaccination that take into account the diversity in inner-city populations. Furthermore, school-based strategies may be an effective strategy in reaching out to inner-city Black and Hispanic mothers and informing them about the HPV vaccine and its availability at the study site.
Last, the study findings indicate the need for more studies to examine sociocultural and behavioral correlates in populations with the greatest disparities in cervical and other HPV-related cancers, particularly Black and Hispanic adolescents in low-income areas. In addition, investigation is needed in the barriers and facilitators for not only HPV vaccine initiation but also for timely completion of the 3-dose series and compliance with recommended dosing intervals.
Limitations
Notwithstanding these implications, our findings should be considered in the context of a few limitations. The study examined 2011 data; however, HPV vaccine uptake may have changed over the past few years and the results may not accurately reflect the current situation.
Although public awareness of the vaccine may have increased over the years,59,60 research is still needed to examine more recent data on HPV vaccine uptake and awareness about the vaccine within the target population. Hence, this study is still pertinent in underserved populations whose awareness of the HPV vaccine and accessibility to HCPs remains low, and in which identifying best ways to educate mothers remains crucial. Furthermore, lack of data on sexual health behaviors, communication with HCPs, and parental health beliefs prevented us from directly assessing the role of these factors.
Conclusions
Improving HPV vaccination is a national priority to reduce cancer burden and eliminate future cancer disparities. Healthy People 2020 objective IID-11.4 is to “Increase the vaccination coverage level of 3 doses of human papillomavirus (HPV) vaccine for females by age 13 to 15 years” to 80% nationally.61 The President’s Cancer Panel work “Accelerating Progress in Cancer Prevention: The HPV Vaccine Example” emphasizes the critical need to achieve widespread HPV vaccine uptake, particularly within racial/ethnic minority groups, among male adolescents, and in specific geographic regions with lower vaccination rates.62 Improving HPV vaccination is critical not only for cancer prevention but also for elimination of disparities in cervical and other HPV-related cancers among Black and Hispanic populations in low-income areas. If we aim to reduce disparities in cervical cancer and have the greatest impact on disease prevention, vaccination rates will need to be optimized in communities with elevated cervical cancer morbidity and mortality rates.
Acknowledgments
We express our sincere appreciation for the staff at Newark Community Health Centers, the study site, for facilitating the process of obtaining and verifying study data.
Human Participant Protection
The study is exempted by the institutional review board at Rutgers University. The study consisted of de-identified electronic health record data and had no direct involvement of human participants.
References
- 1.Centers for Disease Control and Prevention. Human papillomavirus–associated cancers—United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–261. [PubMed] [Google Scholar]
- 2.US Cancer Statistics Working Group. United States cancer statistics: 1999–2007 incidence and mortality web-based report. 2010. Available at: http://www.cdc.gov/uscs. Accessed November 17, 2011.
- 3.Benard VB, Johnson CJ, Thompson TD et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113(10 suppl):2910–2918. doi: 10.1002/cncr.23742. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Factors that contribute to health disparities in cancer. 2014. Available at: http://www.cdc.gov/cancer/healthdisparities/basic_info/challenges.htm. Accessed August 21, 2014.
- 5.US Cancer Statistics Working Group. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. United States cancer statistics: 2006–2010, cervical cancer ranked by state. [Google Scholar]
- 6.Roche LM, Niu X, Paddock L. Use of a GIS to analyze disparities in cervical cancer incidence in New Jersey. Poster presentation at: the North American Association of Central Cancer Registries Annual Conference; June 11–14, 2013; Austin, TX. Available at: http://naaccr.org/LinkClick.aspx?fileticket=l4_t7WepTlQ%3d&tabid=280&mid=761. Accessed March 6, 2015.
- 7. Newark Beth Israel Medical Center. Community health needs assessment. 2013. Available at: http://www.barnabashealth.org/Newark-Beth-Israel-Medical-Center/About-Us/Community-Health-Needs-Assessment.aspx. Accessed October 8, 2014.
- 8. American Cancer Society. Can cervical cancer be prevented? 2011. Available at: http://www.cancer.org/Cancer/CervicalCancer/DetailedGuide/cervical-cancer-prevention. Accessed August 17, 2011.
- 9. Food and Drug Administration. Gardasil. 2011. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM094042. Accessed May 15, 2011.
- 10.Food and Drug Administration. Cervarix. 2011. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186957.htm. Accessed May 15, 2011.
- 11.Markowitz LE, Hariri S, Lin C et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208(3):385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 12.Niccolai LM, Julian PJ, Meek JI, McBride V, Hadler JL, Sosa LE. Declining rates of high-grade cervical lesions in young women in Connecticut, 2008–2011. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1446–1450. doi: 10.1158/1055-9965.EPI-13-0272. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(33):1117–1123. [PubMed] [Google Scholar]
- 14.Gilkey MB, Moss JL, McRee A-L, Brewer NT. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30(41):5928–5934. doi: 10.1016/j.vaccine.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keenan K, Hipwell A, Stepp S. Race and sexual behavior predict uptake of the human papillomavirus vaccine. Health Psychol. 2012;31(1):31–34. doi: 10.1037/a0026812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb SL, Brewer NT, Sternberg MR et al. Human papillomavirus vaccine initiation in an area with elevated rates of cervical cancer. J Adolesc Health. 2009;45(5):430–437. doi: 10.1016/j.jadohealth.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Cook RL, Zhang J, Mullins J et al. Factors associated with initiation and completion of human papillomavirus vaccine series among young women enrolled in Medicaid. J Adolesc Health. 2010;47(6):596–599. doi: 10.1016/j.jadohealth.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Bastani R, Glenn BA, Tsui J et al. Understanding suboptimal human papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1463–1472. doi: 10.1158/1055-9965.EPI-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manhart LE, Burgess-Hull AJ, Fleming CB, Bailey JA, Haggerty KP, Catalano RF. HPV vaccination among a community sample of young adult women. Vaccine. 2011;29(32):5238–5244. doi: 10.1016/j.vaccine.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin RF, Kuttab H-M, Rihani R, Reutzel T. Patient adherence to three dose completion of the quadrivalent human papillomavirus (HPV) vaccine in a private practice. J Community Health. 2012;37(6):1145–1150. doi: 10.1007/s10900-012-9581-9. [DOI] [PubMed] [Google Scholar]
- 21.Moss JL, Gilkey MB, Reiter PL, Brewer NT. Trends in HPV vaccine initiation among adolescent females in North Carolina, 2008–2010. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1913–1922. doi: 10.1158/1055-9965.EPI-12-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chando S, Tiro JA, Harris TR, Kobrin S, Breen N. Effects of socioeconomic status and health care access on low levels of human papillomavirus vaccination among Spanish-speaking Hispanics in California. Am J Public Health. 2013;103(2):270–272. doi: 10.2105/AJPH.2012.300920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluterman NH, Terplan M, Lydecker AD, Tracy JK. Human papillomavirus (HPV) vaccine uptake and completion at an urban hospital. Vaccine. 2011;29(21):3767–3772. doi: 10.1016/j.vaccine.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am J Epidemiol. 2010;171(3):357–367. doi: 10.1093/aje/kwp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pourat N, Jones JM. Role of insurance, income, and affordability in human papillomavirus vaccination. Am J Manag Care. 2012;18(6):320–330. [PubMed] [Google Scholar]
- 26.Staras SAS, Vadaparampil ST, Haderxhanaj LT, Shenkman EA. Disparities in human papillomavirus vaccine series initiation among adolescent girls enrolled in Florida Medicaid programs, 2006–2008. J Adolesc Health. 2010;47(4):381–388. doi: 10.1016/j.jadohealth.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiter PL, Cates JR, McRee AL et al. Statewide HPV vaccine initiation among adolescent females in North Carolina. Sex Transm Dis. 2010;37(9):549–556. doi: 10.1097/OLQ.0b013e3181d73bf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerry SL, De Rosa CJ, Markowitz LE et al. Human papillomavirus vaccine initiation among adolescent girls in high-risk communities. Vaccine. 2011;29(12):2235–2241. doi: 10.1016/j.vaccine.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 29.Reiter PL, McRee AL, Gottlieb SL, Brewer NT. Correlates of receiving recommended adolescent vaccines among adolescent females in North Carolina. Hum Vaccin. 2011;7(1):67–73. doi: 10.4161/hv.7.1.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009 [correction in Pediatrics. 2012;130(1):166–168] Pediatrics. 2011;128(5):830–839. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 31.Brewer NT, Gottlieb SL, Reiter PL et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson R, Brown DR, Boothe MA, Harris CE. Knowledge and acceptability of the HPV vaccine among ethnically diverse Black women. J Immigr Minor Health. 2013;15(4):747–757. doi: 10.1007/s10903-012-9749-5. [DOI] [PubMed] [Google Scholar]
- 33.Lau M, Lin H, Flores G. Factors associated with human papillomavirus vaccine-series initiation and healthcare provider recommendation in US adolescent females: 2007 National Survey of Children’s Health. Vaccine. 2012;30(20):3112–3118. doi: 10.1016/j.vaccine.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Hechter RC, Chun C, Sy LS et al. Quadrivalent human papillomavirus vaccine uptake in adolescent boys and maternal utilization of preventive care and history of sexually transmitted infections. Am J Public Health. 2013;103(9):e63–e68. doi: 10.2105/AJPH.2013.301495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkins RB, Apte G, Marquez C et al. Factors affecting human papillomavirus vaccine use among White, Black and Latino parents of sons. Pediatr Infect Dis J. 2013;32(1):e38–e44. doi: 10.1097/INF.0b013e31826f53e3. [DOI] [PubMed] [Google Scholar]
- 36.Reiter PL, McRee AL, Pepper JK, Gilkey MB, Galbraith KV, Brewer NT. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health. 2013;103(8):1419–1427. doi: 10.2105/AJPH.2012.301189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu PJ, Williams WW, Li J et al. Human papillomavirus vaccine initiation and awareness: US young men in the 2010 National Health Interview Survey. Am J Prev Med. 2013;44(4):330–338. doi: 10.1016/j.amepre.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perkins RB, Tipton H, Shu E et al. Attitudes toward HPV vaccination among low-income and minority parents of sons: a qualitative analysis. Clin Pediatr (Phila) 2013;52(3):231–240. doi: 10.1177/0009922812473775. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(34):685–693. [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(34):671–677. [PubMed] [Google Scholar]
- 41.Perkins RB, Brogly SB, Adams WG, Freund KM. Correlates of human papillomavirus vaccination rates in low-income, minority adolescents: a multicenter study. J Womens Health (Larchmt) 2012;21(8):813–820. doi: 10.1089/jwh.2011.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadigh G, Dempsey AF, Ruffin MT, Resnicow K, Carlos RC. National patterns in human papillomavirus vaccination: an analysis of the National Survey of Family Growth. Hum Vaccin Immunother. 2012;8(2):234–242. doi: 10.4161/hv.18456. [DOI] [PubMed] [Google Scholar]
- 43.Tiro JA, Tsui J, Bauer HM, Yamada E, Kobrin S, Breen N. Human papillomavirus vaccine use among adolescent girls and young adult women: an analysis of the 2007 California Health Interview Survey. J Womens Health (Larchmt) 2012;21(6):656–665. doi: 10.1089/jwh.2011.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galea S, Vlahov D. Urban health: evidence, challenges, and directions. Annu Rev Public Health. 2005;26:341–365. doi: 10.1146/annurev.publhealth.26.021304.144708. [DOI] [PubMed] [Google Scholar]
- 45.Wooten KG, Luman ET, Barker LE. Socioeconomic factors and persistent racial disparities in childhood vaccination. Am J Health Behav. 2007;31(4):434–445. doi: 10.5555/ajhb.2007.31.4.434. [DOI] [PubMed] [Google Scholar]
- 46.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson RM, Brown DR, Carmody DP, Fogarty S. HPV vaccination completion and compliance with recommended dosing intervals among female and male adolescents in an inner-city community health center. J Community Health. 2014 doi: 10.1007/s10900-014-9950-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Agresti A, Finlay B. Statistical Methods for the Social Sciences. 4th ed. Upper Saddle River, NJ: Prentice Hall; 2009. [Google Scholar]
- 49.Hirth JM, Tan A, Wilkinson GS, Berenson AB. Completion of the human papillomavirus (HPV) vaccine series among males with private insurance between 2006 and 2009. Vaccine. 2013;31(8):1138–1140. doi: 10.1016/j.vaccine.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiter PL, McRee AL, Kadis JA, Brewer NT. HPV vaccine and adolescent males. Vaccine. 2011;29(34):5595–5602. doi: 10.1016/j.vaccine.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes J, Cates JR, Liddon N, Smith JS, Gottlieb SL, Brewer NT. Disparities in how parents are learning about the human papillomavirus vaccine. Cancer Epidemiol Biomarkers Prev. 2009;18(2):363–372. doi: 10.1158/1055-9965.EPI-08-0418. [DOI] [PubMed] [Google Scholar]
- 52.Kobetz E, Menard J, Hazan G et al. Perceptions of HPV and cervical cancer among Haitian immigrant women: implications for vaccine acceptability. Educ Health (Abingdon) 2011;24(3):479. [PubMed] [Google Scholar]
- 53.Sanders Thompson VL, Arnold LD, Notaro SR. African American parents’ HPV vaccination intent and concerns. J Health Care Poor Underserved. 2012;23(1):290–301. doi: 10.1353/hpu.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown DR, Wilson RM, Boothe MA, Harris CE. Cervical cancer screening among ethnically diverse Black women: knowledge, attitudes, beliefs, and practices. J Natl Med Assoc. 2011;103(8):719–728. doi: 10.1016/s0027-9684(15)30411-9. [DOI] [PubMed] [Google Scholar]
- 55.Scarinci IC, Garcés-Palacio IC, Partridge EE. An examination of acceptability of HPV vaccination among African American women and Latina immigrants. J Womens Health (Larchmt) 2007;16(8):1224–1233. doi: 10.1089/jwh.2006.0175. [DOI] [PubMed] [Google Scholar]
- 56.Han CS, Ferris DG, Waller J, Tharp P, Walter J, Allmond L. Comparison of knowledge and attitudes toward human papillomavirus, HPV vaccine, pap tests, and cervical cancer between US and Peruvian women. J Low Genit Tract Dis. 2012;16(2):121–126. doi: 10.1097/LGT.0b013e31823a05a3. [DOI] [PubMed] [Google Scholar]
- 57.Cui Y, Baldwin SB, Wiley DJ, Fielding JE. Human papillomavirus vaccine among adult women: disparities in awareness and acceptance. Am J Prev Med. 2010;39(6):559–563. doi: 10.1016/j.amepre.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Marlow LAV, Wardle J, Forster AS, Waller J. Ethnic differences in human papillomavirus awareness and vaccine acceptability. J Epidemiol Community Health. 2009;63(12):1010–1015. doi: 10.1136/jech.2008.085886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darden PM, Thompson DM, Roberts JR et al. Reasons for not vaccinating adolescents: National Immunization Survey of Teens, 2008–2010. Pediatrics. 2013;131(4):645–651. doi: 10.1542/peds.2012-2384. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt S, Parsons HM. Vaccination interest and trends in human papillomavirus vaccine uptake in young adult women aged 18 to 26 years in the United States: an analysis using the 2008–2012 National Health Interview Survey. Am J Public Health. 2014;104(5):946–953. doi: 10.2105/AJPH.2013.301828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Healthy People 2020. Washington, DC: US Department of Health and Human Services; 2012. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=23. Accessed May 15, 2013. [Google Scholar]
- 62.President’s Cancer Panel. Bethesda, MD: National Cancer Institute; 2014. Accelerating HPV vaccine uptake: urgency for action to prevent cancer. A report to the President of the United States from the President’s Cancer Panel. Available at: http://deainfo.nci.nih.gov/advisory/pcp/annualReports/HPV/index.htm. Accessed October 8, 2014. [Google Scholar]