Supplemental Digital Content is available in the text.
Keywords: epidemiology, dengue fever, dengue hemorrhagic fever, children, hospitalization
Background:
The burden of dengue is high in the Philippines but the prevalence of confirmed cases is unknown, and the disease is subject to underreporting because surveillance of suspected cases is passive. We conducted a prospective epidemiological study to estimate the proportion of laboratory-confirmed dengue among clinically suspected hospitalized cases in the pediatric wards of 3 regional hospitals in the Philippines and to describe the clinical and laboratory features, age distributions, case fatality rates and serotype distributions of these hospitalized cases.
Methods:
Patients ≤18 years and hospitalized for suspected dengue were included if they had an axillary temperature ≥38°C for 2−7 days and 2 or more dengue-associated symptoms. Dengue infection was confirmed in acute blood samples by serotype-specific reverse transcription-polymerase chain reaction and IgM immunoassay.
Results:
We confirmed dengue infection in 1809 (86.1%) cases of 2103 suspected cases between November 2009 and November 2010. The 6- to 10-year-old age group had the highest proportion of cases overall (36.7%). Fever, anorexia, myalgia, abdominal pain and headache were the most common symptoms at admission. Hemorrhagic manifestations, signs of plasma leakage, thrombocytopenia and leucopenia were all significantly more common in confirmed than in nonconfirmed cases. Most cases (76.5%) developed dengue hemorrhagic fever or dengue shock syndrome, and the overall case fatality rate was 0.94%. Distributions of all 4 virus serotypes varied at each hospital.
Conclusions:
The clinical burden of pediatric dengue continues to be substantial in the Philippines. Most hospitalized cases of suspected pediatric dengue can be laboratory confirmed and most develop severe disease.
Dengue fever (DF) is caused by infection with 1 of 4 dengue virus (DENV) serotypes (1−4).1,2 Although many dengue infections are asymptomatic or cause only mild to moderate fever for 2−7 days, severe cases can progress to dengue hemorrhagic fever (DHF), which may lead to shock, organ failure or death. Currently, there are no dengue-specific treatments or vaccines available, although one vaccine candidate is in the late phases of clinical development.3–7 Additional and improved vector control measures are also needed to prevent disease.
The global burden of dengue is already large and continues to grow.8–11 Recent estimates put the annual number of dengue infections at 390 million, of which 96 million are symptomatic.8 According to an evidence-based consensus study, DENV transmission occurs in 128 countries worldwide, with 3.97 billion people at risk of dengue infection, of which at least 70% live in the Asia-Pacific region.8,12
Dengue disease is hyperendemic in the Philippines.13 All 4 serotypes circulate throughout the country, and children aged 5−14 years are primarily affected.13–15 To monitor disease patterns and outbreaks, the Philippine Integrated Disease Surveillance and Response program implemented in 2008 includes virological and serological testing. However, because of limited resources and laboratory capacity, only 3%–5% of all reported cases are submitted for laboratory confirmation.16 Consequently, little serotype distribution information is available for different regions of the Philippines. In addition, dengue has been historically subject to underreporting, and the epidemiology of laboratory-confirmed dengue disease in children has not been well described.
We conducted an epidemiological study of all suspected dengue cases in the pediatric wards of 3 sentinel hospitals in the Philippines to estimate the proportion of laboratory-confirmed disease among hospitalized cases, to describe the clinical features of confirmed dengue in children and the age groups most affected and to determine regional serotype distributions.
MATERIALS AND METHODS
Study Design
This was a descriptive, prospective, epidemiological study of dengue disease in the pediatric wards of 3 sentinel regional hospitals in the Philippines (see Fig., Supplemental Digital Content 1, http://links.lww.com/INF/C197) using data collected between November 2009 and November 2010. The 3 hospitals were the José B. Lingad Memorial Regional Hospital (JBL); Davao Medical Center (DMC; now the Southern Philippines Medical Center), both of which care for patients aged ≤15 years, and the Western Visayas Medical Center (WVMC), which cares for patients aged ≤18 years. These 3 hospitals are located on the 3 major islands of the country: Luzon, Mindanao and the Visayas. The primary objective of this study was to estimate the proportion of laboratory-confirmed dengue cases among the clinically suspected dengue cases hospitalized in these pediatric wards. Secondary objectives were to describe the clinical and laboratory features of suspected and laboratory-confirmed dengue; to describe the age distributions, case fatality rates and serotype distributions of laboratory-confirmed dengue and to assess the feasibility of case detection and laboratory confirmation.
Ethical Considerations
This study was conducted in accordance with the latest revision of the Declaration of Helsinki (Seoul, Republic of Korea, October 2008), the Guidelines for Good Epidemiology Practices and local regulatory requirements. Before the enrollment of the first patient, the protocol was approved by the Institutional and Ethical Review Board of the Research Institute for Tropical Medicine (RITM) and by the Hospital Research Committee of the 3 hospitals. All patients (or parents or legal representatives if aged <18 years) provided written and signed informed consent.
Study Population
To be considered for inclusion, patients had to be ≤18 years and hospitalized in the pediatric ward of a study hospital, and to have currently or to report having within the previous several days, an axillary temperature ≥38°C for 2–7 days. Patients also had to have at least 2 of the following dengue-associated signs or symptoms: a positive tourniquet test, hemorrhagic manifestations (eg, epistaxis, petechiae, ecchymosis, gum bleeding, hematemesis, melena or other bleeding), flushing, abdominal pain, anorexia, headache, retro-orbital pain, myalgia, congested pharynx, injected conjunctivae, restlessness, hemoconcentration (hematocrit >20% over baseline for age and sex), thrombocytopenia (platelet count <100,000/mm3), signs of circulatory collapse (eg, shock, rapid weak pulse, narrow pulse pressure, hypotension, cold clammy skin) or other signs or symptoms suggesting dengue, as specified by the physician. Patients were excluded from the study if they had a known diagnosis other than dengue (eg, bronchopneumonia, gastroenteritis, meningitis).
Clinical Procedures
At inclusion, a member of the hospital’s medical staff performed a physical examination and recorded patient characteristics, vital signs, features of dengue-associated illness and clinical signs of any concomitant disease. A blood sample (5 mL) was also collected to obtain a complete blood count and to confirm dengue disease by virological and serological analyses. After admission, patients received the hospital’s standard medical care and treatment for dengue. During hospitalization, the clinical development of the case was recorded, including any signs of severity that appeared during hospitalization (hemorrhagic manifestations, signs of plasma leakage and signs of shock) and any further hematological findings (elevated hematocrit or thrombocytopenia).
Clinical Diagnosis at Discharge
Clinical diagnosis of dengue disease was made according to 1997 World Health Organization guidelines.17 Patients were diagnosed with DHF if, along with a history of fever, they had or developed 3 of the following signs: (1) hemorrhagic tendencies indicated by a positive tourniquet test; petechiae, ecchymoses or purpura; bleeding from the mucosa, gastrointestinal tract, injection site or other site; hematemesis or melena; (2) thrombocytopenia (≤100,000/mm3); and (3) plasma leakage resulting in a hematocrit >20% above average for the age and population or >20% lower after fluid infusion treatment, or resulting in other signs (ascites, hypoproteinemia or pleural effusion). Patients with DHF were considered to have dengue shock syndrome (DSS) if they had or developed circulatory failure resulting in all 3 of the following: (1) rapid and weak pulse; (2) narrow pulse pressure (≤20 mm Hg) or hypotension and (3) cold, clammy skin and restlessness. Patients with DHF or DSS were considered to have severe dengue disease.
Laboratory Assessments
Complete blood counts were performed on the same day it was collected. Serological and virological testing for dengue was performed by the Research Institute for Tropical Medicine virology laboratory (Muntinlupa City, Philippines). Serological confirmation was determined by an absorbance value >1.1-fold over the cutoff value in an anti-dengue IgM capture enzyme-linked immunosorbent assay (PanBio; Alere, Brisbane, Australia). Absorbance values were considered equivocal if they were 0.9–1.1 times the cutoff value and negative if <0.9 times the cutoff value. For virological analysis, RNA was extracted from serum using a QIAamp Viral RNA Mini Kit (Qiagen, San Diego, CA). Viral RNA was then amplified by reverse transcription-polymerase chain reaction (RT-PCR) using dengue- and serotype-specific primers18 and visualized on an agarose gel. Virological confirmation was determined by the presence of bands corresponding to viral RNA and to 1 or more DENV serotypes. Nonconfirmed cases were negative for RT-PCR and for serology, negative for RT-PCR with an equivocal serology result or no serology test performed or had no RT-PCR or serology test performed.
Statistical Analyses
Statistical analyses were performed using Stata 10 software (StataCorp LP, College Station, TX). Normally distributed data were compared using Student’s t test for continuous variables and the χ2 test for categorical variables. Fisher exact test was used for small sample sizes. P values ≤ 0.05 were considered to indicate significant differences.
RESULTS
Study Population
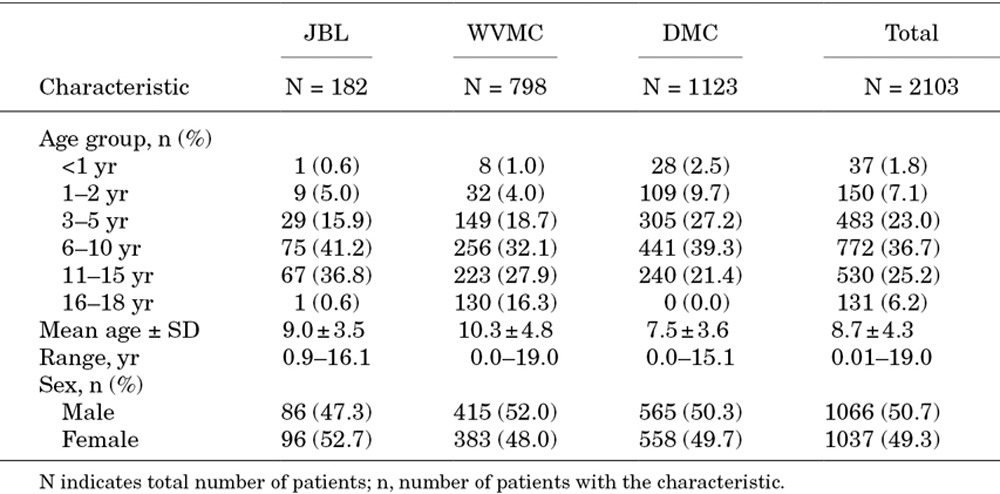
Overall, 2103 suspected dengue cases were enrolled between November 2009 and November 2010 in the pediatric wards of the study hospitals, with 182 cases at JBL, 798 at WVMC and 1123 at DMC (Table 1). Patient ages ranged from infants <1 years to 18-year-old adolescents, with 85% of all suspected cases between 3 and 15 years of age. Mean age was 9.0 ± 3.5 years at JBL, 7.5 ± 3.6 years at DMC and 10.3 ± 4.8 years at WVMC (the only hospital where patients aged 16–18 years were admitted). Sex ratios were generally equivalent at each hospital.
TABLE 1.
Study Population by Center
Laboratory-Confirmed Cases
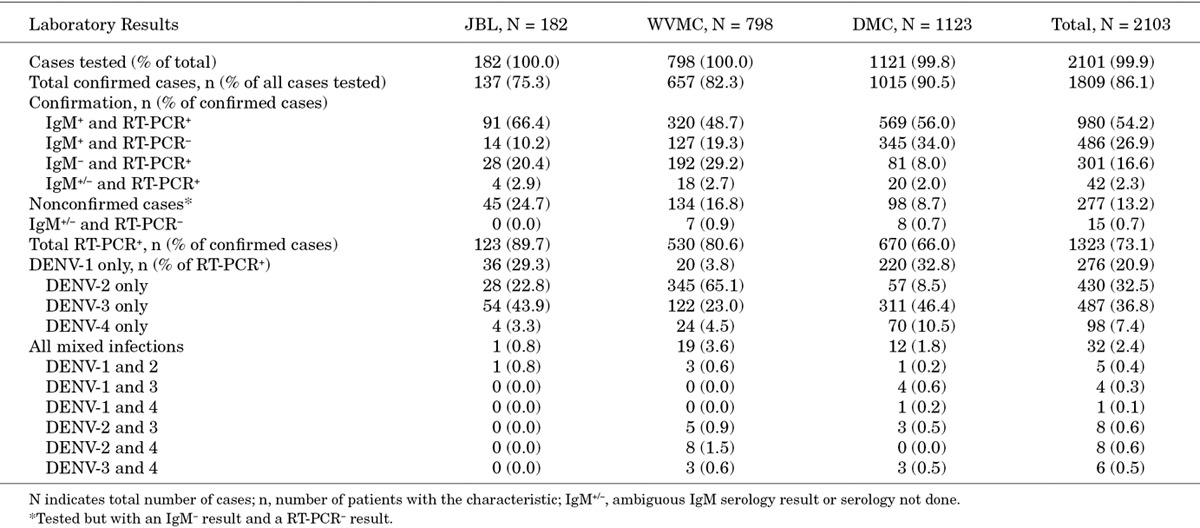
Of the 2101 cases with blood samples available for analysis, 1809 (86.1%) were confirmed by the presence of anti-dengue IgM, viral RNA or both (Table 2). Of the remainder, 277 (13.2%) lacked detectable levels of either IgM or viral RNA and were considered nonconfirmed, and 15 (0.7%) had an ambiguous or missing serology result and undetectable levels of viral RNA. Overall, 81.1% of the confirmed cases were positive for IgM, 73.1% were positive for viral RNA and 54.2% were positive for both. Confirmation rates for the individual hospitals were between 75.3% and 90.5% although the corresponding proportions of IgM-positive and viral RNA-positive cases varied at each hospital. Between 68.0% and 90.0% of confirmed cases were IgM-positive and between 66.0% and 89.8% were viral RNA-positive.
TABLE 2.
Results of Laboratory Confirmation Analyses
The distributions of the suspected and confirmed cases among the pediatric age groups were similar for the 3 hospitals (Fig. 1). The age groups with the highest number of confirmed cases were the 11 to 15-year group at JBL and the 6 to 10-year group at DMC and WVMC. Confirmation rates were high for nearly all age groups at each hospital, were over 70% for each age group overall, and did not correlate with age.
FIGURE 1.
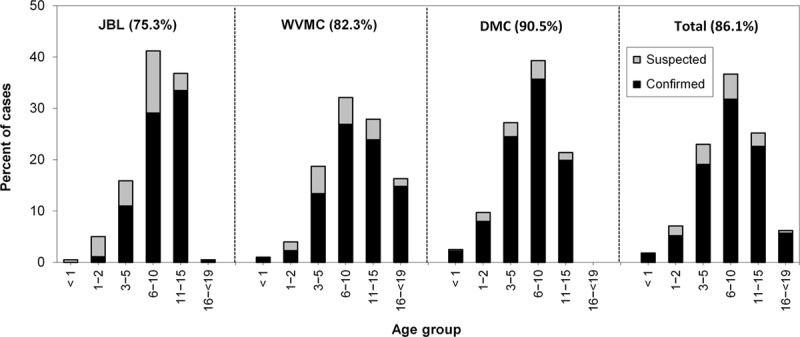
Age-group distributions of suspected and confirmed dengue at the three sentinel hospitals. Cases of suspected (gray bars) and confirmed (black bars) dengue in each age group for each hospital and overall are shown as a percentage of the total number of cases. The values in parentheses are the case-confirmation rates at each hospital and overall. JBL, José B. Lingad Memorial Regional Hospital; WVMC, Western Visayas Medical Center; DMC, Davao Medical Center.
Dengue Serotype Distributions
All 4 DENV serotypes were found at each hospital (Table 2). Overall, in patients infected with a single serotype, DENV-3 was the most common followed by DENV-2 and DENV-1. Serotype distributions also differed at each hospital, with DENV-3 the most common at JBL and DMC, and DENV-2 the most common at WVMC. Thirty-two patients overall had mixed infections with 2 DENV different serotypes.
Fever Patterns in Confirmed Dengue
Only 26.1% of the confirmed cases overall and 24.9–32.3% at each hospital had fever at admission, with the majority of cases reporting a prior episode of fever (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/C198). More cases overall (44.0%) and at each hospital (38.7%–52.7%) had fever during hospitalization, indicating that fever returned or spiked during hospitalization for at least 324 cases (17.9%). Fever began 3–5 days before admission for most confirmed cases overall (71.0%) and at each hospital (61.0%–78.7%; see Fig., Supplemental Digital Content 3, http://links.lww.com/INF/C199).
Clinical Features of Confirmed Dengue at Admission and Signs of Deterioration During Hospitalization
At admission, anorexia, myalgia, abdominal pain, headache and hemorrhage were the most common signs and symptoms and were present in >55% of the confirmed and nonconfirmed cases overall and in >50% of the confirmed cases at each hospital (Fig. 2). Myalgia (P = 0.001), abdominal pain (P < 0.001), flushing (P = 0.019) and signs of circulatory collapse (P = 0.002) were all significantly more common in confirmed than in nonconfirmed cases. Some signs of hemorrhage, such as a positive tourniquet test (45.5% vs 34.7%; P = 0.001) and petechiae (20.8% vs 13.4%; P = 0.004), were significantly more common in confirmed than in nonconfirmed cases, but signs of hemorrhage overall were not. At admission, shock was reported for only 8 confirmed cases. Retro-orbital pain, hepatomegaly and pleural effusion were infrequent among all cases overall.
FIGURE 2.
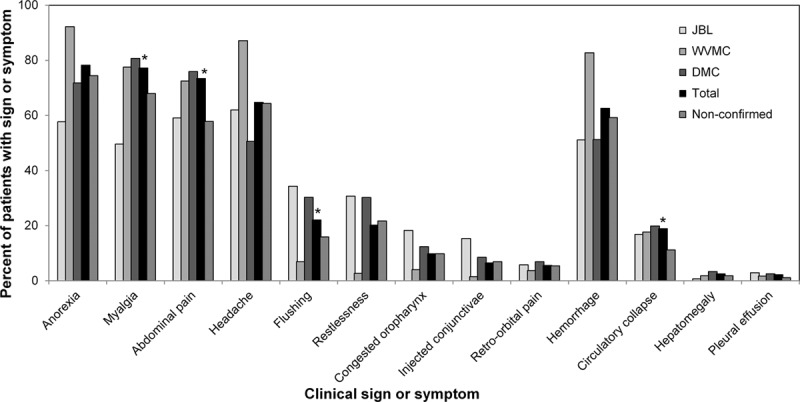
Clinical signs and symptoms at admission for confirmed and non-confirmed dengue. The percentage of cases with each clinical sign or symptom at admission are shown for the confirmed cases at each hospital, for the total confirmed cases, and for the total non-confirmed cases. Asterisks indicate signs or symptoms with significantly different percentages (P< 0.05) for the total confirmed cases vs the total non-confirmed cases.
Elevated hematocrits (>42% and >45%), thrombocytopenia (<100,000/mm3) and leucopenia (<5000/mm3) were all significantly more common in confirmed cases than in nonconfirmed cases (all P < 0.001).
During hospitalization, between 17.7% and 45.4% of the confirmed cases at each hospital presented with dengue-related signs of deterioration, which were significantly more common overall in confirmed (28.4%) than in nonconfirmed cases (18.1%; P < 0.001; see Table, Supplemental Digital Content 4, http://links.lww.com/INF/C200). Hemorrhagic manifestations (especially petechiae and epistaxis) and signs of plasma leakage (especially hemoconcentration) were significantly more common in confirmed than in nonconfirmed cases. Signs of shock developed in 3.6%–13.1% of the confirmed cases at each hospital. Among the mixed serotype infections, 11 cases had signs of deterioration, including hemorrhagic manifestations (7 cases), signs of plasma leakage (6 cases) and shock (2 cases).
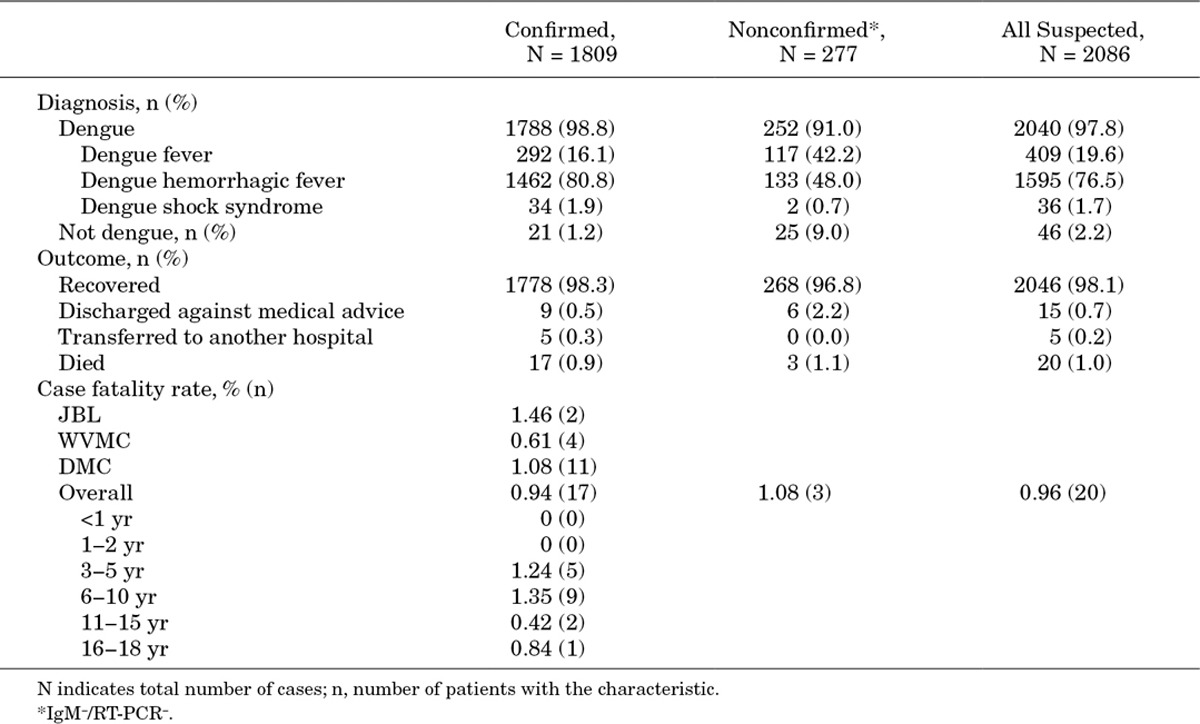
Clinical Diagnosis at Discharge, Outcome and Case Fatality Rate
Overall, 98.8% of the confirmed cases were clinically diagnosed with dengue disease at discharge (Table 3). Most (82.7%) were severe dengue (DHF or DSS), and 16.1% were DF. Of the mixed infections, 26 (81.3%) were severe, and 6 (18.7%) were DF. Despite laboratory confirmation (not known at time of discharge), 21 confirmed cases were clinically diagnosed with an illness other than dengue. Many of these cases were diagnosed with acute viral infections (7 cases) or pneumonia (6 cases) or other infections that involve fever. Nearly all of the nonconfirmed cases were also diagnosed with dengue. Overall, 98.3% of the confirmed cases recovered and 20 patients died, 17 of which had laboratory-confirmed dengue. None of the mixed serotype infections were fatal. Case fatality rates (CFR) for confirmed dengue were between 0.61% and 1.46% at each hospital and 0.94% overall. Most fatal confirmed cases were in patients aged 4–16 years, with a mean age of 8.0 ± 3.4 years. Six (35.3%) were boys, and 11 (64.7%) were girls.
TABLE 3.
Clinical Diagnoses, Outcomes and Case Fatality Rates of Confirmed and Nonconfirmed Dengue
Seasonal Infection Patterns
During the study, infection numbers at all 3 hospitals peaked during the Philippine rainy season (June–November) for all 4 DENV serotypes (Fig. 3).
FIGURE 3.
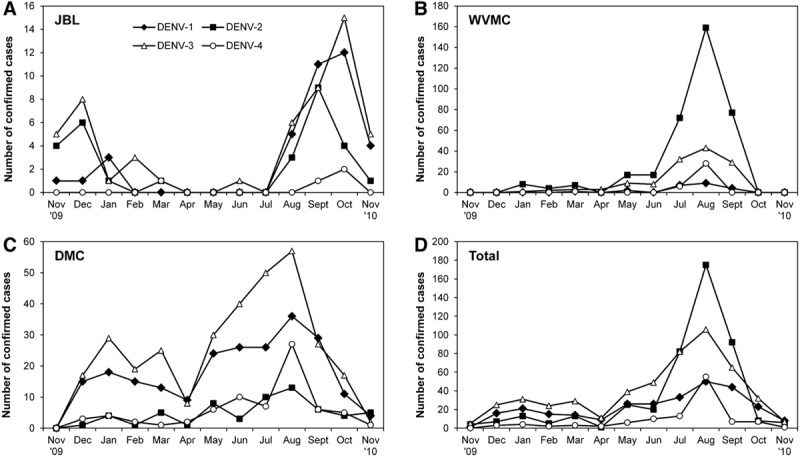
Seasonal prevalence of dengue serotypes. Numbers of cases in which the infecting serotype was identified by RT-PCR are shown for each hospital and for the overall (total) for each month of the study (November 2009 to November 2010).
DISCUSSION
In the Philippines, children and adolescents are at the highest risk of dengue infection. In this study of 2103 hospitalized pediatric cases of suspected dengue, 86.1% were laboratory confirmed, and over 80% were considered severe dengue. The clinical signs and symptoms at admission were those typically associated with dengue. Almost one-third of the confirmed cases worsened during hospitalization and 17 were fatal, although the overall case fatality rate was <1%. The distributions of all 4 DENV serotypes were seasonal and varied at the three hospitals.
This study yielded valuable regional age distribution information for pediatric dengue in the Philippines. The proportions of confirmed infections tended to be highest in the 6- to 10-year-old age group and to decrease in the older age groups. These results are consistent with data from the Philippine Department of Health showing that the highest proportions of DF and DHF cases were in the 5- to14-year-old age group in 2000–2009.13 Although we are unable to compute the age group incidence rates for these areas and did not investigate adult transmission rates, these results suggest that dengue susceptibility in the Philippines peaks between 6 and 10 years of age and then decreases. These findings are consistent with the hyperendemicity and the high burden of dengue disease in the Philippines, where dengue immunity increases with age.19
A high proportion of the suspected cases in the study were laboratory confirmed, indicating that the clinical diagnosis at admission was generally accurate and that the combination of laboratory analyses was an effective means of confirming dengue disease. Indeed, both assays were important for case confirmation, and we calculate that 343 (18.9%) case confirmations would have been missed using only serological analysis and 486 (26.9%) would have been missed using only virological analysis. That the study included only cases severe enough to warrant hospitalization likely also contributed to the high confirmation rate. Severe cases tend to have higher viral loads and are more likely to be confirmed than mild cases.20–22
Many of the confirmed cases had no fever at admission but instead had reported previous fever. Fever in dengue may be short-lived (2–7 days), so fever for some patients may have already subsided during the natural course of the infection. However, at least 10.2%–26.0% of the patients with confirmed dengue at each hospital experienced a second bout of fever while hospitalized. This may have also occurred as a natural course of the infection as fever waned and spiked.
Nearly all of the confirmed cases received a clinical diagnosis of dengue. For those that did not, some may have had a false positive test, whereas a dengue diagnosis may have been justified for others but was missed because of the unavailability of test results or to an unrelated coinfection (eg, typhoid or ascariasis). With many of the same nonspecific clinical features as the confirmed cases, 91% of the nonconfirmed cases were also diagnosed with dengue despite the absence of laboratory confirmation. It is possible that some of these cases were authentic dengue infections that produced false negative test results because of cleared or low viremia or a weak or excessively late IgM response. Another possibility is that some cases were misdiagnosed because of bias or overreporting, which is common in endemic areas and during outbreaks.23
The overall CFR (0.94%) was comparable with the national values reported by the Philippine Department of Health for 2008 (0.94%) and 2009 (0.95%), however, it was higher than that reported for 2010 (0.59%).24 The higher CFR found in this study during the same period may be related to the pediatric age groups studied and to the hospitalization requirement, which would include the most severe cases. Indeed, the highest CFRs were in the 3- to 5-year and 6- to 10-year age groups. By comparison, CFRs rarely exceed 1% in endemic countries in the Western Pacific.1,2
Our study may be the first to describe concurrent serotype distributions at 3 different locations in the Philippines. All 4 DENV serotypes were found at all 3 hospitals, but the distributions and seasonal patterns were different at each one. As disease severity may vary with serotype,20,25 these results may not reflect the true proportions of the serotypes circulating in the local populations but only the proportions associated with hospitalization.
The 32 mixed infections with multiple serotypes we detected was another indication of the high burden of disease and hyperendemicity in the Philippines. Although a mixed infection was not detected until 1982,26 they are not uncommon in hyperendemic regions if appropriate virological techniques, including serotype-specific RT-PCR, are used for surveillance.27 Indeed, mixed infections have been reported in many countries.27–32 It is unlikely that mixed infections in this study resulted from sample contamination or different technological standards because all samples from the 3 hospitals were tested at the same laboratory. Determining the outcome and impact of these types of infections will require detailed virological testing and follow-up of suspected cases in hyperendemic areas, although most mixed infections appear to be no more severe than single infections.27,32,33
This study is strengthened by the accurate clinical diagnosis and comprehensive laboratory testing, which resulted in confirmation of >85% of suspected cases. We also investigated dengue in 3 distinct geographical areas in the Philippines, allowing the clinical features, age distributions and serotype distributions of dengue to be compared across regions. We present some of the first serotype distribution information for the islands of Visayas and Mindanao. We also recorded signs of deterioration, which allowed us to follow the course of infections during hospitalization. One limitation was the lack of catchment information for the 3 hospitals, which prevented us from estimating the incidence and burden of dengue hospitalization in these areas, and from determining why enrolment levels were different at each hospital. Finally, the study included only hospitalized cases, which are more severe than outpatient cases, so we are unable to estimate laboratory confirmation rates for outpatient symptomatic cases or burdens of disease in these areas.
In conclusion, most hospitalized cases of pediatric dengue in the Philippines can be laboratory confirmed. Over 85% of the cases we studied were in children 3–15 years of age, and over 80% of these cases were severe dengue with classic signs and symptoms. Regional and seasonal variations in the distributions of all 4 DENV serotypes were detected at each hospital, but transmission was highest during the rainy season. Finally, the very high seasonal caseloads of dengue at each hospital, the age-group distributions and the abundance of mixed infections all point to an extremely high clinical disease burden in the Philippines and emphasize the need for additional control measures.
ACKNOWLEDGMENTS
The authors thank Anamae Obcena for study coordination and all study participants, their families and the medical and laboratory staff of the 3 sentinel hospitals. The authors also thank Florence Coux for study monitoring; Céline Zocchetti for project management; Christine Luxemburger for support in study conceptualization; Laurence Pollissard for contributions to study design and Ruby Dizon. Medical writing services were provided by Kurt Liittschwager (4Clinics, Paris, France). Support for medical writing assistance and financial support for this study were provided by Sanofi Pasteur. Sanofi Pasteur participated in study design; the collection, analysis, and interpretation of data; writing of the report and the decision to submit the article for publication.
Supplementary Material
Footnotes
This study was funded by Sanofi Pasteur, Lyon, France. M.L. is an employee of Sanofi Pasteur. The authors have no other funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.World Health Organization. Global Strategy for Dengue Prevention and Control. Geneva: WHO Press; 2012. [Google Scholar]
- 2.World Health Organization. Dengue and severe dengue. 2014. Available at: http://www.who.int/mediacentre/factsheets/fs117/en/index.html#. Accessed April 15, 2014. [Google Scholar]
- 3.Capeding RZ, Luna IA, Bomasang E, et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine. 2011;29:3863–3872. doi: 10.1016/j.vaccine.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 4.Poo J, Galan F, Forrat R, et al. Live-attenuated tetravalent dengue vaccine in dengue-naïve children, adolescents, and adults in Mexico city: randomized controlled phase 1 trial of safety and immunogenicity. Pediatr Infect Dis J. 2011;30:e9–e17. doi: 10.1097/INF.0b013e3181fe05af. [DOI] [PubMed] [Google Scholar]
- 5.Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai school children: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 6.Thomas SJ, Endy TP. Vaccines for the prevention of dengue: development update. Hum Vaccin. 2011;7:674–684. doi: 10.4161/hv.7.6.14985. [DOI] [PubMed] [Google Scholar]
- 7.Thomas SJ. Developing a dengue vaccine: progress and future challenges. Ann N Y Acad Sci. 2014;1323:140–159. doi: 10.1111/nyas.12413. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ. 2002;324:1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapia-Conyer R, Betancourt-Cravioto M, Méndez-Galván J. Dengue: an escalating public health problem in Latin America. Paediatr Int Child Health. 2012;32(Suppl 1):14–17. doi: 10.1179/2046904712Z.00000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bravo L, Roque VG, Brett J, et al. Epidemiology of dengue disease in the Philippines (2000-2011): a systematic literature review. PLoS Negl Trop Dis. 2014;8:e3027. doi: 10.1371/journal.pntd.0003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes CG, Manaloto CR, Gonzales A, et al. Dengue infections in the Philippines: clinical and virological findings in 517 hospitalized patients. Am J Trop Med Hyg. 1988;39:110–116. doi: 10.4269/ajtmh.1988.39.110. [DOI] [PubMed] [Google Scholar]
- 15.Republic of Philippines Department of Health. Philippine Integrated Disease Surveillance and Response - Annual Reports, 2009–2011. Manila: National Epidemiology Center; 2011. [Google Scholar]
- 16.Republic of Philippines Department of Health. Philippine Integrated Disease Surveillance and Response, Manual of Procedures. Manila: National Epidemiology Center; 2008. [Google Scholar]
- 17.World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 18.Lanciotti RS, Calisher CH, Gubler DJ, et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Barraquer I, Cordeiro MT, Braga C, et al. From re-emergence to hyperendemicity: the natural history of the dengue epidemic in Brazil. PLoS Negl Trop Dis. 2011;5:e935. doi: 10.1371/journal.pntd.0000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 21.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis. 1997;176:322–330. doi: 10.1086/514048. [DOI] [PubMed] [Google Scholar]
- 22.Wang WK, Chao DY, Kao CL, et al. High levels of plasma dengue viral load during defervescence in patients with dengue hemorrhagic fever: implications for pathogenesis. Virology. 2003;305:330–338. doi: 10.1006/viro.2002.1704. [DOI] [PubMed] [Google Scholar]
- 23.Undurraga EA, Halasa YA, Shepard DS. Use of expansion factors to estimate the burden of dengue in Southeast Asia: a systematic analysis. PLoS Negl Trop Dis. 2013;7:e2056. doi: 10.1371/journal.pntd.0002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization WPRO. Annual dengue data in the Western Pacific Region, 2000–2010. 2015. Available at: http://www.wpro.who.int/emerging_diseases/annual.dengue.data.wpr/en/. Accessed January 15, 2015. [Google Scholar]
- 25.Fried JR, Gibbons RV, Kalayanarooj S, et al. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis. 2010;4:e617. doi: 10.1371/journal.pntd.0000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gubler DJ, Kuno G, Sather GE, et al. A case of natural concurrent human infection with two dengue viruses. Am J Trop Med Hyg. 1985;34:170–173. doi: 10.4269/ajtmh.1985.34.170. [DOI] [PubMed] [Google Scholar]
- 27.Loroño-Pino MA, Cropp CB, Farfán JA, et al. Common occurrence of concurrent infections by multiple dengue virus serotypes. Am J Trop Med Hyg. 1999;61:725–730. doi: 10.4269/ajtmh.1999.61.725. [DOI] [PubMed] [Google Scholar]
- 28.Colombo TE, Vedovello D, Mondini A, et al. Co-infection of dengue virus by serotypes 1 and 4 in patient from medium sized city from Brazil. Rev Inst Med Trop Sao Paulo. 2013;55:275–281. [Google Scholar]
- 29.Figueiredo RM, Naveca FG, Oliveira CM, et al. Co-infection of dengue virus by serotypes 3 and 4 in patients from Amazonas, Brazil. Rev Inst Med Trop Sao Paulo. 2011;53:321–323. doi: 10.1590/s0036-46652011000600004. [DOI] [PubMed] [Google Scholar]
- 30.Vinodkumar CS, Kalapannavar NK, Basavarajappa KG, et al. Episode of coexisting infections with multiple dengue virus serotypes in central Karnataka, India. J Infect Public Health. 2013;6:302–306. doi: 10.1016/j.jiph.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Bharaj P, Chahar HS, Pandey A, et al. Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J. 2008;5:1. doi: 10.1186/1743-422X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pongsiri P, Themboonlers A, Poovorawan Y. Changing pattern of dengue virus serotypes in Thailand between 2004 and 2010. J Health Popul Nutr. 2012;30:366–370. doi: 10.3329/jhpn.v30i3.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamani E, Figueroa D, García MP, et al. Concurrent infections by two dengue virus serotypes during an outbreak in northwestern Peru, 2008. Rev Peru Med Exp Salud Publica. 2010;27:16–21. doi: 10.1590/s1726-46342010000100004. [DOI] [PubMed] [Google Scholar]


