Abstract
Two-component signal transduction systems are the primary mechanisms by which bacteria perceive and respond to changes in their environment. The Hk1/Rrp1 two-component system (TCS) in B. burgdorferi consists of a hybrid histidine kinase and a response regulator with diguanlyate cyclase activity, respectively. Phosphorylated Rrp1 catalyzes the synthesis of c-di-GMP, a second messenger associated with bacterial life-style control networks. Spirochetes lacking either Hk1 or Rrp1 are virulent in mice but destroyed within feeding ticks. Activation of Hk1 by exogenous stimuli represents the seminal event for c-di-GMP signaling. We reasoned that structural characterization of Hk1's sensor would provide insights into the mechanism underlying signal transduction and aid in the identification of activating ligands. The Hk1 sensor is composed of three ligand-binding domains (D1-3), each with homology to periplasmic solute-binding proteins (PBPs) typically associated with ABC transporters. Herein, we determined the structure for D1, the most N-terminal PBP domain. As expected, D1 displays a bilobed Venus Fly Trap-fold. Similar to the prototypical sensor PBPs HK29S from Geobacter sulfurreducens and VFT2 from Bordetella pertussis, apo-D1 adopts a closed conformation. Using complementary approaches, including SAXS, we established that D1 forms a dimer in solution. The D1 structure enabled us to model the D2 and D3 domains. Differences in the ligand-binding pockets suggest that each PBP recognizes a different ligand. The ability of Hk1 to recognize multiple stimuli provides spirochetes with a means of distinguishing between the acquisition and transmission blood meals and generate a graded output response that is reflective of the perceived environmental threats.
Introduction
Two-component signal transduction systems (TCS) are a principal molecular mechanism by which bacteria sense and adapt to perturbations in their surroundings (Capra and Laub, 2012; Gao and Stock, 2009; Mascher et al., 2006). Prototypical TCSs are composed of a sensory histidine kinase (HK) and a response regulator (RR) (Gao and Stock, 2009). The vast majority of HKs, contain a highly variable periplasmic (or, with Gram-positive bacteria, extracellular) sensor and a well-conserved cytoplasmic histidine kinase core that is comprised of (i) an N-terminal HisKA domain involved in histidine phosphorylation/dimerization (DHp) and (ii) a C-terminal catalytic and ATP-binding (CA) domain (Gao and Stock, 2009; Krell et al., 2010; Mascher et al., 2006). Although less common, HKs also can be entirely cytoplasmic. The RR component contains a readily identifiable receiver (REC) and a variable effector domain (i.e., DNA binding, enzymatic activity) (Galperin, 2010; Gao and Stock, 2009). Activation of bacterial HKs most often begins with a ligand-induced conformational change within the sensor which, when transmitted across the cytoplasmic membrane via one or more flanking transmembrane (TM) domains, stimulates autophosphorylation of a conserved His residue within the histidine kinase core (Cheung and Hendrickson, 2010; Gao and Stock, 2009; Krell et al., 2010; Lowe et al., 2012b). The high-energy phosphoryl group is then transferred to an Asp residue within the corresponding RR REC domain (Galperin, 2010; Gao and Stock, 2009). Hybrid sensory HKs contain both histidine kinase core and REC domains, enabling them to function as “stand alone” systems or as part of more elaborate signal transduction networks (Gao and Stock, 2009; Krell et al., 2010). While tens of thousands of HK sensor domains have been identified in bacterial genomes, only a handful of activating molecules have been defined (Gao and Stock, 2009; Mascher et al., 2006; Ulrich and Zhulin, 2010).
Periplasmic-sensing HKs represent the largest group of membrane-bound sensory HKs and, in general, fall into one of four structural classes (Cheung and Hendrickson, 2010; Mascher et al., 2006): (i) PhoQ/CitA/DcuS (PDC)-type sensor domains adopt a “PAS-like” α+β fold and respond to a variety of environmental stimuli, including divalent cations (Ca2+ and Mg2+), antimicrobial peptides, and citrate and C4-dicarboxylates (i.e., succinate, fumarate, and malate) (Cheung and Hendrickson, 2010; Gao and Stock, 2009; Krell et al., 2010; Mascher et al., 2006). (ii) NarX- and TorS-type sensors consist of single or double four-helix bundles (Baraquet et al., 2006; Cheung and Hendrickson, 2009; Moore and Hendrickson, 2009) and respond to nitrite/nitrate and trimethylamine-N-oxide, respectively (Jourlin et al., 1996; Lee et al., 1999). (iii) Reg-prop-type sensors display a β-propeller fold and appear to respond primarily to sugars (Lowe et al., 2012a; Zhang et al., 2014). (iv) PBP-type sensor domains which display strong structural similarity to high-affinity type 2 periplasmic binding proteins (PBPs) for ABC transporters (Berntsson et al., 2010; Mascher et al., 2006; Tam and Saier, 1993). First described in 2009 by Cheung et al. (Cheung et al., 2009), sensor PBPs, represented by HK29S from Geobacter sulfurreducens (Cheung et al., 2009) and VFT1 and VFT2 from Bordetella pertussis (Dupré et al., 2015; Herrou et al., 2010), are thought to function by the Venus Fly Trap (VFT) mechanism most often associated with ABC transporters (Berntsson et al., 2010; Mao et al., 1982; Quiocho and Ledvina, 1996). With ABC transporter PBPs, occupancy of the ligand binding pocket induces a transition from an open to a closed conformation, allowing the liganded-PBP to interact with its cognate membrane-bound permease (Berntsson et al., 2010; Mao et al., 1982; Quiocho and Ledvina, 1996). In the case of sensor PBPs, the open-to-closed conformational change induced by ligand binding is translated across the cytoplasmic membrane via a piston-like or rotational motion within the TM domains, which in turn, activates (or antagonizes) the kinase core (Cheung and Hendrickson, 2010; Lowe et al., 2012b; Mascher et al., 2006; Moore and Hendrickson, 2009; Neiditch et al., 2006; Sevvana et al., 2008).
Borrelia burgdorferi, the Lyme disease spirochete, is maintained in nature via an obligate enzootic cycle involving Ixodes ticks and mammalian reservoir hosts (Pal and Fikrig, 2010; Radolf et al., 2012; Steere et al., 2004). The transmission, survival and pathogenic potential of B. burgdorferi depend on the bacterium's ability to modulate its transcriptome as it transits between its arthropod vector and mammalian reservoir hosts (Groshong and Blevins, 2014; Iyer et al., 2015; Pal and Fikrig, 2010; Radolf et al., 2012; Skare et al., 2010). Remarkably, differential gene expression in B. burgdorferi is orchestrated by just two Two-Component Systems (TCS) (Fraser et al., 1997). One, Hk2/Rrp2, is composed of an entirely cytoplasmic HK with a PAS-like sensor and a cytoplasmic response regulator, respectively (Galperin, 2010; Xu et al., 2010; Yang et al., 2003). Although the contribution of Hk2 to phosphorylation of Rrp2 remains uncertain (Xu et al., 2010), Rrp2-P acts in concert with the DNA-binding Fur ortholog BosR to promote RpoN-dependent transcription of rpoS at the onset of the nymphal blood meal (Hyde et al., 2009; Katona et al., 2004; Ouyang et al., 2008; Ouyang et al., 2015; Ouyang et al., 2012; Radolf et al., 2012; Samuels, 2011; Smith et al., 2007; Yang et al., 2003). RpoS, in turn, controls the reciprocal expression of genes involved in tick transmission and mammalian infection (Caimano et al., 2007; Dunham-Ems et al., 2012; Fisher et al., 2005; Groshong and Blevins, 2014; Hubner et al., 2001; Ouyang et al., 2012; Xu et al., 2012). B. burgdorferi's other TCS, Hk1/Rrp1, consists of a membrane-bound hybrid sensory HK and a cytoplasmic RR with diguanlyate cyclase activity, respectively (Caimano et al., 2011; He et al., 2011; Kostick et al., 2011). Upon phosphorylation by Hk1, Rrp1 catalyzes the synthesis of c-di-GMP (Caimano et al., 2015; Kostick et al., 2011; Ryjenkov et al., 2005), a second messenger associated with a wide range of bacterial life-style control networks, including the transition from planktonic to sessile states during biofilm formation (Povolotsky and Hengge, 2012; Romling et al., 2013). B. burgdorferi lacking either Hk1 or Rrp1 are virulent in mice by needle-inoculation but are destroyed within the midguts of feeding ticks during acquisition and transmission (Caimano et al., 2015; Caimano et al., 2011; He et al., 2011; Kostick et al., 2011). These phenotypic data, combined with results from transcriptomics analyses of Rrp1-deficient organisms grown in vitro (Caimano et al., 2015; He et al., 2011; Rogers et al., 2009), suggest that the Hk1/Rrp1 TCS activated in response to exogenous ligands encountered during tick feeding and functions to promote utilization of alternate substrates for energy generation and biosynthesis of intermediary metabolites and/or protective remodeling of the spirochete cell envelope.
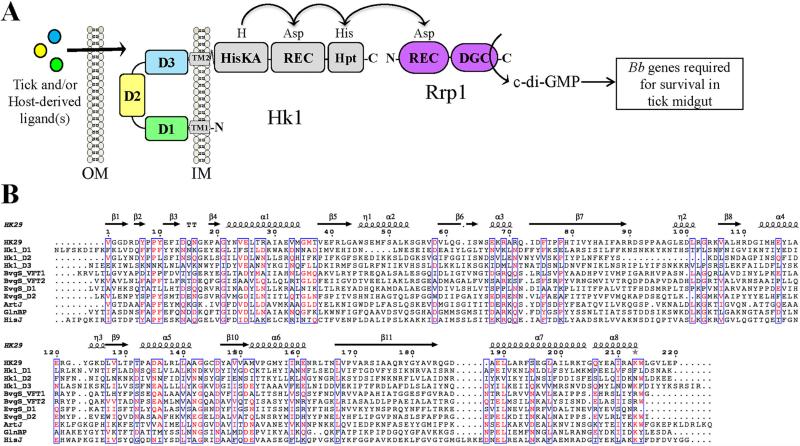
Phosphorylation of Rrp1 by Hk1 represents the seminal event for c-di-GMP signaling by B. burgdorferi (Caimano et al., 2015; Caimano et al., 2011; He et al., 2011; Kostick et al., 2011; Rogers et al., 2009; Ryjenkov et al., 2005). The periplasmic sensor for Hk1 is composed of three sensor PBP domains (D1, D2 and D3; Fig. 1A), all of which could be involved in mediating signal transduction. We reasoned that a detailed structural analysis of this sensor would provide valuable insight into the molecular mechanisms underlying Hk1-mediated signal transduction and aid in the identification of its activating ligand(s). Toward this end, we determined the crystal structure for D1, the most N-terminal PBP. As expected, D1 displays the bilobed VFT-fold that is characteristic of both ABC transporter and sensor PBPs. Similar to the HK29S and VFT2, the prototypes for sensor PBPs (Cheung et al., 2009; Herrou et al., 2010), D1 adopts a closed conformation in the absence of ligand. Using complementary approaches (e.g., size-exclusion chromatography and small angle X-ray scattering [SAXS]), we established that D1 forms a stable homodimer in solution. Using D1 as a guide, we modeled Hk1's other two sensor PBPs, D2 and D3. Despite their overall structural similarity, non-synonymous differences in the residues lining the putative ligand binding pockets for D1, D2 and D3 suggest that each recognizes a different ligand. The ability of Hk1 to perceive multiple exogenous stimuli provides Lyme disease spirochetes with a facile means of distinguishing the acquisition and transmission blood meals and calibrating c-di-GMP-dependent gene expression according to the perceived environmental threats.
Figure 1. The B. burgdorferi Hk1 periplasmic sensor is comprised of three domains, each with sequence similarity to HK sensor PBPs and ABC transporter substrate-binding proteins.
A. Schematic representation of sensor domains of B. burgdorferi Hk1. Based on bioinformatics, Hk1 is predicted to be anchored to the inner membrane by an uncleaved signal sequence (residues 6-26; TM1) and an internal transmembrane domain (residues 716-737; TM2). Conserved domains were identified using CD-Search (Marchler-Bauer and Bryant, 2004). B. Multiple sequence alignment of Hk1 sensor domains D1, D2 and D3 and prototypical sensor PBPs and ABC transporter substrate-binding proteins performed using a BLOSUM matrix. Conserved residues (≥60%) are highlighted in red and outlined in blue. The α-helices and β-strands are marked with coils and arrows, respectively, and are based on the secondary structure of HK29s from Geobacter sulfurreducens (PDB id: 3H7M). BvgS_VFT1 and BvgS_VFT2, periplasmic sensor domains from Bordatella pertussis BvgS (GenBank accession AAA22970); EvgS_D1 and EvgS_D2, periplasmic sensor domains from Escherichia coli EvgS (GenBank accession BAA03108); ArtJ, Arginine-, Histidine-, Lysine-binding protein ArtJ from Geobacillus stearothermophilus (GenBank accession KFX35677); GlnBP, GlnP Substrate Binding Domain 2 (sbd2) from Lactococcus lactis (GenBank accession WP_003132767); HisJ, ABC transporter substrate-binding protein HisJ from E. coli (GenBank accession AAA85769).
Materials and Methods
Cloning, overexpression and purification of recombinant D1
The Hk1 (BB0420) domain D1 was amplified from B. burgdorferi strain 5A4 NP1 genomic using oligonucleotide primers D1-5’-NheI (5’-TAGCTAGCAACGTCAATTTATTTTCTAAGGAT-3’) and D1-3’-XhoI (5’-GTGCTCGAGCTTAGCATTGCTATCTAAAAAAGA-3’). The resulting amplicon was cloned into NheI/XhoI-digested pET28a (Novagen, San Diego, CA) for overexpression in Escherichia coli Overexpress™ C41 (DE3) cells (Lucigen, Middleton, WI). A hexahistidine affinity tag was fused in-frame at the Hk1 N-terminus to facilitate purification by metal affinity chromatography. The nucleotide sequence of the resulting construct, pMC2748, was confirmed by sequencing (Genewiz, Inc., South Plainfield, NJ). For overexpression of recombinant protein, an overnight culture of MC2748 was used to inoculate 4-L of LB-Miller broth containing kanamycin (50 μg ml−1). Cultures were incubated at 37°C with shaking at 200 rpm. At A600 = ~0.6, expression was induced by the addition of isopropyl β-D-1-thiogalactopyranoside (final concentration, 0.5 mM), followed by further growth for 3-4 h. Cells were harvested by centrifugation at 6000 × g for 30 min at 4°C and the resulting pellets were suspended in buffer containing 20 ml of 50 mM Tris (pH 7.5), 10% glycerol, 100 μg of lysozyme (Sigma-Aldrich, St. Louis, MO) and 100 μl of protease inhibitor mixture (Sigma-Aldrich) and lysed by sonication. The sonicated lysate was centrifuged at 20,000 × g for 30 min at 4°C followed by filtration through a 0.22 μM filter before loading onto a 5-ml nickel-nitrilotriacetic acid (Ni-NTA)(Qiagen, Valencia, CA) column equilibrated with Buffer A (50 mM Tris [pH 7.5], 100 mM NaCl, and 10 mM imidazole). The column was washed once with 25-mls of Buffer A, followed by successive washes with Buffer A containing 20 mM and 40 mM imidazole. Recombinant D1 was cleaved off the Ni-NTA column by digestion with thrombin (100 units) for 3-4 h at 22°C. Cleaved D1 was loaded onto a SP-Sepharose (Bio-Rad, Hercules, CA) cation-exchange column, which was washed extensively with 25 mM Tris (pH 7.5) and 10% glycerol. D1 was eluted with a gradient of 0–1.0 M NaCl. Fractions containing D1 were pooled and subjected to size-exclusion chromatography over a Superdex 200 10/300 Tricorn column (GE Healthcare Life Sciences, Pittsburgh, PA) equilibrated with 50 mM Tris (pH 7.5) and 100 mM NaCl. Selenomethionine (SeMet)-substituted D1-His was purified as described above using from E. coli grown in M9 minimal medium supplemented with a mixture of 100 mg each of lysine, phenylalanine and threonine, 50 mg of isoleucine, leucine and valine, and 60 mg of SeMet. Far-UV circular dichroism spectroscopy was performed as previously described (Luthra et al., 2011) to ensure that the purified recombinant D1 protein remained properly folded.
Analytical size exclusion chromatography
Size-exclusion chromatography was carried out on a Superdex 200 10/300 Tricorn column (GE Healthcare Life Sciences, Pittsburgh, PA) on a BioLogic Duoflow FPLC workstation (Bio-Rad) using the following calibration standards: Thyroglobulin (Stokes radius [Rs] 8.5 nm; 670 Da), Ferritin (Rs 6.1 nm; 440 Da), Aldolase (Rs 4.8 nm; 158 Da), Conalbumin (Rs 3.6 nm; 75 kDa), Chymotrypsinogen (Rs 2 nm; 25 kDa) and Ribonuclease A (Rs 1.6 nm; 13.7 kDa). The column was equilibrated and run in desired buffer at 25°C. The relative elution volume was determined using Prism Graph (Graph Pad, LaJolla, CA) from regression analysis of the log10 values for protein standards as a function of the available partition coefficient (Kav) using the equation: Kav = Ve – V0/Vg – V0, where Ve is the elution volume, V0 is the void volume determined by elution of Blue Dextran (2000 kDa) and Vg is the geometric column volume. To assess the stability of the D1 dimer, protein samples (1 mg/ml) incubated with 3 M urea at room temperature for 1 hour before being applied to a Superdex 75 16/60 column pre-equilibrated with buffer containing 50 mM Tris-HCl (pH 7.0), 0.1 M NaCl, 3 M urea and 1 mM TCEP. Protein was eluted at room temperature in the same buffer.
Crystallization, data collection and processing
Crystallization of the recombinant Hk1 SBP domain D1 (D1) was carried out at 22°C using the sitting-drop vapor-diffusion method. D1 samples were concentrated to 1.7 mg/ml in 50 mM Tris (pH 7.5), 50 mM NaCl and 1 mM Tris (2-carboxyethyl) phosphine (TCEP). Initial crystallization was performed using conditions derived by the high-throughput screening (HTS) laboratory at the Hauptman-Woodward Medical Research Institute (Luft et al., 2011). Diffraction quality crystals were obtained for the native D1 protein in conditions containing 0.1 M Tris (pH 8.0), 0.1 M Mg(NO3)2 and 20% PEG 20,000. Crystallization drops were set up by adding 2 μl of protein solution to 2 μl of mother liquor. Streak-seeding was used to improve crystal quality and reproducibility. Before flash cooling in liquid N2, crystals were cryoprotected by transferring through a 3:1 mixture of Paratone-N (Hampton Research) and Paraffin oil (Hampton Research). SeMet-substituted protein was concentrated to 1.7 mg/ml in 50 mM HEPES (pH 7.0) and 50 mM NaCl and crystallized by streak seeding as described above for the native crystals in conditions containing 0.1 M Tris-HCl (pH 8.0), 0.1 M Mg(NO3)2 and 18% PEG 20,000. Crystals of SeMet-substituted D1 were cryo-cooled using a method similar to the native crystals. All data sets were collected remotely at 100 K on beamline 17-ID-B of IMCA-CAT at the Advanced Photon Source (APS) at Argonne National Laboratory (Argonne, Illinois) using a Dectris Pilatus 6M Pixel Array. Diffraction data were measured at absorption, inflection and remote wavelengths for multiwavelength anomalous dispersion (MAD) phasing and subsequently processed with DENZO and SCALEPACK in HKL-2000 (Otowinski and Minor, 1997). Details of the data collection statistics are summarized in Table 1.
Table 1.
Data collection and refinement statistics
| Native | Inflection | Peak | Remote | |
|---|---|---|---|---|
| Data Collection | ||||
| Wavelength (Å) | 0.97910 | 0.97935 | 0.97910 | |
| Resolution range (Å) | 37.66 – 2.05 | 34.42 – 3.00 | 34.42 – 3.05 | |
| Space group | P 1 21 1 | P 1 21 1 | P 1 21 1 | P 1 21 1 |
| Unit cell | 58.30, 62.85, 111.81, 90, 101.32, 90 | 67.08, 63.27, 107.10, 90, 106.89, 90 | 66.89, 63.17, 106.58, 90, 106.58, 90 | 67.08, 63.15, 106.98, 90, 106.77, 90 |
| Rsym | 0.063 (0.554)1 | 0.064 (0.368) | 0.083 (0.662) | 0.051 (0.353) |
| Completeness (%) | 99.55 (95.0) | 91.70 (91.4) | 100 (100) | 99.3 (92.1) |
| Mean I/σ(I) | 8.82 (1.29) | 13.9 (3.87) | 14.63 (4.54) | 14.30 (4.21) |
| Redundancy | 3.3 | 2.4 | 13.3 | 2.5 |
| Refinement Statistics | ||||
| R (work/free) | 0.194510.2206 | |||
| No. of Unique Reflections | 49534 (4772) | |||
| Number of Residues | 796 | |||
| Molecules / ASU | 4 | |||
| Number of Atoms: | ||||
| Non-hydrogen | 6644 | |||
| Protein | 6354 | |||
| Ligands | 33 | |||
| Water | 257 | |||
| Wilson B-factor (Å2) | 38.64 | |||
| RMS (bonds) | 0.003 | |||
| RMS (angles) | 0.650 | |||
| Ramachandran favored (%) | 96.0 | |||
| Ramachandram outliers (%) | 0 | |||
| Clashscore | 0.32 | |||
| Overall Molprobity Score | 0.94 | |||
| PDB Deposit | 5BWJ |
Parentheses denote statistics for the outer shell.
RMS, root mean square deviations from ideal geometry of bonded angles.
Crystal structure determination, refinement and validation
SeMet-substituted D1 crystals diffracted to 3.0 Å and exhibited different cell dimensions compared to native crystals. The SeMet-substituted D1 domain construct contained 233 residues including four SeMet residues per monomer with a total of 4 monomers. Only two out of a possible 16 SeMet peaks were identified in the asymmetric unit by PHENIX (ver. 1.8.4-1496) (Adams et al., 2010). Therefore, only one Se anomalous signal was used per 466 residues in the structural determination. As a result, the initial electron density maps were not of sufficient quality to manually or automatically build a model. The electron density for one copy of D1 within the asymmetric unit was cut out using the programs Mapmask and Maprot from the CCP4 suite of programs (Collaborative Computational Project, 1994) and subsequently used as a search model for molecular replacement for both the SeMet and native D1 datasets using PHASER (Storoni et al., 2004). Successive cycles of map cutting, MR, and then NCS averaging were performed in order to improve phases. The quality of the map was manually inspected after each round of refinement to ensure that the map averaging was successful and to redefine the map boundaries in an attempt to identify the region corresponding to a single molecule. Once the maps were of a sufficient quality to clearly identify three of the molecules in the asymmetric unit, six-fold, multi-crystal averaging was performed with DMMulti from CCP4 to improve map quality and ultimately transfer phases from the low-resolution Se-Met data to the high-resolution native data set. After molecular replacement, NCS averaging was applied to improve the quality of the maps. Utilizing this improved map, an initial model of the high-resolution native data was built automatically using the AutoBuild program in PHENIX (Adams et al., 2010) with a NCS matrix enforced. Initial model building revealed the presence of the fourth molecule in the asymmetric unit. With new the four-fold NCS matrix enforced, AutoBuild was able to place approximately 80% of the total number of residues automatically. Iterative rounds of model building and restrained refinement were performed using Coot (Emsley et al., 2010) and PHENIX (ver. 1.8.4-1496) (Adams et al., 2010), respectively. NCS restraints were used during initial stages of refinement, but were released for later stages. The final refinement protocol included the addition of riding hydrogens, individual B-factor, optimized X-ray/stereochemistry and X-ray/ADP weights, and TLS refinement. Amino acid residues for D1 are numbered according to the full-length Hk1 protein. All crystallographic figures were generated in PyMOL (http://www.pymol.org/). The stereochemistry and quality of the model was validated using MolProbity (Adams et al., 2010). Dimer interaction analysis was aided by the use of PDBSum (http://www.ebi.ac.uk/pdbsum) (de Beer et al., 2014). Data processing and refinement statistics are summarized in Table 1. Coordinates and structure factors have been deposited in the PDB (PDB ID 5BWJ).
Small angle X-ray scattering (SAXS) data acquisition and analysis
SAXS and WAXS data were collected simultaneously at beamline X9 at the National Synchrotron Light Source (NSLS, Upton, NY) at 10°C by two overlapping detectors, a Mar 165 CCD SAXS detector 3.4 m from the sample, and a custom built Photonic Science CCD WAXS detector. The two-dimensional scattering images collected on the CCD detectors were circularly averaged using software developed at the beamline to yield one-dimensional scattering profiles as a function of momentum transfer q (q = 4π sin(θ)/λ, where 2θ is the scattering angle and λ is the wavelength). The X-ray wavelength was 0.855 Å and the angular range collected was 0.008 ≤q≥ 1.0060. The sample cell contained a glass capillary sealed across the evacuated chamber. Protein samples and matching buffer solutions were flowed through the capillary during exposure to reduce radiation damage. For data collection, 90 μl of the protein sample at 1, 3 and 6 mg/ml or matching buffer (50 mm Tris [pH 7.5], 100 mM NaCl) was exposed for 90 s, subdivided into three 30 s exposures of 30 μl. The raw scattering data were scaled and buffer was subtracted using pyXS developed at X9 beamline at Brookhaven National Laboratory (Allaire and Yang, 2011). Individual scattering curves were visually inspected for radiation damage and aggregation prior to merging. Scattering curves for the three concentrations were scaled and merged in PRIMUS (Konarev et al., 2003) using predominantly the low Q data for the low concentrations, the high Q range data for the highest concentrations, and the mid-range intensities for the second highest concentration. Radii of gyration (Rg) and I(0) values, extrapolated from the Guinier region of the Guinier plot, were computed using PRIMUS (Konarev et al., 2003). P(r) functions were calculated using the program GNOM (Svergun, 1992). Theoretical scattering curves were computed from the D1 crystal structure and compared to experimental scattering curves using the program FoXS module (Schneidman-Duhovny et al., 2010) built in UCSF chimera (Pettersen et al., 2004). The relative molecular weight was determined from the SAXS data using AUTOPOROD (Petoukhov et al., 2012).
Bioinformatics, homology modeling and multiple sequence alignment
Conserved domains within Hk1 were identified using CD-Search (Marchler-Bauer and Bryant, 2004). Cellular location and topology for Hk1 was predicted using SignalP v 3.0 (Bendtsen et al., 2004), TMpred (Hofmann and Stoffel, 1993) and TOPCONS (Tsirigos et al., 2015). Dimeric interfaces between PBP domains were determined using PDBsum (http://www.ebi.ac.uk/pdbsum) (de Beer et al., 2014; Laskowski et al., 1997). Homology models of Hk1 D2 and D3 domains were generated by comparative modeling (Marti-Renom et al., 2000) approaches using MODELLER 9.13 (http://salilab.org/modeller) (Sali and Blundell, 1993). Multiple sequence alignments and graphical displays were generated using ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and ESPript (Gouet et al., 1999), respectively. The BLOSUM scoring matrix option was used for all alignments (Henikoff and Henikoff, 1992). Secondary structure prediction for all three sensor PBPs were generated using PsiPred (Buchan et al., 2013; McGuffin et al., 2000). Opening angles were calculated as previously described (Herrou et al., 2010) and are based on the angle formed between the vectors defined by α carbons of three residues (one on the lip of Lobe 1, one in the hinge region, and one on the lip of Lobe 2. Except where noted, opening angles obtained herein were equivalent or highly similar to those previously reported for the same structure (Herrou et al., 2010).
Results
The Hk1 periplasmic sensor is comprised of three tandem sensor PBP domains
Previously we reported that the periplasmic sensor for Hk1 contained two tandem domains (D1 [residues 23-249] and D2 [residues 255-461]), both of which share sequence similarity to the Cluster 3 family of bacterial type 2 periplasmic binding proteins (PBPs) (Caimano et al., 2011; Tam and Saier, 1993). Despite their low overall sequence similarity (Fig. 1B), type 2 PBPs display a high degree of structural similarity (Cheung et al., 2009; Herrou et al., 2010; Tam and Saier, 1993). Reanalysis of the Hk1 full length periplasmic sensor (residues 23-717) by CD-Search (Marchler-Bauer and Bryant, 2004) revealed a third PBP domain, D3 (residues 471-684; E value=1.29e-17). As expected, D1-3 are predicted by PsiPred to have similar secondary structures (Supplementary Fig. 1). A search of the Pfam database identified ~400 known or putative HKs containing 1-2 sensor PBP domains. However, only a handful of putative HKs contain ≥3 sensor PBPs; all of these appear to be with hybrid HKs (i.e., contain both histidine kinase and REC domains). Interestingly, with the exception of Hk1 and its orthologs in related Borrelia species (i.e., relapsing fever spirochetes), almost all of the hybrid HKs containing 3 or more PBPs are found in marine bacteria (data not shown).
Overall structure of Hk1 sensor domain D1
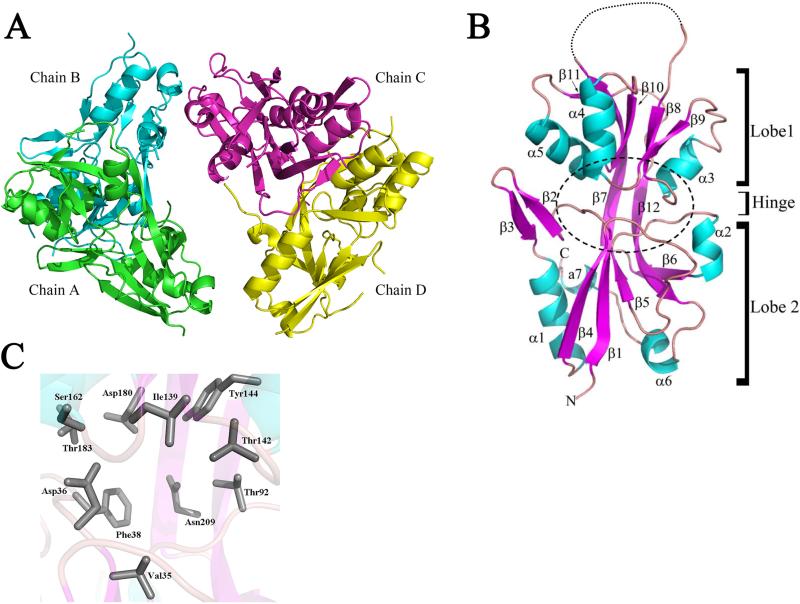
To better understand how exogenous stimuli are perceived by Hk1, we overexpressed and affinity-purified D1, the most N-terminal PBP domain, from Escherichia coli for structural characterization. The D1 crystal structure, determined by multiple-wavelength anomalous dispersion (MAD) phasing, was refined at 2.05Å. Details of the data collection parameters and refinement statistics are summarized in Table 1. Crystals of D1 grew in the monoclinic space group P21, with four molecules in the asymmetric unit arranged as a dimer of dimers (Chains A/B and C/D; Fig. 2A). Using Chain A as a reference, the root mean square deviation (RMSD) values for the B, C and D chains were 1.1 Å, 1.0 Å and 1.0 Å, respectively (also see Supplemental Table 1). In the final refined MAD structure (data not shown), it was revealed that ten of the SeMet residues were completely disordered or had disordered side chains. In the final native structure, only Met70 was consistently ordered in all chains. Met233 was present in 3 of the 4 chains (A, C and D) and Met235 was only ordered in Chain D. The high degree of disorder of the MSE residues complicated the data analysis and reduced the overall anomalous signal. The structure was ultimately solved by using the MAD dataset maps as a molecular replacement model by cutting out the density and using molecular replacement and NCS averaging over several cycles (see Materials and Methods).
Figure 2. Refined crystal structure of B. burgdorferi Hk1 sensor domain D1.
A. Ribbon drawing of the Hk1 D1 asymmetrical unit (dimer of dimers; Chains A/B and C/D). Chain A, green; Chain B, cyan; Chain C, magenta; and Chain D, yellow. B. Ribbon drawing depicting Lobe 1 [residues 27-109, 208-234] and Lobe 2 [residues 110-207] of Chain A. β strands and helices are indicated in magenta and cyan, respectively. The dashed line between β strands 7 and 8 indicates an unstructured loop. The putative ligand binding pocket (LBP) positioned between two lobes is indicated by a dashed circle. C. Side chains of residues predicted to form the D1 LBP are shown in gray. Amino acid residue numbering is based on full length native Hk1.
The Hk1 D1 monomer adopts the bilobed Venus Fly Trap (VFT) architecture that is common to ABC transporter and HK sensor PBPs (Cheung et al., 2009; Dupré et al., 2015; Fukami-Kobayashi et al., 1999; Fulyani et al., 2013; Herrou et al., 2010). The VFT-fold is characterized as having two mixed α/β globular domains (Lobes 1 and 2), each comprised of a 5-stranded β-sheet enclosed in 3-4 α-helices, connected by a two β-strand hinge region (Fig. 2B). Using the D1 structure as a query, we searched for D1 structural homologs within the RSCB Protein Data Bank (PDB) (Berman et al., 2000) using the DALI server (Holm and Rosenström, 2010). As expected, HK29S (PDB id: 3H7M) and VFT2 (PDB id: 3MPL), the prototypes for bacterial sensor PBPs and numerous amino acid ABC transporter PBPs, were among the highest scoring matches (Supplemental Table 2).
The putative ligand binding pocket (LBP) for D1, located in the cleft between Lobes 1 and 2 (Fig. 2B and C), contained only ordered water molecules (Table 1). All three subdomains (Lobe 1, Lobe 2 and the β strand hinge region) contribute amino acid side chains potentially involved in ligand binding (Lobe 1: Asp36, Phe38, Thr92, Asn209; Hinge: Ile139, Thr142, Ser162; Lobe 2: Tyr144, Asp180, Thr183). While the residues lining the LBP are predominantly acidic (Asp36, Asp180) or proton donors (Thr92, Thr142, Ser162, Tyr144, Thr183), the cavity also includes both polar (Asn209) and hydrophobic (Phe38) side chains. Many of the side chains facing the interior of the cavity participate in a water-mediated hydrogen-bonding network. We saw little to no amino acid similarity between residues forming the putative LBPs for D1 and the ABC transporter PBPs ArtJ, GlnBP and HisJ (data not shown), strongly suggesting that preferred ligand(s) for D1 differs from those of its structural homologs. On the other hand, we did see a higher degree of similarity between the D1 and VFT2 LBPs, including conservation of a phenylalanine residue (Phe38 and Phe317 in D1 and VFT2, respectively) involved in ligand binding by VFT2 (Herrou et al., 2010), although, overall, the D1 LBP is much more negatively charged (Supplemental Fig. 2).
Apo-D1 adopts a closed conformation
As per the Venus Fly Trap model, ABC transporter PBPs undergo a substantial conformational change upon ligand binding, which enables the liganded protein to interact with its cognate membrane-bound permease (Felder et al., 1999; Mao et al., 1982; Quiocho and Ledvina, 1996). Based on DALI search results, D1 most closely resembled the closed-liganded forms of ABC transporter PBPs (Supplemental Table 2). Of note, the two previously characterized sensor PBPs, HK29S and VFT2, also crystallized in a closed conformation in the absence of ligand (Cheung et al., 2009; Herrou et al., 2010). We next determined the opening angles for D1 along with a diverse panel of 10 ABC transporter and 2 HK sensor PBPs (Supplemental Fig. 3 and Supplemental Table 2). The value of the opening angle for D1 (22.3°) was the smallest of the 12 PBPs examined, strongly suggesting that this domain is in a closed conformation when unliganded. However, opening angles for unliganded transporter PBPs can vary substantially depending on the orientation of the domain (Wilkinson and Verschueren, 2003). Thus, it is possible that the opening angle for D1 in the crystal structure may not be representative of the native domain within the full length membrane-bound sensor.
Hk1 D1 forms a stable dimer
Although HKs typically function as homodimers in vivo, periplasmic sensor domains expressed without their corresponding TM and/or cytoplasmic domains are usually monomeric or dimerize only at very high concentration (Cheung and Hendrickson, 2010). Analysis of the D1 crystal structure using PDBsum (Laskowski et al., 1997) and PISA (Xu et al., 2008) indicated that the dimeric conformation is the most stable form; the free energies of dissociation (ΔGdiss) for the dimeric interfaces between Chains A/B and C/D, estimated based on the crystal structure, are 8.3 and 9.0 kcal/mol, respectively. Using PDBsum, we identified the residues responsible for D1 dimer formation (Supplemental Fig. 4). The dimeric interfaces between Chains A/B and C/D are stabilized by numerous hydrogens bonds and van der Waals interactions mainly contributed by helix α5 (residues 181-187) (Supplemental Figs. 4 and 5A). The putative LBPs for each domain are oriented on opposite sides of the dimer and tilted by ~45° relative to each other (Supplemental Fig. 5A). Using complementary approaches, we confirmed that D1 also forms a dimer in solution: (i) By analytical size-exclusion chromatography (SEC), D1 eluted at 14.4 mls with an estimated Stokes radius (Rs) of ~3.35 nm (Supplemental Fig. 5B and C), which is consistent with that of a globular protein having a molecular mass of ~60 kDa, twice that of the monomer (27.2 kDa). (ii) The SEC elution profile of D1 did not change when the protein was purified under denaturing conditions (i.e., 3 M urea) (Supplemental Fig. 5D). (iii) As described below, the ab initio envelope model for D1, determined by SAXS, most closely resembled that of the Chain A/B dimer. The ratio between the Stokes radius and radius of gyration (Rs/Rg) for D1 determined by SAXS (see below and Table 2) was ~0.81, which is consistent with D1 being a homogenous sphere (Rizos et al., 2006).
Table 2.
Summary of SAXS analysis.
| Protein Conc.a | Rg(Å)b,c | I(0)c | I(0)/Conc (μM) | MMd (Daltons) | |
|---|---|---|---|---|---|
| 1 | 18 μM | 26.05 ± 0.78 | 23.1 ± 0.08 | 1.28 | 65,641 |
| 3 | 54 μM | 27.74 ± 1.1 | 57.6 ± 0.28 | 1.06 | 55,829 |
| 6 | 108 μM | 29.68 ± 0.6 | 79.1 ± 0.28 | 0.75 | 59,795 |
| Merged datae | 27.70 ± 1.03 | 56.6 ± 1.09 | 60,422 | ||
Protein concentration determined using Nanodrop. Molarity is based on the dimeric conformation
The radius of gyration (Rg) is the root mean square distance from the center of mass to each electron.
Determined by Guinier approximation in PRIMUS (Konarev et al., 2003).
Molecular masses (MM) are based on Porod volumes calculated using AUTOPOROD (Petoukhov et al., 2012).
Values represented the merged normalized data for all three protein concentrations.
Envelope structure of D1 determined by SAXS
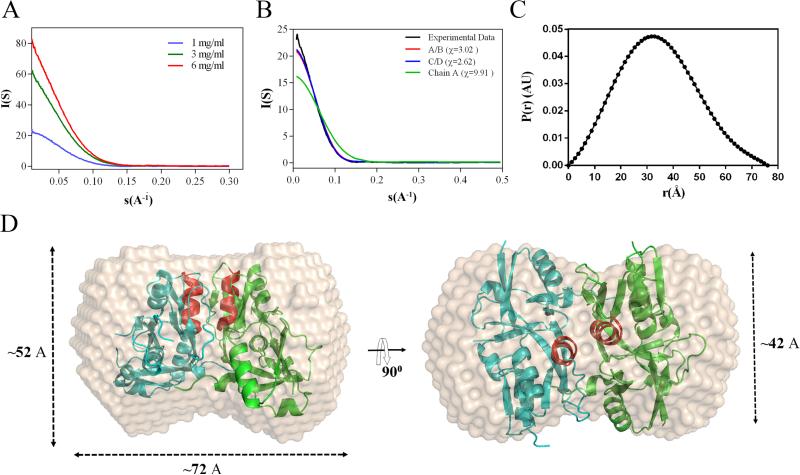
We next employed small angle X-ray scattering (SAXS) to define the oligomeric state and envelope structure of at three different sample concentrations (1, 3 and 6 mg/ml) (Fig. 3A). Radii of gyration (Rg) and I(0) values, extrapolated from the Guinier region of the Guinier plot (Supplemental Fig. 6) using PRIMUS (Konarev et al., 2003), were highly similar at all three concentrations (Table 2), indicating that D1 is monodisperse in solution. Molecular mass values computed at all three sample concentrations are consistent with that of a dimer. The merged experimental scattering curve for D1 showed excellent agreement with the theoretical scattering curves for the crystal structure dimers (χ2 =3.02 and 2.62 for Chains A/B and C/D, respectively) calculated with FoXS implemented in Chimera (http://www.cgl.ucsf.edu/chimera) (Fig. 3B). In contrast, the theoretical scattering curve for the Chain A monomer correlated poorly (χ2=9.92) with the experimental curve (Fig. 3B). The distance distribution functions P(r), derived using an indirect Fournier transformation of the scattering curve, as implemented in the program GNOM (Svergun, 1992), showed a unimodal peak at ~32 Å, which is consistent with D1 having an inflexible globular shape. The maximum distance (Dmax) for D1 (~76 Å) (Fig. 3C) was similar to the theoretical Dmax (70 Å) calculated from the crystal structure dimer.
Figure 3. SAXS analysis of domain.
A. Experimental scattering curves for D1 collected at 1, 3 and 6 mg/ml. B. Experimental curve for D1 (black) represents the merged scattering data collected at all three protein concentrations. Theoretical scattering curves were computed using the crystal structure coordinates for the Chain A monomer (green) and Chain A/B (red) and C/D (blue) dimers. C. P(r) function plot of D1 calculated from averaged experimental curves in panel A using GNOM (Svergun, 1992). D. Ab initio reconstruction of the D1 molecular envelope calculated using DAMMIF and DAMAVER (Franke and Svergun, 2009) overlaid with the crystal structure Chain A/B dimer. Helix α5 in each monomer is shown in red.
Ab initio shape reconstructions from the SAXS data were performed using DAMMIN (Svergun, 1999) and DAMAVER (Volkov and Svergun, 2003) using ten independent models with (P2) and without enforcing symmetry (P1) (Fig. 3D). The mean normalized spatial discrepancy (NSD) values for the P1 and P2 consensus models were similar (0.518 ± 0.04 and 0.545 ± 0.04, respectively); the NSD value comparing the P1 and P2 consensus models was 0.45. Thus, both symmetries consistently yielded stable reconstructions of similar shapes. The molecular mass estimated from the excluded volume of the DAMMIF models (57.4 kDa) agrees with the expected mass for the D1 dimer (54.4 kDa) based on amino acid sequence. Using COLORES implemented within SITUS, we performed rigid body docking of monomeric and dimeric D1 into the ab initio envelope model. As shown in Fig. 3D, the SAXS-reconstructed envelope most closely resembled that of the Chain A/B dimer (NSD = 2.9); the NSD value for the monomer was 5.5.
Homology modeling of Hk1 D2 and D3 PBP domains
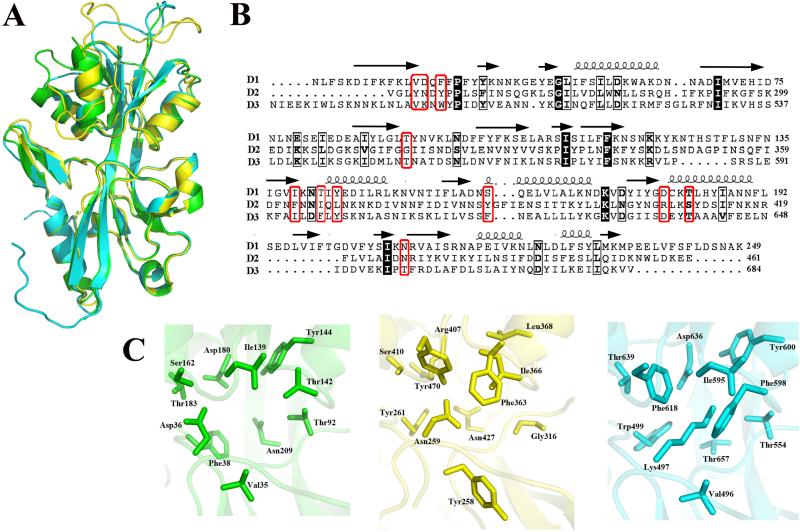
Using the D1 structure as a template, we generated homology models for D2 and D3, Hk1's two other sensor PBPs (Fig. 1A). RMSD values between D1 Chain A and the homology models for D2 and D3 were calculated by the secondary structure matching (SSM) method implemented in Coot (Emsley and Cowtan, 2004; Emsley et al., 2010). As expected, the structures for D2 and D3 overlapped very closely with D1 (1.2 Å and 0.96 Å, respectively) (Fig. 4A). Single amino acid changes in the LBPs for sensor and ABC transporter PBPs have been shown to dramatically affect ligand specificity and/or binding affinity (Bjorkman and Mowbray, 1998; Fulyani et al., 2013; Herrou et al., 2010). We next compared the putative LBPs for all three Hk1 sensor PBPs; as shown in Fig. 4B and Table 3, we saw greater overall sequence conservation between D1 and D3. Most notably, substitution of a positively charged residue (Arg407) in D2 for conserved negatively charged residues in D1 and D3 (Asp180 and Asp636, respectively) presumably alters the electrostatic environment of the corresponding LBP cavities substantially (Fig. 4C). Likewise, non-synonymous substitution of Asp36 in D1 for Asn259 and Lys497 in D2 and D3, respectively, would be expected to affect ligand binding. Based on these analyses, we conjecture that Hk1's three sensor PBPs recognize different ligand(s).
Figure 4. Homology modeling of Hk1 sensor domains D2 and D3.
A. Homology model of D2 (yellow) and D3 (cyan) overlaid with D1 (green). B. Multiple sequence alignment of all three Hk1 sensor PBPs was generated using Clustal W (Thompson et al., 1994) and ESPript (Gouet et al., 1999). Residues that are identical or similar in all three PBPs are highlighted and outlined in black, respectively. Residues predicted to form the LBPs for each domain are outlined in red. Cartoon depictions of α helices (loops) and β strands (arrows) in the D1 crystal structure are shown above the corresponding residues. C. Predicted ligand binding pockets for Hk1 D1 (green), D2 (yellow) and D3 (cyan) sensor PBP domains. Amino acid numbering is based on full length native Hk1.
Table 3.
Residues lining the LBPs for each PBP.1
| D1 | D2 | D3 |
|---|---|---|
| Val35 | Tyr258 | Val496 |
| Asp36 | Asn259 | Lys497 |
| Phe38 | Tyr261 | Trp499 |
| Thr92 | Gly316 | Thr554 |
| Ile139 | Phe363 | Ile595 |
| Thr142 | Ile366 | Phe598 |
| Tyr144 | Leu368 | Tyr600 |
| Ser162 | Tyr386 | Phe618 |
| Asp180 | Arg407 | Asp636 |
| Thr183 | Ser410 | Thr639 |
| Asn209 | Asn427 | Thr657 |
Residues are based on the full length Hk1.
Discussion
Bacterial HK sensor PBPs are thought to function by analogy to the Venus Fly Trap mechanism first described for ABC transporters (Cheung et al., 2009; Herrou et al., 2010; Mascher et al., 2006). According to this model, ligand binding by sensor PBPs induces a conformational change within flanking TM domain(s) that is required for activation (or antagonism) of the cytoplasmic kinase core domain (Cheung and Hendrickson, 2010; Lowe et al., 2012b; Mascher et al., 2006; Moore and Hendrickson, 2009; Neiditch et al., 2006; Sevvana et al., 2008). Recently, Dupré et al. (Dupré et al., 2015) provided compelling evidence supporting this model; using a mutagenesis approach, they demonstrated that ligand binding by the B. pertussis BvgS VFT1 and/or VFT2 sensor domains had a negative effect on kinase activity, presumably by inducing a conformational change within the adjacent transmembrane domain. Structural differences between Hk1 and BvgS suggest that the molecular mechanism underlying signal transduction by these HKs differs: (i) Signal transduction by BvgS is mediated primarily by inter-domain interactions between VFT1 and VFT2 (Dupré et al., 2015). The presence of a third PBP in the Hk1 sensor substantially increases the number and complexity of intermolecular interactions that could be involved in regulating kinase activity, and, ultimately, synthesis of c-di-GMP by Rrp1. (ii) In contrast to BvgS, Hk1's periplasmic sensor is predicted to be tethered to the cytoplasmic membrane by both N- and C-terminal TM domains. Being constrained at both ends, presumably, decreases the overall flexibility of the full-length sensor and/or help maintain one or more of the PBPs in a more open conformation. (iii) Unlike VFT1 and VFT2 (Dupré et al., 2015; Herrou et al., 2010), D1 forms a dimer in solution; dimerization of D1 in the native membrane-bound Hk1 homodimer could provide an important “control point” for kinase activation by limiting movement of the N-terminal TM domain. Collectively, the above features could confer on Hk1 the ability to sense and respond to the variety of small molecules that B. burgdorferi encounters during its enzootic cycle and generate a graded output response that is reflective of the concentration and nature of the ligands bound.
Hk1's sensor PBPs share sequence and structural similarity to the substrate-binding proteins for ABC transporters of polar amino acids (Berntsson et al., 2010; Cheung and Hendrickson, 2010; Cheung et al., 2009; Mascher et al., 2006; Tam and Saier, 1993). The fact that Hk1 is active when B. burgdorferi is cultivated in BSK-II medium (Caimano et al., 2015; Caimano et al., 2011; He et al., 2011; Kostick et al., 2011; Rogers et al., 2009), a complex, semi-defined growth medium contains abundant free amino acids (provided by the medium component CMRL 1066), bovine serum albumin and rabbit serum (Barbour, 1984), strongly suggests that one or more of Hk1's sensor PBPs also could recognize amino acids and/or small peptides. Non-synonymous substitutions in the ligand binding pockets suggest, however, that their preferred ligands for D1-3 differ from their transporter orthologs. In an effort to identify the ligands that activate Hk1 in vitro, we incubated recombinant D1, which we showed herein is purified from E. coli in an unliganded state, with complete BSK-II medium and individual medium components. Using liquid chromatography-mass spectrometry, however, we were unable to detect a media-derived ligand bound to the recombinant protein (data not shown). Likewise, using a thermal shift assay, a technique often applied to ligand-PBP interactions (Giuliani et al., 2008; Pantoliano et al., 2001), we did not detect any binding interactions between recombinant D1 and a panel of potential ligands (i.e., BSK-II, medium components and free amino acids) (data not shown). We envision at least three possible explanations for these results. First, the preferred ligand(s) for D1 may not have been among those tested. Second, activation of Hk1 by BSK-II in vitro may be mediated by D2 and/or D3. Third, the LBP for recombinant D1 may be inaccessible given this domain's closed conformation. Distinguishing between these possibilities will require structural and/or biochemical characterization of D2 and D3 and, conceivably, presentation of all three sensor PBP domains within a more native, preferably membrane-bound, configuration.
Activation of the Hk1/Rrp1 TCS is required for B. burgdorferi to survive both the acquisition and transmission blood meals (Caimano et al., 2015; Caimano et al., 2011; He et al., 2011; Kostick et al., 2011). During larval acquisition, chemotactic signals emanating from the bite site induce spirochetes to migrate from the skin of the reservoir host into the tick midgut where they colonize the epithelial cell surfaces (Pal and Fikrig, 2010; Tilly et al., 2008; Zung et al., 1989). During transmission, B. burgdorferi, revived from a state of nutrient-deprivation by the influx of the nymphal blood meal, undergo exponential replication and traverse the midgut epithelium en route to a new host (de Silva and Fikrig, 1995; Dunham-Ems et al., 2009; Ohnishi et al., 2001; Pal and Fikrig, 2010; Ribeiro et al., 1987). Structural analysis and modeling of D1-3 suggest that the Hk1 sensor has the requisite versatility to distinguish between these two feeding states. These data also raise a key question -- What types of ligands might be used by B. burgdorferi to differentiate between acquisition and transmission? Spirochetes within mammalian tissues are exposed continuously to free amino acids within interstitial fluid, thus it seems unlikely that these molecules would be solely responsible for activating Hk1. The Hk1 sensor, most likely, has the ability to recognize host- and/or tick-derived ligands that are unique to the feeding process. One provocative possibility is that one or more of Hk1's PBPs bind neurotransmitters, which are derived from amino acids and would be released by the host in response to tissue injury (i.e., formation of the feeding lesion) (Askenase et al., 1995; Dai et al., 2010; Theoharides et al., 2012). Tick saliva also contains a plethora of bioactive molecules, including dopamine and dopamine sulfate (Koci et al., 2014; Simo et al., 2012; Simo et al., 2011; Yalcin et al., 2010). Interestingly, using qRT-PCR, we recently reported significantly greater induction of c-di-GMP-dependent genes by B. burgdorferi in feeding nymphs compared to larvae (Caimano et al., 2015). These data suggest that the Hk1 calibrates the synthesis of c-di-GMP by Rrp1 according to the level of protection required within different tick life stages. The structural data presented herein make it possible to devise a mutagenesis-based strategy to: (i) identify residues involved in ligand binding by each PBP; (ii) determine when during the enzootic cycle each domain is required; and (iii) establish whether these sensor domains act independently or cooperatively to promote Hk1-mediated signal transduction.
Supplementary Material
Acknowledgements
This work was supported by PHS Grants AI 29735 (to J.D.R. and M.J.C.) and GM 094611 (to M.G.M.). Use of IMCA-CAT beamline 17-ID at the Advanced Photon Source was supported by companies of the Industrial Macromolecular Crystallography Association through a contract with the Hauptman-Woodward Medical Research Institute. We also thank Lin Yang (National Synchrotron Light Source, Brookhaven National Laboratory) for assistance with SAXS data collection and Dr. Thomas Grant (Hauptman-Woodward Institute, Buffalo, NY) for his helpful suggestions regarding the SAXS data analysis and interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire M, Yang L. Biomolecular solution X-ray scattering at the National Synchrotron Light Source. Journal of synchrotron radiation. 2011;18:41–44. doi: 10.1107/S0909049510036022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenase PW, Geba GP, Levin J, Ratzlaff RE, Anderson GM, Ushio H, Ptak W, Matsuda H. A role for platelet release of serotonin in the initiation of contact sensitivity. Int. Arch. Allergy Immunol. 1995;107:145–147. doi: 10.1159/000236958. [DOI] [PubMed] [Google Scholar]
- Baraquet C, Theraulaz L, Guiral M, Lafitte D, Mejean V, Jourlin-Castelli C. TorT, a member of a new periplasmic binding protein family, triggers induction of the Tor respiratory system upon trimethylamine N-oxide electron-acceptor binding in Escherichia coli. J. Biol. Chem. 2006;281:38189–38199. doi: 10.1074/jbc.M604321200. [DOI] [PubMed] [Google Scholar]
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsson RP, Smits SH, Schmitt L, Slotboom DJ, Poolman B. A structural classification of substrate-binding proteins. FEBS Lett. 2010;584:2606–2617. doi: 10.1016/j.febslet.2010.04.043. [DOI] [PubMed] [Google Scholar]
- Bjorkman AJ, Mowbray SL. Multiple open forms of ribose-binding protein trace the path of its conformational change. J. Mol. Biol. 1998;279:651–664. doi: 10.1006/jmbi.1998.1785. [DOI] [PubMed] [Google Scholar]
- Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 2013;41:W349–357. doi: 10.1093/nar/gkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy MR, Radolf JD. c-di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface and mediates a cell survival program that appears to involve spirochete cell envelope remodeling. Infect. Immun. 2015;83 doi: 10.1128/IAI.00315-15. In process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, Akins DR, Pal U, Radolf JD. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect. Immun. 2011;79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra EJ, Laub MT. Evolution of two-component signal transduction systems. Annu. Rev. Microbiol. 2012;66:325–347. doi: 10.1146/annurev-micro-092611-150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J, Hendrickson WA. Structural analysis of ligand stimulation of the histidine kinase NarX. Structure. 2009;17:190–201. doi: 10.1016/j.str.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J, Hendrickson WA. Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 2010;13:116–123. doi: 10.1016/j.mib.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung J, Le-Khac M, Hendrickson WA. Crystal structure of a histidine kinase sensor domain with similarity to periplasmic binding proteins. Proteins. 2009;77:235–241. doi: 10.1002/prot.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the Lyme disease agent. PLoS Pathog. 2010;6:e1001205. doi: 10.1371/journal.ppat.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer TA, Berka K, Thornton JM, Laskowski RA. PDBsum additions. Nucleic Acids Res. 2014;42:D292–296. doi: 10.1093/nar/gkt940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Eggers CH, Radolf JD. Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog. 2012;8:e1002532. doi: 10.1371/journal.ppat.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, Radolf JD. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré E, Herrou J, Lensink MF, Wintjens R, Vagin A, Lebedev A, Crosson S, Villeret V, Locht C, Antoine R, Jacob-Dubuisson F. Virulence regulation with Venus flytrap domains: Structure and function of the periplasmic moiety of the sensor-kinase BvgS. PLoS Pathog. 2015;11:e1004700. doi: 10.1371/journal.ppat.1004700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr.D.Biol.Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CB, Graul RC, Lee AY, Merkle H-P, Sadee W. The Venus flytrap of periplasmic binding proteins: An ancient protein module present in multiple drug receptors. AAPS PharmSci. 1999;1:1–20. doi: 10.1208/ps010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Nat. Acad. Sci. USA. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke D, Svergun DI. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J Appl Crystallogr. 2009;42:342–346. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Fukami-Kobayashi K, Tateno Y, Nishikawa K. Domain dislocation: a change of core structure in periplasmic binding proteins in their evolutionary history. J. Mol. Biol. 1999;286:279–290. doi: 10.1006/jmbi.1998.2454. [DOI] [PubMed] [Google Scholar]
- Fulyani F, Schuurman-Wolters Gea K., Žagar Andreja V., Guskov A, Slotboom D-J, Poolman B. Functional diversity of tandem substrate-binding domains in ABC transporters from pathogenic bacteria. Structure. 2013;21:1879–1888. doi: 10.1016/j.str.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Galperin MY. Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol. 2010;13:150–159. doi: 10.1016/j.mib.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani SE, Frank AM, Collart FR. Functional assignment of solute-binding proteins of ABC transporters using a fluorescence-based thermal shift assay. Biochemistry (Mosc) 2008;47:13974–13984. doi: 10.1021/bi801648r. [DOI] [PubMed] [Google Scholar]
- Gouet P, Courcelle E, Stuart DI, Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- Groshong AM, Blevins JS. Insights into the biology of Borrelia burgdorferi gained through the application of molecular genetics. Adv. Appl. Microbiol. 2014;86:41–143. doi: 10.1016/B978-0-12-800262-9.00002-0. [DOI] [PubMed] [Google Scholar]
- He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, Norgard MV, Gomelsky M, Yang XF. Cyclic-di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 2011;7:e1002133. doi: 10.1371/journal.ppat.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrou J, Bompard C, Wintjens R, Dupré E, Willery E, Villeret V, Locht C, Antoine R, Jacob-Dubuisson F. Periplasmic domain of the sensor-kinase BvgS reveals a new paradigm for the Venus flytrap mechanism. Proc. Nat. Acad. Sci. USA. 2010;107:17351–17355. doi: 10.1073/pnas.1006267107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K, Stoffel W. Tmbase, a database of membrane spanning protein segments. Biol.Chem.Hoppe Seyler. 1993;347:166–166. [Google Scholar]
- Holm L, Rosenström P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Nat. Acad. Sci. USA. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Shaw DK, Smith Iii R, Trzeciakowski JP, Skare JT. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol. Microbiol. 2009;74:1344–1355. doi: 10.1111/j.1365-2958.2009.06951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Caimano MJ, Luthra A, Axline D, Jr., Corona A, Iacobas DA, Radolf JD, Schwartz I. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol. Microbiol. 2015;95:509–538. doi: 10.1111/mmi.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourlin C, Bengrine A, Chippaux M, Mejean V. An unorthodox sensor protein (TorS) mediates the induction of the tor structural genes in response to trimethylamine N-oxide in Escherichia coli. Mol. Microbiol. 1996;20:1297–1306. doi: 10.1111/j.1365-2958.1996.tb02648.x. [DOI] [PubMed] [Google Scholar]
- Katona LI, Tokarz R, Kuhlow CJ, Benach J, Benach JL. The fur homologue in Borrelia burgdorferi. J. Bacteriol. 2004;186:6443–6456. doi: 10.1128/JB.186.19.6443-6456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koci J, Simo L, Park Y. Autocrine/paracrine dopamine in the salivary glands of the blacklegged tick Ixodes scapularis. J. Insect Physiol. 2014;62:39–45. doi: 10.1016/j.jinsphys.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. Journal of Applied Crystallography. 2003;36:1277–1282. [Google Scholar]
- Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol. Microbiol. 2011;81:219–231. doi: 10.1111/j.1365-2958.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell T, Lacal J, Busch A, Silva-Jiménez H, Guazzaroni M-E, Ramos JL. Bacterial sensor kinases: Diversity in the recognition of environmental signals. Annu. Rev. Microbiol. 2010;64:539–559. doi: 10.1146/annurev.micro.112408.134054. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Hutchinson EG, Michie AD, Wallace AC, Jones ML, Thornton JM. PDBsum: a web-based database of summaries and analyses of all PDB structures. Trends Biochem. Sci. 1997;22:488–490. doi: 10.1016/s0968-0004(97)01140-7. [DOI] [PubMed] [Google Scholar]
- Lee AI, Delgado A, Gunsalus RP. Signal-dependent phosphorylation of the membrane-bound NarX two-component sensor-transmitter protein of Escherichia coli: nitrate elicits a superior anion ligand response compared to nitrite. J. Bacteriol. 1999;181:5309–5316. doi: 10.1128/jb.181.17.5309-5316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe EC, Basle A, Czjzek M, Firbank SJ, Bolam DN. A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proc. Natl. Acad. Sci. U. S. A. 2012a;109:7298–7303. doi: 10.1073/pnas.1200479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe EC, Baslé A, Czjzek M, Firbank SJ, Bolam DN. A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proceedings of the National Academy of Sciences. 2012b;109:7298–7303. doi: 10.1073/pnas.1200479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft JR, Snell EH, Detitta GT. Lessons from high-throughput protein crystallization screening: 10 years of practical experience. Expert Opin Drug Discov. 2011;6:465–480. doi: 10.1517/17460441.2011.566857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra A, Zhu G, Desrosiers DC, Eggers CH, Mulay V, Anand A, McArthur FA, Romano FB, Caimano MJ, Heuck AP, Malkowski MG, Radolf JD. The transition from closed to open conformation of Treponema pallidum outer membrane-associated lipoprotein TP0453 involves membrane sensing and integration by two amphipathic helices. J. Biol. Chem. 2011;286:41656–41668. doi: 10.1074/jbc.M111.305284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Pear MR, McCammon JA, Quiocho FA. Hinge-bending in L-arabinose-binding protein. The “Venus's-flytrap” model. J. Biol. Chem. 1982;257:1131–1133. [PubMed] [Google Scholar]
- Marchler-Bauer A, Bryant SH. CD-Search: protein domain annotations on the fly. Nucleic Acids Res. 2004;32:W327–W331. doi: 10.1093/nar/gkh454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- Mascher T, Helmann JD, Unden G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 2006;70:910–938. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- Moore JO, Hendrickson WA. Structural analysis of sensor domains from the TMAO-responsive histidine kinase receptor TorS. Structure. 2009;17:1195–1204. doi: 10.1016/j.str.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, Bassler BL, Hughson FM. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Nat. Acad. Sci. USA. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otowinski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology. 2008;154:2641–2658. doi: 10.1099/mic.0.2008/019992-0. [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Zhou J, Brautigam CA, Deka RK, Norgard MV. Identification of a core sequence for the binding of BosR to the rpoS promoter region in Borrelia burgdorferi. Microbiology. 2015;161:931. doi: 10.1099/mic.0.000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Narasimhan S, Neelakanta G, Kumar M, Pal U, Fikrig E, Norgard MV. Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC Microbiol. 2012;12:44. doi: 10.1186/1471-2180-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U, Fikrig E. Tick Interactions. In: Samuels DS, Radolf JD, editors. Borrelia. Molecular Biology, Host intercations and Pathogenesis, Caister Academic Press; Norfolk, UK.: 2010. pp. 279–298. [Google Scholar]
- Pantoliano MW, Petrella EC, Kwasnoski JD, Lobanov VS, Myslik J, Graf E, Carver T, Asel E, Springer BA, Lane P, Salemme FR. High-density miniaturized thermal shift assays as a general strategy for drug discovery. Journal of Biomolecular Screening. 2001;6:429–440. doi: 10.1177/108705710100600609. [DOI] [PubMed] [Google Scholar]
- Petoukhov MV, Franke D, Shkumatov AV, Tria G, Kikhney AG, Gajda M, Gorba C, Mertens HDT, Konarev PV, Svergun DI. New developments in the ATSAS program package for small-angle scattering data analysis. Journal of Applied Crystallography. 2012;45:342–350. doi: 10.1107/S0021889812007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Povolotsky TL, Hengge R. 'Life-style' control networks in Escherichia coli: Signaling by the second messenger c-di-GMP. J. Biotechnol. 2012;160:10–16. doi: 10.1016/j.jbiotec.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Quiocho FA, Ledvina PS. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol. Microbiol. 1996;20:17–25. doi: 10.1111/j.1365-2958.1996.tb02484.x. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JM, Mather TN, Piesman J, Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae). J. Med. Entomol. 1987;24:201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- Rizos AK, Tsikalas I, Morikis D, Galanakis P, Spyroulias GA, Krambovitis E. Characterization of the interaction between peptides derived from the gp120/V3 domain of HIV-1 and the amino terminal of the chemokine receptor CCR5 by NMR spectroscopy and light scattering. Journal of Non-Crystalline Solids. 2006;352:4451–4458. [Google Scholar]
- Rogers EA, Terekhova D, Zhang H-M, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol. Microbiol. 2009;71:1551–1573. doi: 10.1111/j.1365-2958.2009.06621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Samuels DS. Gene regulation in Borrelia burgdorferi. Annu. Rev. Microbiol. 2011 doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny D, Hammel M, Sali A. FoXS: a web server for rapid computation and fitting of SAXS profiles. Nucleic Acids Res. 2010;38:W540–544. doi: 10.1093/nar/gkq461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevvana M, Vijayan V, Zweckstetter M, Reinelt S, Madden DR, Herbst-Irmer R, Sheldrick GM, Bott M, Griesinger C, Becker S. A ligand-induced switch in the periplasmic domain of sensor histidine kinase CitA. J. Mol. Biol. 2008;377:512–523. doi: 10.1016/j.jmb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Simo L, Zitnan D, Park Y. Neural control of salivary glands in ixodid ticks. J. Insect Physiol. 2012;58:459–466. doi: 10.1016/j.jinsphys.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo L, Koci J, Zitnan D, Park Y. Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS One. 2011;6:e16158. doi: 10.1371/journal.pone.0016158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Carroll JA, Yang XF, Samuels DS, Akins DR. Gene Regulation, Transcriptomics, and Proteomics. In: Samuels DS, Radolf JD, editors. Borrelia. Molecular Biology, Host Interactions and Pathogenesis, Calister Academic Press; Norfolk, UK.: 2010. [Google Scholar]
- Smith AH, Blevins JS, Bachlani GN, Yang XF, Norgard MV. Evidence that RpoS (σS) in Borrelia burgdorferi is controlled directly by RpoN (σ54/σN). J. Bacteriol. 2007;189:2139–2144. doi: 10.1128/JB.01653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J.Clin.Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr D Biol Crystallogr. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- Svergun DI. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. Journal of Applied Crystallography. 1992;25:495–503. [Google Scholar]
- Svergun DI. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 1999;76:2879–2886. doi: 10.1016/S0006-3495(99)77443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam R, Saier MH., Jr. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim. Biophys. Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through weighting positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Rosa PA, Stewart PE. Biology of infection with Borrelia burgdorferi . Infect.Dis.Clin.North Am. 2008;22:217–234. v. doi: 10.1016/j.idc.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirigos KD, Peters C, Shu N, Kall L, Elofsson A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 2015;43:W401–407. doi: 10.1093/nar/gkv485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich LE, Zhulin IB. The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res. 2010;38:D401–407. doi: 10.1093/nar/gkp940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov VV, Svergun DI. Uniqueness of ab initio shape determination in small-angle scattering. Journal of Applied Crystallography. 2003;36:860–864. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson AJ, Verschueren KHG. Crystal structures of periplasmic solute-binding proteins in ABC transport complexes illuminate their function. In: Holland IB, et al., editors. ABC Proteins. From Bacteria to Man, Academic Press; Boston: 2003. pp. 187–208. [Google Scholar]
- Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, Gherardini F, Wolfe AJ, Yang XF. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 2010;6:e1001104. doi: 10.1371/journal.ppat.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Shi Y, Dadhwal P, Liang FT. RpoS regulates essential virulence factors remaining to be identified in Borrelia burgdorferi. PLoS One. 2012;7:e53212. doi: 10.1371/journal.pone.0053212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Canutescu AA, Wang G, Shapovalov M, Obradovic Z, Dunbrack Jr RL. Statistical analysis of interface similarity in crystals of homologous proteins. J. Mol. Biol. 2008;381:487–507. doi: 10.1016/j.jmb.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin EB, Stangl H, Pichu S, Mather TN, King RS. Monoamine neurotransmitters as substrates for novel tick sulfotransferases, homology modeling, molecular docking, and enzyme kinetics. ACS Chemical Biology. 2010;6:176–184. doi: 10.1021/cb100266g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Nat. Acad. Sci. USA. 2003;100:1100–1106. doi: 10.1073/pnas.1834315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu Q, Hendrickson WA. Crystal structures of apparent saccharide sensors from histidine kinase receptors prevalent in a human gut symbiont. The FEBS journal. 2014;281:4263–4279. doi: 10.1111/febs.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung JL, Lewengrug S, Mrdzibnska MA, Spielman A. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini Can. J. Zool. 1989;67:1737–1748. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.