BACKGROUND:
There remains a proportion of patients with unfavorable outcomes after aneurysmal subarachnoid hemorrhage, of particular relevance in those who present with a good clinical grade. A forewarning of those at risk provides an opportunity towards more intensive monitoring, investigation, and prophylactic treatment prior to the clinical manifestation of advancing cerebral injury.
OBJECTIVE:
To assess whether biochemical markers sampled in the first days after the initial hemorrhage can predict poor outcome.
METHODS:
All patients recruited to the multicenter Simvastatin in Aneurysmal Hemorrhage Trial (STASH) were included. Baseline biochemical profiles were taken between time of ictus and day 4 post ictus. The t-test compared outcomes, and a backwards stepwise binary logistic regression was used to determine the factors providing independent prediction of an unfavorable outcome.
RESULTS:
Baseline biochemical data were obtained in approximately 91% of cases from 803 patients. On admission, 73% of patients were good grade (World Federation of Neurological Surgeons grades 1 or 2); however, 84% had a Fisher grade 3 or 4 on computed tomographic scan. For patients presenting with good grade on admission, higher levels of C-reactive protein, glucose, and white blood cells and lower levels of hematocrit, albumin, and hemoglobin were associated with poor outcome at discharge. C-reactive protein was found to be an independent predictor of outcome for patients presenting in good grade.
CONCLUSION:
Early recording of C-reactive protein may prove useful in detecting those good grade patients who are at greater risk of clinical deterioration and poor outcome.
ABBREVIATIONS:
ALP, alkaline phosphatase
ALT, alanine aminotransferase
CK, creatine kinase
CRP, C-reactive protein
EVD, external ventricular drainage
ICH GCP, International Conference on Harmonisation guidelines for good clinical practice
mRS, modified Rankin Scale
SAH, subarachnoid hemorrhage
STASH, Simvastatin in Aneurysmal Subarachnoid Hemorrhage Trial
WBC, white blood cells
WFNS, World Federation of Neurological Surgeons
KEY WORDS: Aneurysm, C-reactive protein, Inflammation, Subarachnoid hemorrhage
Subarachnoid hemorrhage (SAH) from a ruptured intracranial aneurysm affects approximately 600 000 patients worldwide1 and 7000 patients within the United Kingdom2 each year. Despite considerable advances in treatment, the condition is associated with a disproportionate (25%) loss of productive life and cost to society affecting a young age group.3 Combined morbidity and mortality reach 50%, with one-third of survivors becoming dependent.4
Late clinical deterioration is associated with morbidity and mortality, the causes of which are multifactorial. The most recognized contributor to clinical deterioration and unfavorable outcome is delayed cerebral ischemia. Although phase II randomized controlled trials suggested a potential role for statin therapy in SAH,5-8 a large placebo-controlled and blinded phase III study showed no benefit for simvastatin 40 mg in either the long-term or short-term outcome of patients with aneurysmal subarachnoid hemorrhage (STASH Trial).9
In addition to delayed cerebral ischemia, there are a number of non-neurological systemic complications that often occur post SAH and which can contribute to poor outcome, including sepsis, anemia, hypertension, hypotension, hyperglycemia, hypernatremia, hyponatremia, hypomagnesemia, and cardiac complications.2,10 Many of these complications could be attributed to poor neurological status on admission. However, there remains a group of patients who present with a good World Federation of Neurological Surgeons (WFNS) grade who unpredictably deteriorate with a poor outcome. In this group there is a need for early recognition and close monitoring, opening up the opportunity to earlier intervention.
In a post hoc study of the STASH data, we explored whether biochemical markers sampled soon after the time of initial hemorrhage are predictive of a subsequent poor outcome, hence providing a simple and readily accessible indication of who in the population of good grade SAH patients are likely to worsen.
METHODS
Study Population
Patients were recruited from 35 neurosurgical units (23 in the United Kingdom and 12 non-United Kingdom sites) between 2007 and 2013.9 Laboratory data were extracted from the STASH database. Approval for the study was granted by the Berkshire Research Ethics Committee (06/MRE12/26) and the Medicine and Healthcare products Regulatory Agency, MHRA (2006-000277-30). Appropriate ethics and regulatory approvals were obtained for all participating sites not in the United Kingdom in accordance with the International Conference on Harmonisation guidelines for good clinical practice (ICH GCP). Written informed consent was obtained from all patients or their legal representative. The study conformed to CONSORT guidelines (trial profile in Simvastatin in aneurysmal subarachnoid hemorrhage [STASH] publication9).
Inclusion criteria were radiological confirmatory evidence of an SAH, age 18 to 65 years, and presentation less than 96 hours from ictus. Exclusion criteria were patient taking statin therapy at presentation, pregnancy, no reasonable prospect of survival, known renal or hepatic impairment, patient not fully independent before bleed, strong suspicion of drug or alcohol misuse, patient unlikely to be amenable to follow-up, patient taking warfarin-type drugs or contraindicated medications or suspected additional life-threatening disease.
Baseline Data
Baseline data included age, sex, ethnic origin, and WFNS grade on presentation to the neurosurgical unit. Radiological data included Fisher grade on computed tomography (CT), presence of intraparenchymal or intraventricular bleed, presence of hydrocephalus (requiring external ventricular drainage [EVD]), and aneurysm location. The Fisher grading was determined as described in the original publication,9 in conjunction with a radiologist at each local participating site, after review of the imaging. The extent of intracerebral or intraventricular involvement was not prescriptive; however, it was assumed to be diagnostic of grade 4.
Laboratory Tests
All patients had biochemical profiles taken at trial entry, prior to randomization (baseline values) and again between days 9 to 12 post randomization (late values). Recorded parameters were liver function: C-reactive protein (CRP), bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin, glucose, and magnesium; kidney function: creatine kinase (CK), sodium and potassium; lipid profile: total cholesterol, low-density lipoprotein, high-density lipoprotein and triglycerides; and blood profile: hematocrit, hemoglobin, WBC (white blood cells) and platelets and fibrinogen. Patients who were discharged prior to day 9 did not receive a second biochemical profile. Suspected adverse events were reviewed on an individual patient basis.
Outcome Measure
The modified Rankin Scale (mRS) was dichotomized to good (mRS 0-2) and poor outcome (mRS 3-6) at discharge and again at 6 months post ictus. The scale was in the form of a simplified questionnaire which was assessed by a clinician at outcome and by postal questionnaire at 6 months, which was verified using the Lindsay Wilson structured questionnaire.
Statistical Analysis
All analyses were on an intention-to-treat basis. Data were presented as mean ± standard deviation and 95% confidence interval. Analyses were performed using SPSS statistical software (IBM Corporation, Armonk, New York). The t-test was used to compare the biochemical markers between outcome groups. The biochemical parameters that reached significance along with recognized clinical factors known to affect outcome were assessed using a backwards likelihood ratio (LR) stepwise multivariable binary logistic regression to determine independent predictors of unfavorable outcome at discharge and at 6 months. The odds ratio was calculated and presented with 95% CI. P values less than 0.05 were considered to be statistically significant.
RESULTS
Demographics
Data from 803 patients are outlined in Table 1. For the study, 2066 patients did not meet the entry criteria; the major exclusions were age (over 65 years), taking statin therapy at presentation, and time from ictus to admission >96 hours. The majority of patients were good grade on admission (WFNS grades 1 or 2, 588 out of 803 patients, 73%); however, 84% (673 out of 803) had a Fisher grade 3 or 4 on a CT scan.
TABLE 1.
Baseline Patient Characteristicsa
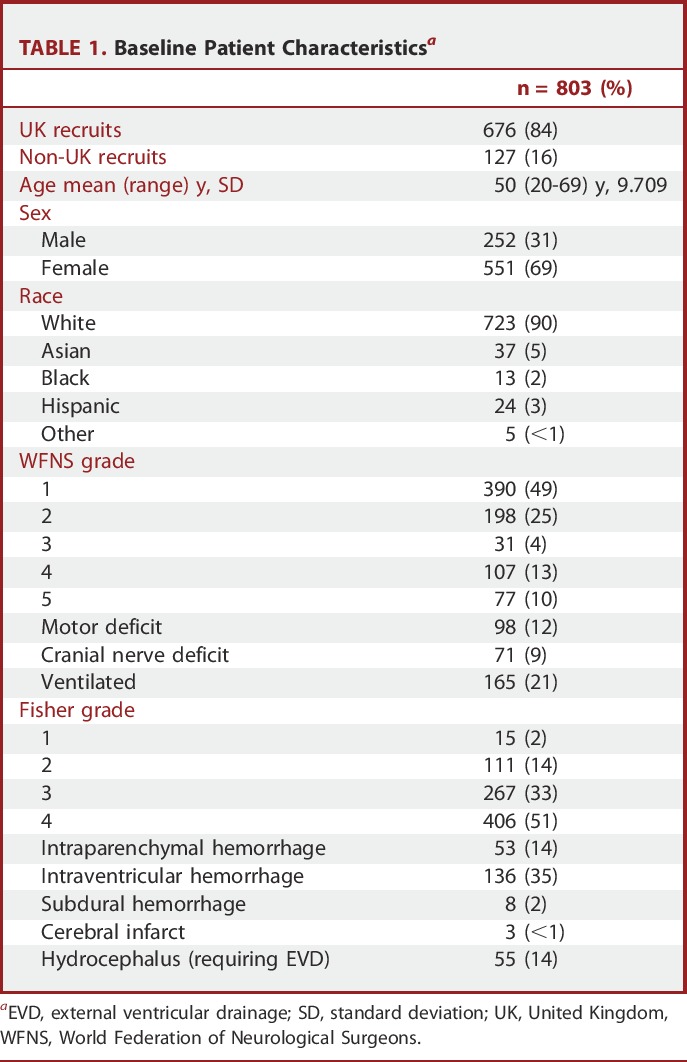
Observational Sample Data
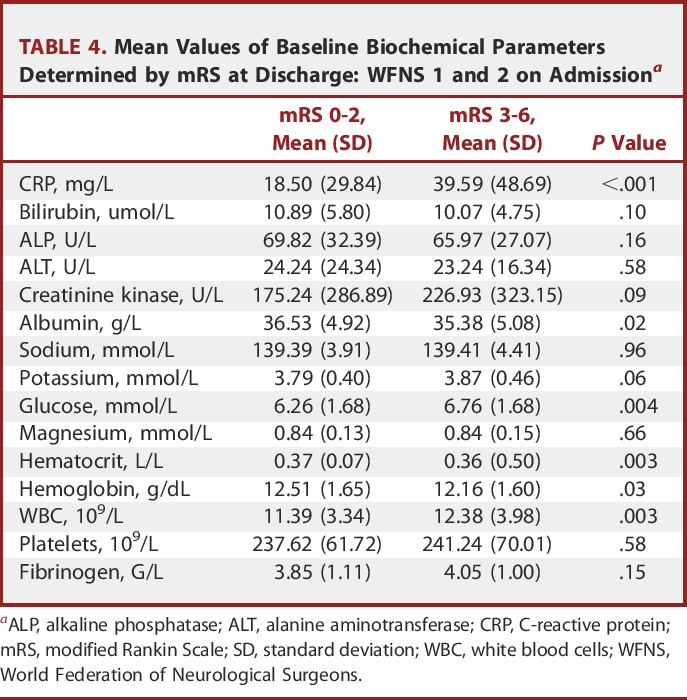
Baseline biochemical data were obtained in approximately 91% of cases (Table 2). The mean time from ictus to randomization was 2.1 days (SD 1.0). Table 3 shows the mean values of the baseline biochemical parameters for patients who were classified as good outcome (mRS 1-2) vs poor outcome (mRS 3-6) at discharge. Baseline measures of CRP, CK, sodium, potassium, glucose, magnesium, and WBC showed elevated levels in patients with poor outcome compared to good outcome at discharge. Likewise, lower levels of bilirubin and albumin were associated with poor outcome. For those patients presenting with good grade on admission (Table 4), higher baseline levels of CRP, glucose, and WBC and lower levels of hematocrit, albumin, and hemoglobin were the biochemical measures associated with an eventual poor outcome.
TABLE 2.
Baseline Biochemical Dataa
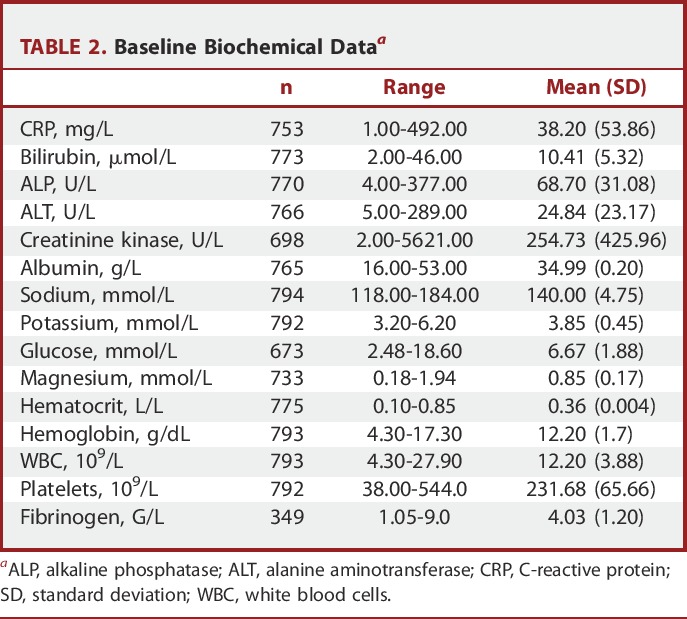
TABLE 3.
Mean Values of Baseline Biochemical Parameters Determined by mRS at Dischargea
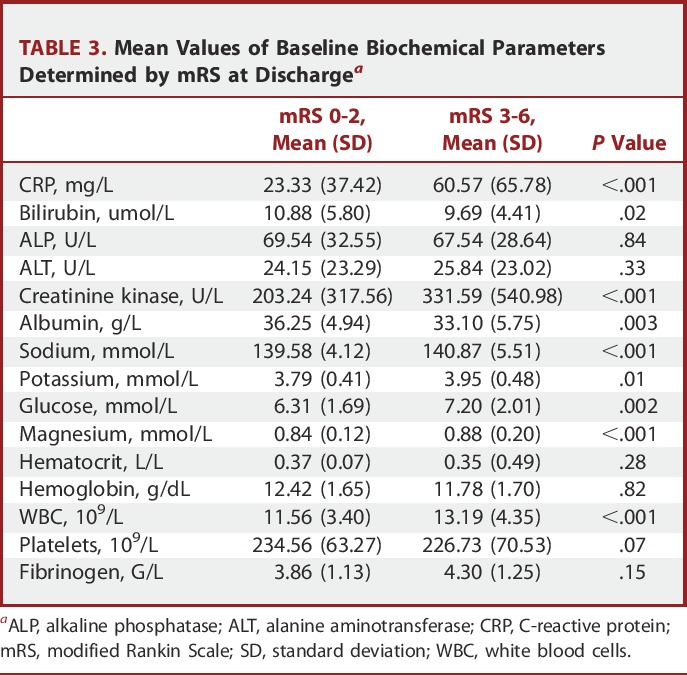
TABLE 4.
Mean Values of Baseline Biochemical Parameters Determined by mRS at Discharge: WFNS 1 and 2 on Admissiona
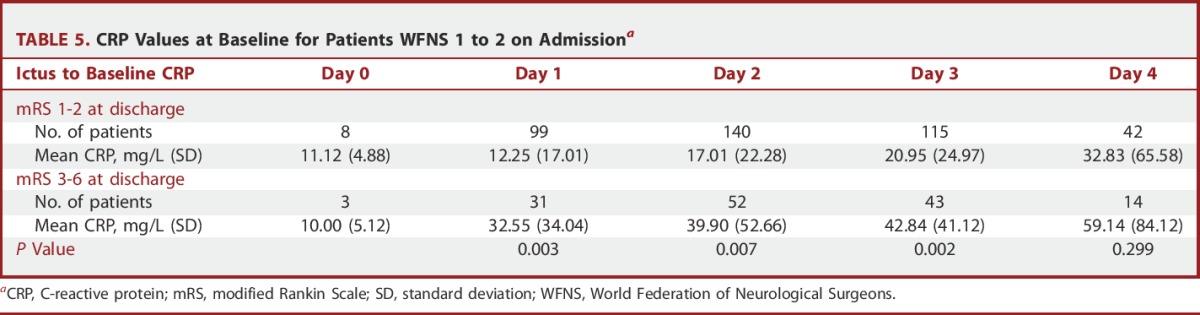
The mean CRP levels for good grade patients categorized by outcome and grouped by post-ictal day are shown in Table 5. For the good outcome group, the values were 12.25 mg/L and 20.95 mg/L on post-ictal day 1 and 3, respectively. The corresponding values for patients with poor outcome were 32.55 mg/L and 42.84 mg/L. The difference in these values was highly significant on days 1, 2, and 3, but not day 4 post ictus (P = .003, 0.01, 0.002, and 0.30, respectively).
TABLE 5.
CRP Values at Baseline for Patients WFNS 1 to 2 on Admissiona
Regression Analysis
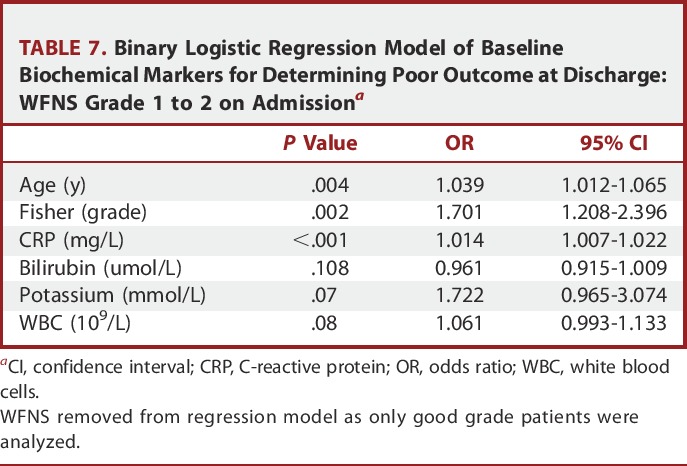
Binary logistic regression identified baseline CRP, potassium, glucose, and WBC to be independently related to poor outcome at discharge, along with age, WFNS status, and Fisher CT grading on admission (Table 6). Other imaging parameters related to the magnitude of the bleed such as intraventricular and intraparenchymal hemorrhage were not found to be predictive of outcome. Importantly, when only patients presenting with WFNS 1 and 2 were analyzed, only baseline CRP was found to be an independent biochemical predictor of outcome (Table 7).
TABLE 6.
Binary Logistic Regression Model of Baseline Parameters for Determining Poor Outcome at Discharge: All Patientsa
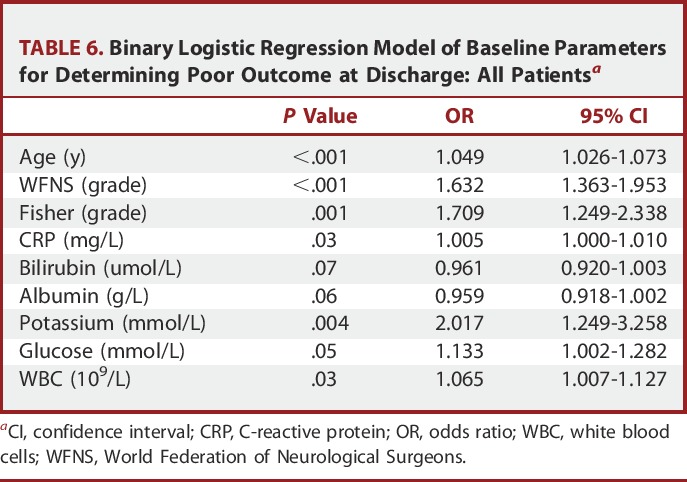
TABLE 7.
Binary Logistic Regression Model of Baseline Biochemical Markers for Determining Poor Outcome at Discharge: WFNS Grade 1 to 2 on Admissiona
An additional multivariate model was applied, including sepsis, statin and placebo, white cell count, and CRP on day 9, to determine whether elevated CRP is related to outcome independent of infection and sepsis. We found baseline CRP (odd ratio [OR] 1.02; 95% CI 1.01-1.02; P = .002) to be an independent predictor of outcome at discharge. This was not the case for late day 9 CRP values (OR 1.00; 95% CI 1.00-1.01; P = .053). Statins showed no significant effect on CRP (mean day 9 CRP in statin group, 33.67 mg/L and in the placebo group 31.76 mg/L, P = .64).
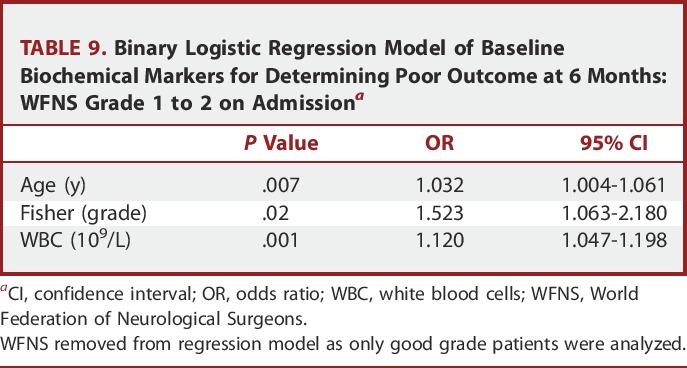
For clinical outcome at 6 months, CRP remained an independent predictor for all patients, but not for good grade patients alone (Tables 8 and 9).
TABLE 8.
Binary Logistic Regression Model of Baseline Biochemical Markers for Determining Poor Outcome at 6 Months: All Patientsa
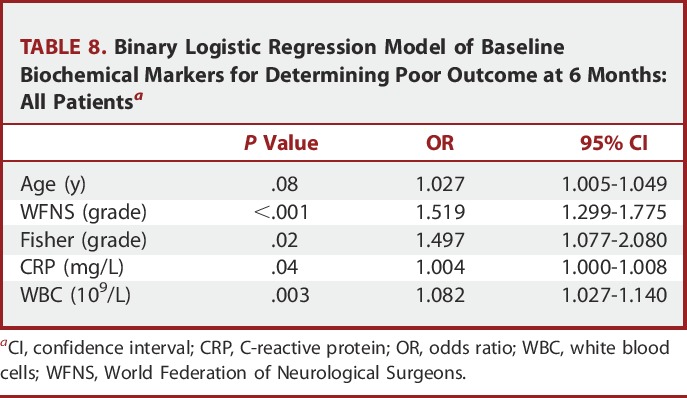
TABLE 9.
Binary Logistic Regression Model of Baseline Biochemical Markers for Determining Poor Outcome at 6 Months: WFNS Grade 1 to 2 on Admissiona
Defining CRP Thresholds
To select an arbitrary CRP threshold for outcome prediction, receiver operating characteristic (ROC) curve analysis determined sensitivity and specificity for each epoch post ictus (Figure). Patients who had samples taken within 24 hours of ictus and on day 4 post ictus were omitted from the ROC curve analysis, as the numbers in these groups were small (11 and 56, respectively). Table 10 shows the corresponding values for baseline CRP in each epoch with the associated predictive sensitivity and specificity values. For example, a baseline CRP level of 17.5 mg/L on day 1 post ictus would predict poor outcome at discharge with a confidence level of 58% sensitivity and 79% specificity, whereas on day 3 post ictus, a threshold of 20.5 mg/L would predict poor outcome with a confidence level of 65% sensitivity and 68% specificity.
TABLE 10.
Sensitivity and Specificity for C-Reactive Protein Sampled at Baseline, Days Post Ictus
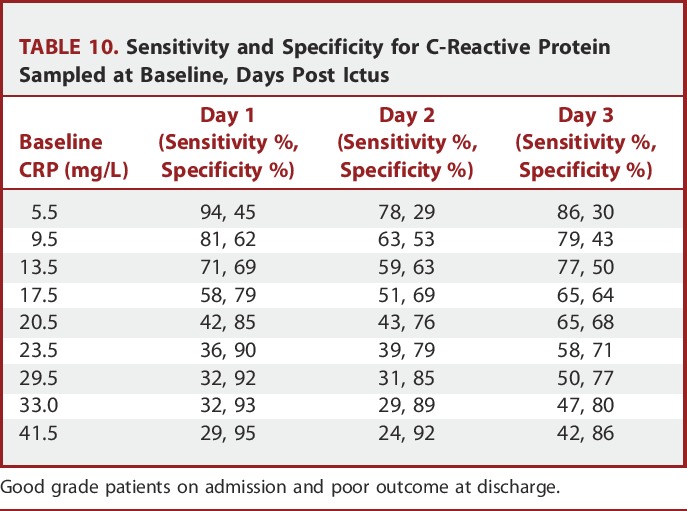
FIGURE.
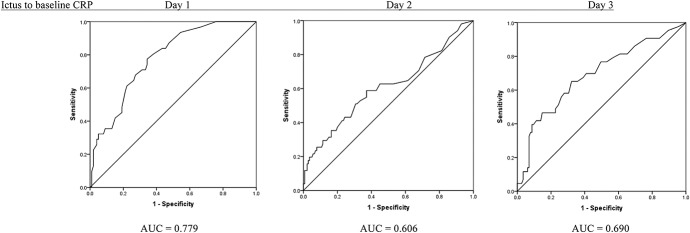
Receiver operating characteristic curves for baseline C-reactive protein values. Good grade patients on admission and poor outcome at discharge. AUC, area under the curve.
DISCUSSION
While the outcomes after SAH have significantly improved during the past 2 decades, the condition is still associated with relatively high levels of mortality and morbidity.11,12 Providing a simple and reliable method for earlier prediction of clinical deterioration, particularly in good grade patients, would have a significant bearing on targeted escalation of observation and therapy.
In this study, using data from the STASH trial, we have identified a number of biochemical parameters that showed differences in patients achieving favorable vs unfavorable outcome. Of these, CRP remained the dominant independent variable associated with poor outcome at discharge, independently of age, WFNS grade, and the Fisher grade. Because CRP is a sensitive marker of a systemic inflammatory reaction,13,14 this observation has a common and partly understood pathophysiological basis. Inflammation is regarded to be an important factor influencing outcome after aneurysmal SAH and has been demonstrated to play a role in early brain injury after SAH.15 Although elevated baseline CRP in relation to poor outcome after an SAH has been previously documented,16-20 the relevance of an elevated baseline CRP on outcome in good grade patients has not been previously demonstrated.
We recognize that there are numerous potential explanations for increased CRP after SAH, most notably development of sepsis, which is known to adversely influence outcome. In the present study we aimed to analyze the admission parameters at a time point where infection is unlikely to be present. When assessed in a multivariate model, baseline CRP and sepsis were both independent predictors of outcome. Furthermore, a continuous rise in CRP on consecutive days after SAH was seen in both the good as well as the poor outcome groups, suggesting that a systemic inflammatory response is playing an active part independently of sepsis.
The actual time-from-ictus to baseline sampling ranged from <24 hours (day 0) to 96 hours (day 4) post ictus. This is in contrast to previous studies such as Juvela et al20 where CRP values were obtained within 48 hours of ictus or Romero et al19 where CRP values were obtained <24 hours post ictus. These time epochs explain the differences in absolute values of CRP recorded in the respective studies. We have not included later (post day 4) CRP values in this analysis, as our objective was to identify early biochemical markers. Furthermore, later samples are more likely to be contaminated by evolving sepsis.
We examined ROC curves to determine a clinical baseline CRP threshold as a predictor of poor outcome. As expected, selecting higher baseline CRP thresholds caused a decrease in sensitivity and increase in specificity; however, the sensitivity range at each time interval was not consistent. The likelihood of predicting poor outcome at most CRP levels seemed to have higher sensitivity on day 1 and day 3 when compared to day 2, no doubt a reflection of the relatively small numbers in each epoch.
The presented data suggest that an elevated baseline CRP was both an early and an independent predictor of outcome. Importantly, this observation was apparent when only the good grade patients were analyzed. This suggests that the observed inflammatory response was not only a reflection of the severity of the initial ictus determining presentation grade, but also of a potentially progressive condition in good grade patients. Larger numbers with consistent timing in CRP evaluation is required to identify a more definitive level of CRP that predicts deterioration in the early stage post hemorrhage. Clearly, the earlier the measurable rise in CRP, the more likely it will serve as a valuable predictor, allowing time for intervention. On that note, we are encouraged that rises in CRP were seen so early after the initial ictus.
As these data was derived from the STASH trial,9 we included the potential effect of statin therapy on the measured parameters. Statins had no influence, and given that the trial drug was started after the baseline blood samples were taken, these tests were not contaminated by any theoretical statin effect.
A number of publications describe the role of biomarkers as a precursor to clinical deterioration after a subarachnoid hemorrhage. However, their role is unclear. Lad et al21 reviewed 27 articles in relation to a number of potential markers, none of which have been established as a biomarker for diagnosing preclinical vasospasm or monitoring its progression. In contrast to our findings, McMahon et al22 determined the incidence of raised biomarkers including IL-6 and CRP as a precursor to cerebral vasospasm. They analyzed samples up to 13 days post angiography and found an elevation in interleukin-6 but not CRP reflected impending cerebral ischemia; however, their endpoint was delayed cerebral ischemia, not outcome, and the numbers were relatively small.
Limitations
A limitation of this study is that the data presented were the result of a post hoc analysis. However, the results imply that prospective early recording of CRP may prove useful in detecting those good grade patients who are at an increased risk of clinical deterioration. If verified, besides providing an obvious clinical utility, identification of patients at risk of deterioration using early measures of CRP could prove useful in the design of clinical trials in SAH. Another limitation is the timing of the outcome measure. It is well recognized that patients improve their outcome score between discharge and 6 months; hence, any early treatment gain is diluted. This may explain why baseline CRP was only predictive of outcome in the good grade patients at discharge. Early measures of clinical well-being may prove a more useful way to measure outcomes in studies of this type, affording the opportunity of demonstrating influences on speed of recovery, need for rehabilitation, and time to discharge home.
CONCLUSION
The post hoc analysis of biochemical data derived from the STASH study has shown that baseline CRP levels may have predictive value in identifying good grade patient who are at greater risk of a clinical deterioration. A targeted prospective study is required to validate and quantify this observation.
Disclosures
British Heart Foundation (SP/08/003/24065), Wellcome Trust (Charities Open Access Fund). Financial support is provided by the British Heart Foundation. Mr Kirkpatrick and Professor Hutchinson are supported by the Cambridge National Institute for Health Research Biomedical Research Centre, and Professor Hutchinson is supported by a National Institute for Health Research Professorship. We also acknowledge the support of the Cambridge Clinical Trials Unit, United Kingdom Clinical Research Network for all 35 participating sites. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
STASH Investigators—Writing Committee: P.J. Kirkpatrick, C.L. Turner, G.D. Murray, P.J. Hutchinson. Steering Committee: Professor Sir G. Teasdale, Chairman; Professor G.D. Murray, Statistician; Mr P.J. Kirkpatrick, Chief Investigator; Professor A.D. Mendelow, Co-Investigator; Professor K. Muir, Independent; Professor M. Smith, Independent; Mr P. McCabe, Lay Member, CEO Headway, London; Professor J. Pearson, BHF Representative. Data Management Committee: Professor G. Ford, Chairman; Mr A. Vail, Statistician; Mr A. King, Independent; Dr P. Tyrrell, Independent; Dr H. Richards, Statistician; Dr S. Bond, Independent Statistician. Trial Management Team: Mr P.J. Kirkpatrick, Chief Investigator; Mrs C.L. Turner, Trial Manager; Dr C. Smith, Trial Coordinator; Dr E. Warburton, Independent Medical Advisor. Center Investigators: P.J. Kirkpatrick, C.L. Turner, C. Smith (Addenbrooke's Hospital, Cambridge); A. Belli, D. Bulters, M. Brown (Southampton University Hospital, Southampton); G. Critchley, G. Spurling, J. Gaylard (Hurstwood Park Neurological Centre, Brighton); M. Javadpour, P. Eldridge, L. Murray (Walton Centre, Liverpool); R. Nelson, R. Taylor, S. Hierons (Frenchay Hospital, Bristol); A.D. Mendelow, B. Tobin, K. Storey (Royal Victoria Infirmary, Newcastle); D. Walsh, B. Mistry, J. Aeron-Thomas (King's College Hospital, London); C. Puppo (Montevideo, Uruguay); M. Papadopoulos, L. Montague (St George's Hospital, London); P. Gan, G. Flint, J. Hurley (Queen Elizabeth Hospital, Birmingham); E. Ronne, I. Stjernling (Uppsala, Sweden); E. Wang, E.L. Cheng, K.W. Low (NNI Singapore); S. Ross, R. Bellfield, L. Mandizvidza (Leeds General Infirmary, Leeds); P. Whitfield, N. Persad (Derriford Hospital, Plymouth); N. Suttner, M. Teo, K. McGuigan, L. Cloughley (Southern General Hospital, Glasgow); H. Patel, A. Ingham, K. Shaw (Salford Royal Infirmary, Manchester); R. Vindlacheruvu (Queen's, Romford); J. Millo, O. Warner, R. Teal (John Radcliffe, Oxford); F. Bernard, C. Sirois (Hopital Sacre Coeur, Montreal); S. Joshi, S. Nyabadza (University Hospital Coventry and Warwickshire, Coventry); J. Grieve, N. Kitchen, V. Bassan, P. Rayson (National Hospital for Neurology and Neurosurgery, London); A. Alasheev, A. Zeitlin (Burdenko Institute, Moscow); M. Findlay, L. Sonnema, B. Poworoznik (University of Alberta, Edmonton, Canada); J. Quintero (Medellin, Colombia); S. Eljamel, M. Teo (Ninewells Hospital, Dundee); F. Rasulo (Brescia, Italy); I. Ng, J.L. Lai, K.W. Low (Singapore General Hospital, Singapore); B. Mathew, J. Grieg (Hull Royal Infirmary, Hull); R. Hanel, A. Richie (Mayo Clinic, Florida); I. Fleetwood, E. Reardon-White, G. Hampton (Queen Elizabeth11, Halifax, Canada); S. Lewis, L. Miralia (Shands Hospital, Gainesville, Florida); H. Brydon, H. Maguire (University Hospital of North Staffordshire, Stoke-on-Trent); U. Patel, H. Sanderson, K. Birchall, P. Bayliss (Royal Hallamshire Hospital, Sheffield); K. O'Neill, T. Sachs (Charing Cross Hospital, London); R. Kett-White, L. Quinn (Morriston Hospital, Swansea).
COMMENTS
Because aneurysmal subarachnoid hemorrhage (SAH) is still a serious disease despite improvement of treatment options, all available data should be used to reveal prognostic factors for outcome as well as for knowledge of pathophysiological mechanisms of clinical deterioration. Common inexpensive laboratory markers that are in routine clinical use may be more beneficial than expensive methods without validation.
C-reactive protein (CRP) is an acute phase sensitive, nonspecific inflammatory marker and initiating factor of inflammation and infection. Previous large studies including meta-analyses indicate that elevated CRP levels increase modestly and independent of confounding factors risk for coronary heart disease as well as for both vascular and nonvascular mortality.1,2 CRP levels also predict risk for ischemic stroke, but no more after adjustment for risk factors (hypertension, cigarette smoking, diabetes, and heart disease), as CRP levels correlate with these risk factors.2
Elevated CRP values seen within 2 days after bleeding correlate with outcome, but do not seem to predict delayed cerebral ischemia or infarction after SAH.3,4 Similar correlation between CRP levels obtained within 24 hours after primary intracerebral hemorrhage and outcome has been observed.5 These associations have been independent of clinical and radiological severity of bleeding and of prehemorrhage confounding factors (hypertension, cigarette smoking, diabetes, and heart disease). Prognostic value of CRP levels obtained later than 3 to 4 days after bleeding decreases significantly because the levels are affected by severity of clinical symptoms and infections (sepsis and pneumonia).
The value of the present study is that patients with World Federation of Neurological Surgeons grades 1 to 2 who later have a poor outcome have a clearly steeper increase of CRP within 2 days after SAH. Therefore, routine measurement of CRP within 2 days after SAH is also recommended in good grade patients, not only in poor grade patients.
Seppo Juvela
Helsinki, Finland
- 1.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(7):483-495. [DOI] [PubMed] [Google Scholar]
- 2.Elkind MS, Luna JM, Moon YP, et al. High-sensitivity C-reactive protein predicts mortality but not stroke: the Northern Manhattan Study. Neurology. 2009;73(16):1300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juvela S, Kuhmonen J, Siironen J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 2012;154(3):397-404. [DOI] [PubMed] [Google Scholar]
- 4.Csajbok LZ, Nylén K, Öst M, Sonander H, Nellgård B. In-hospital C-reactive protein predicts outcome after aneurysmal subarachnoid haemorrhage treated by endovascular coiling. Acta Anaesthesiol Scand. 2015;59(2):255-264. [DOI] [PubMed] [Google Scholar]
- 5.Löppönen P, Qian C, Tetri S, et al. Predictive value of C-reactive protein for the outcome after primary intracerebral hemorrhage. J Neurosurg. 2014;121(6):1374-1379. [DOI] [PubMed] [Google Scholar]
The authors present a post hoc analysis of the STASH trial data to identify biomarkers that could be predictive of poor outcome among patients with favorable grade aneurysmal subarachnoid hemorrhage. This study identified elevations in C-reactive protein (CRP) to be associated with poor outcome after subarachnoid hemorrhage. Although CRP has also been identified as an important biomarker by other recent studies,1-3 the authors are to be congratulated for further demonstrating this association, specifically among patients with favorable grade hemorrhages. Using biomarkers to identify patients with an increased likelihood of poor outcome is a worthwhile pursuit, but this information will be especially useful among patients that would otherwise be expected to progress well, based on having a small volume of blood on computed tomographic imaging. This study will also be useful in the drafting of future aneurysmal hemorrhage biomarker studies, which might further validate these results through a prospective design.
G. Edward Vates
Stephen Sandwell
Rochester, New York
- 1.Fountas KN, Tasiou A, Kapsalaki EZ, et al. Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2009;26(5):E22. [DOI] [PubMed] [Google Scholar]
- 2.Jeon YT, Lee JH, Lee H, et al. The postoperative C-reactive protein level can be a useful prognostic factor for poor outcome and symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2012;24(4):317-324. [DOI] [PubMed] [Google Scholar]
- 3.Juvela S, Kuhmonen J, Siironen J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 2012;154(3):397-404. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Callo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8(4):355-369. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid hemorrhage. Lancet. 2007;369(9558):306-318. [DOI] [PubMed] [Google Scholar]
- 3.The Across Group. Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke. 2000;31(8):1843-1850. [DOI] [PubMed] [Google Scholar]
- 4.van Gijn J, Rinkel GJ. Subarachnoid hemorrhage: diagnosis, causes and management. Brain. 2001;124(pt 2):249-278. [DOI] [PubMed] [Google Scholar]
- 5.Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with Pravastatin on cerebral vasospasm, autoregulation and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage. A phase 11 randomised placebo-controlled trial. Stroke. 2005;36(8):1627-1632. [DOI] [PubMed] [Google Scholar]
- 6.Lynch JR, Wang H, McGirt MJ, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomised clinical trial. Stroke. 2005;36(9):2024-2026. [DOI] [PubMed] [Google Scholar]
- 7.McGirt MJ, Lynch JR, Parra A, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33(12):2950-2956. [DOI] [PubMed] [Google Scholar]
- 8.Parra A, Kreiter KT, Williams S, et al. Effect of prior statin use on functional outcome and delayed vasospasm after acute aneurysmal subarachnoid hemorrhage: a matched controlled cohort study. Neurosurgery. 2005;56(3):476-484. [DOI] [PubMed] [Google Scholar]
- 9.Kirkpatrick P, Turner C, Smith C, Hutchinson PJ, Murray GD; for the STASH Collaborators. Simvastatin in aneurysmal subarachnoid hemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol. 2014;13(7):666-675. [DOI] [PubMed] [Google Scholar]
- 10.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243-2246. [DOI] [PubMed] [Google Scholar]
- 11.Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage: new strategies for prevention and management. Curr Opin Crit Care. 2006;12(2):78-84. [DOI] [PubMed] [Google Scholar]
- 12.Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population based study and systematic review. Neurology. 2010;74(19):1494-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller BA, Turan N, Chau M, Pradilla G. Inflammation, vasospasm and brain injury after subarachnoid hemorrhage. Biomed Res Int. 2014;2014:384342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Clos TW. Pentraxins: structure, function and role in inflammation. ISRN Inflamm. 2013;2013:379040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97(1):14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindgren C, Hultin M, Koskinen LO, Lindvall P, Borota L, Naredi S. ADMA levels and arginine/ADMA ratios reflect severity of disease and extent of inflammation after subarachnoid hemorrhage. Neurocrit Care. 2014;21(1):91-101. [DOI] [PubMed] [Google Scholar]
- 17.Fountas KN, Tasiou A, Kapsalaki EZ, et al. Serum and cerebrospinal fluid C-reactive protein levels as predictors of vasospasm in aneurysmal subarachnoid hemorrhage. Clinical article. Neurosurg Focus. 2009;26(5):E22. [DOI] [PubMed] [Google Scholar]
- 18.Romero FR, Bertolini Ede F, Figueiredo EG, Teixeira MJ. Serum C-reactive protein levels predict neurological outcome after aneurysmal subarachnoid hemorrhage. Arq Neuropsiquiatr. 2012;70(3):202-205. [DOI] [PubMed] [Google Scholar]
- 19.Romero FR, Cataneo DC, Cataneo AJ. C-reactive protein and vasospasm after aneurysmal subarachnoid hemorrhage. Acta Cir Bras. 2014;29(5):340-345. [DOI] [PubMed] [Google Scholar]
- 20.Juvela S, Kuhmonen J, Siironen J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). 2012;154(3):397-404. [DOI] [PubMed] [Google Scholar]
- 21.Lad SP, Hegen H, Gupta G, Diesenhammer F, Steinberg GK. Proteomic biomarker discovery in cerebrospinal fluid for cerebral vasospasm following subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2012;21(1):30-41. [DOI] [PubMed] [Google Scholar]
- 22.McMahon CJ, Hopkins S, Vail A, et al. Inflammation as a predictor for delayed cerebral ischemia after subarachnoid hemorrhage. J Neurointerv Surg. 2013;5(6):512-517. [DOI] [PMC free article] [PubMed] [Google Scholar]


