Abstract
Background
The surgical management of giant hepatocellular carcinoma (G-HCC), or HCC of ≥10 cm in diameter, remains controversial. The aim of this study was to compare the outcomes of surgical resection of, respectively, G-HCC and small HCC (S-HCC), or HCC measuring <10 cm.
Methods
A retrospective review of all patients (n = 86) diagnosed with HCC and submitted to resection in a tertiary hospital during the period from January 2007 to June 2012 was conducted. Overall survival (OS), recurrence rates and perioperative mortality at 30 days were compared between patients with, respectively, G-HCC and S-HCC. Prognostic factors for OS were analysed.
Results
The sample included 23 patients with G-HCC (26.7%) and 63 with S-HCC (73.3%) based on histological tumour size. Patient demographics and comorbidities were comparable. Median OS was 39.0 months in patients with G-HCC and 65.0 months in patients with S-HCC (P = 0.213). Although size did not affect OS in this cohort, the presence of satellite lesions [hazard ratio (HR) 3.70, P = 0.012] and perioperative blood transfusion (HR 2.85, P = 0.015) were negative predictors for OS.
Conclusions
Surgical resection of G-HCC provides OS comparable with that after resection of S-HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in Southeast Asia.1 For patients with HCCs of <5 cm in diameter, surgery or transplant has been established as the first line of treatment. However, the treatment of giant HCCs (G-HCCs) of ≥10 cm in diameter is more controversial and different centres advocate different modalities of treatment. In patients with HCC, lesions of ≥10 cm are often deemed to be non-amenable to surgery because they indicate an unfavourable prognosis in which morbidity rates range from 25% to 50% and mortality rates from 0% to 8%.2–4 Conflicting data have emerged from several individual centres,5,6 suggesting that tumour size is not critical and that physiological parameters and the characteristics of the liver remnant are the main determinants of treatment outcomes.
These controversies exist because data on the management of G-HCC are limited. As surgery is the only viable modality of cure in patients with G-HCC, it is essential that further clarification of the role of surgery in these patients is obtained.
The aims of this study were to compare survival after the surgical resection of G-HCC and small HCC (S-HCC), respectively, and to evaluate the prognostic factors influencing outcomes in these patients.
Materials and methods
Patient selection
A retrospective review of patients who underwent surgical resection of HCC was performed at the National University Hospital, Singapore, a tertiary health care institution. All patients (n = 86) who underwent liver resection for histologically proven HCC between January 2007 and June 2012 were included. Patients were divided into two categories according to whether they exhibited G-HCC (≥10 cm) or S-HCC (<10 cm) based on the size of tumour detailed in the pathology report. Patients who underwent ablative therapies such as radiofrequency ablation (RFA) or percutaneous ethanol injection as the only treatment were excluded from this study.
Preoperative assessment
All patients underwent routine preoperative investigations (full blood count, electrolytes panel, liver function test, viral serology testing, chest X-ray and electrocardiography) prior to operation. Triphasic computed tomography (CT) of the liver and alpha-fetoprotein (AFP) levels were used to help make the preoperative diagnosis and to assess the extent of the primary tumour. For lesions that did not have typical CT features, liver magnetic resonance imaging (MRI) was performed. Hepatic reserve was assessed using the Child–Pugh classification.7 Indocyanine green (ICG) clearance was used when remnant liver function was equivocal. Retention of <15% ICG at 15 min was considered adequate for major liver resection.8 Routine biopsy of liver lesions were not performed if the lesion had typical features of HCC on radiological imaging and biochemistry analysis.9
Surgical technique
The extent of resection was classified according to the Brisbane 2000 Guidelines for Liver Anatomy and Resection.10 The abdomen was entered through a Kocher's or rooftop incision. Tumour visualization was achieved with intraoperative ultrasound with subsequent mobilization of the liver. When conventional mobilization was not feasible, an anterior approach11 or liver hanging maneuvre12 was performed. Blood loss was minimized by using Pringle's manoeuvre or by hemihepatic vascular inflow occlusion while maintaining a low13 central venous pressure during parenchymal transection. Parenchymal transection was performed with the Cavitron Ultrasonic Surgical Aspirator (CUSA; Valleylab, Inc., Boulder, CO, USA) or LigaSure (Covidien, Inc., Mansfield, MA, USA). Concomitant RFA was performed if there were small contralateral lesions.
Follow-up
Postoperative mortality was defined as death within 30 days of surgery. Postoperative surveillance included a clinical examination and monitoring of the liver function panel and AFP level at intervals of 3 months during the first year and 6 months thereafter. Surveillance CT scans were also performed at intervals of 3 months in the first year and 6 months in the second year. Telephone interviews and a review of outpatient clinical notes were used to determine longterm outcomes to April 2015.
Histopathological assessment was performed for both the tumour and liver parenchyma. Tumour size was recorded based on gross pathological examination. Surgical margins were considered positive if viable tumour cells were seen within 1 mm on microscopy. Tumours were graded according to the degree of differentiation. Cirrhosis was diagnosed histologically.
The primary endpoint was overall survival (OS). Secondary endpoints were rate of recurrence and perioperative mortality at 30 days. Prognostic factors in the surgical resection of HCC were classified as either positive or negative prognostic factors for OS.
Statistical analysis
Institutional review board approval was obtained from the National University of Singapore Ethics Committee. spss Statistics for Windows Version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Categorical and continuous data were analysed using Fisher's exact test and the Mann–Whitney U-test, respectively. Kaplan–Meier analysis was used to depict OS in the different study groups. Factors found to be significant on univariate analysis for OS were subjected to multivariate analysis using a Cox proportional hazard model to determine the factors of significant prognostic value. A P-value of <0.05 was considered to indicate statistical significance.
Results
Clinical and histopathological data are shown in Tables1 and 2, respectively.
Table 1.
Demographic and clinical data for patients with, respectively, giant (G-HCC) and small (S-HCC) hepatocellular carcinoma
| S-HCC | G-HCC | P-value | |
|---|---|---|---|
| Patients, n | 63 | 23 | |
| Age, years, median (range) | 59 (27–81) | 63 (34–84) | 0.031 |
| Gender, n (%) | |||
| Male | 50 (79.4%) | 20 (87.0%) | 0.541 |
| Female | 13 (20.6%) | 3 (13.0%) | |
| Underlying aetiology, n (%) | |||
| HBV | 44 (69.8%) | 10 (43.5%) | 0.227 |
| HCV | 5 (7.9%) | 1 (4.3%) | |
| Alcoholic | 3 (4.8%) | 0 | |
| Cryptogenic | 0 | 1 (4.3%) | |
| Alcohol and HBV | 0 | 1 (4.3%) | |
| HBV and HCV | 2 (3.2%) | 0 | |
| NASH | 1 (1.6%) | 0 | |
| Comorbidities, n (%) | |||
| Hypertension | 25 (39.7%) | 14 (60.9%) | 0.093 |
| Diabetes mellitus | 20 (31.7%) | 5 (21.7%) | 0.431 |
| Hyperlipidaemia | 13 (20.6%) | 8 (34.8%) | 0.256 |
| Cerebrovascular attacks | 4 (6.3%) | 0 | 0.570 |
| Cardiac abnormalities | 3 (4.8%) | 1 (4.3%) | NA |
| Renal disease | 2 (3.2%) | 1 (4.3%) | NA |
| Pulmonary disease | 1 (1.6%) | 3 (13.0%) | 0.057 |
| Previous abdominal surgery, n (%) | |||
| Yes | 12 (19.0%) | 3 (13.0%) | 0.750 |
| No | 51 (81.0%) | 20 (87.0%) | |
| Preoperative treatment, n (%) | |||
| TACE | 10 (15.9%) | 5 (21.7%) | 0.661 |
| RFA | 1 (1.6%) | 0 | |
| None | 52 (82.5%) | 18 (78.3%) | |
| Presentation of disease, n (%) | |||
| Screening | 39 (61.9%) | 5 (21.7%) | 0.005 |
| Abdominal pain | 10 (15.9%) | 6 (26.1%) | 0.213 |
| Abdominal mass | 11 (17.5%) | 16 (69.6%) | <0.001 |
| Abdominal distension | 5 (7.9%) | 3 (13.0%) | 0.406 |
| Jaundice | 1 (1.6%) | 0 | NA |
| Haematemesis | 1 (1.6%) | 1 (4.3%) | 0.440 |
| Incidental finding | 9 (14.3%) | 5 (21.7%) | 0.324 |
| Liver biopsy performed, n (%) | |||
| Yes | 7 (11.1%) | 1 (4.3%) | 0.676 |
| No | 56 (88.9%) | 22 (95.7%) | |
| BCLC score, n (%) | |||
| A1 | 24 (38.1%) | 0 | <0.001 |
| A2 | 9 (14.3%) | 0 | |
| A3 | 2 (3.2%) | 1 (4.3%) | |
| A4 | 3 (4.8%) | 1 (4.3%) | |
| B | 24 (38.1%) | 19 (82.6%) | |
| C | 1 (1.6%) | 2 (8.7%) | |
| Child–Pugh class, n (%) | |||
| A | 60 (95.2%) | 20 (87.0%) | 0.336 |
| B | 3 (4.8%) | 3 (13.0%) | |
P-values in bold indicate differences of statistical significance at P < 0.05.
BCLC, Barcelona Clinic Liver Cancer Group; HBV, hepatitis B virus; HCV, hepatitis C virus; NA, not applicable; NASH, non-alcoholic steatohepatitis; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Table 2.
Histopathological data for patients with, respectively, giant (G-HCC) and small (S-HCC) hepatocellular carcinoma
| S-HCC n (%) | G-HCC n (%) | P-value | |
|---|---|---|---|
| Patients, n | 63 | 23 | |
| Gross vascular invasion | |||
| (n = 62) | (n = 23) | ||
| Yes | 4 (6.5%) | 0 | 0.570 |
| No | 58 (93.5%) | 23 (100%) | |
| Microvascular invasion | |||
| (n = 62) | (n = 23) | ||
| Yes | 13 (21.0%) | 11 (47.8%) | 0.028 |
| No | 49 (79.0%) | 12 (52.2%) | |
| Perineural invasion | |||
| (n = 62) | (n = 23) | ||
| Yes | 2 (3.2%) | 0 | 1.000 |
| No | 60 (96.8%) | 23 (100%) | |
| Lymphoinvasion | |||
| (n = 62) | (n = 23) | ||
| Yes | 11 (17.7%) | 9 (39.1%) | 0.048 |
| No | 51 (82.3%) | 14 (60.9%) | |
| Presence of tumour capsule | |||
| (n = 63) | (n = 23) | ||
| Yes | 37 (58.7%) | 18 (78.3%) | 0.129 |
| No | 26 (41.3%) | 5 (21.7%) | |
| Tumour capsule invasion | |||
| (n = 36) | (n = 18) | ||
| Yes | 11 (30.6%) | 3 (16.7%) | 0.339 |
| No | 25 (69.4%) | 15 (83.3%) | |
| Presence of satellite lesions | |||
| (n = 62) | (n = 23) | ||
| Yes | 12 (19.4%) | 5 (21.7%) | 0.770 |
| No | 50 (80.6%) | 18 (78.3%) | |
| Presence of tumour rupture | |||
| (n = 61) | (n = 23) | ||
| Yes | 6 (9.8%) | 2 (8.7%) | 1.00 |
| No | 55 (90.2%) | 21 (91.3%) | |
| Margins involved | |||
| (n = 61) | (n = 23) | ||
| Yes | 1 (1.6%) | 3 (13.0%) | 0.061 |
| No | 60 (98.4%) | 20 (87.0%) | |
| Presence of liver cirrhosis | |||
| (n = 63) | (n = 23) | ||
| Yes | 32 (50.8%) | 3 (13.0%) | 0.002 |
| No | 31 (49.2%) | 20 (87.0%) | |
P-values in bold indicate differences of statistical significance at P < 0.05.
In patients with G-HCC, most surgical resections were performed using an open approach (n = 21, 91.3%), whereas this approach was used less often in patients with S-HCC (n = 34, 54.0%) (P = 0.002). Surgery was performed laparoscopically in two (8.7%) patients with G-HCC and in 29 (46.1%) patients with S-HCC (P = 0.002). The rate of major hepatectomy was significantly higher in the G-HCC group (n = 19, 82.6%) compared with the S-HCC group (n = 20, 31.7%) (P = 0.001). Because of the greater incidence of major resection, either or both the liver hanging manoeuvre and anterior approach were used more frequently in the G-HCC group (n = 11, 47.8%) compared with the S-HCC group (n = 3, 4.8%) (P = 0.001).
The incidence of postoperative 30-day mortality in all patients was 3.5% (n = 3). The cause of death in two patients with G-HCC was acute renal failure. The single postoperative mortality in the S-HCC group occurred as a result of disseminated intravascular coagulopathy secondary to sepsis. The median duration of follow-up differed significantly between the S-HCC group (44 months; range: 1–88 months) and the G-HCC group (22 months; range: 1–66 months) (P = 0.046). Eleven of 23 G-HCC patients and 25 of 63 S-HCC patients died during follow-up. The recurrence rate in the G-HCC group (n = 5, 21.7%) was comparable with that in the S-HCC group (n = 21, 33.3%) (P = 0.441). Median OS was 39.0 months (range: 1–66 months) in the G-HCC group and 65.0 months (range: 1–88 months) in the S-HCC group (P = 0.213) (Fig.1).
Figure 1.
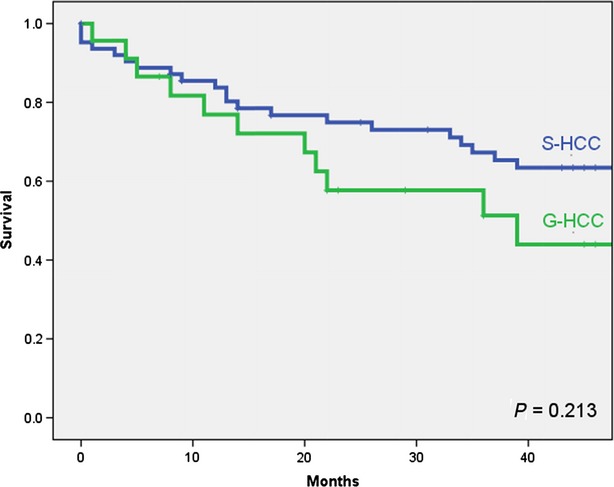
Overall survival in patients submitted to resection of hepatocellular carcinoma (HCC), showing comparisons between subgroups of patients with giant HCC (G-HCC) and small HCC (S-HCC), respectively
Univariate and multivariate analyses of the prognostic factors influencing OS are shown in Table3.
Table 3.
Univariate and multivariate analyses of overall survival in patients with hepatocellular carcinoma
| Factor | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Tumour size ≥10 cm | 1.572 | 0.764–3.232 | 0.213 | |||
| Presence of cirrhosis | 1.227 | 0.595–2.529 | 0.576 | |||
| Positive resection margin | 1.003 | 0.240–4.188 | 0.997 | |||
| Tumour rupture | 3.362 | 1.377–8.208 | 0.004 | 2.048 | 0.623–6.728 | 0.238 |
| Satellite lesions | 2.133 | 1.022–4.450 | 0.038 | 3.700 | 1.335–10.251 | 0.012 |
| Presence of tumour capsule | 0.643 | 0.333–1.242 | 0.183 | |||
| Tumour capsule invasion | 1.025 | 0.369–2.847 | 0.962 | |||
| Lymphoinvasion | 1.986 | 0.921–4.285 | 0.073 | |||
| Perineural invasion | NA | NA | NA | |||
| Microvascular invasion | 2.480 | 1.238–4.968 | 0.008 | 0.902 | 0.336–2.421 | 0.838 |
| Gross vascular invasion | 2.020 | 0.481–8.477 | 0.324 | |||
| Grade of tumour differentiation | 1.854 | 1.071–3.211 | 0.080 | 1.515 | 0.757–3.035 | 0.241 |
| Perioperative blood transfusion | 2.767 | 1.418–5.399 | 0.002 | 2.845 | 1.225–6.609 | 0.015 |
| AFP >400 ng/ml | 2.817 | 1.290–6.152 | 0.006 | 1.442 | 0.509–4.087 | 0.491 |
| Male gender | 1.064 | 0.443–2.558 | 0.889 | |||
P-values in bold indicate differences of statistical significance at P < 0.05.
95% CI, 95% confidence interval; AFP, alpha-fetoprotein; HR, hazard ratio; NA, not applicable.
Discussion
This study analysed whether the size of the primary HCC determines survival- and recurrence-related outcomes after surgical resection. Traditionally, patients with G-HCC were often deemed to be poor candidates for surgery2 and locoregional therapy such as transarterial chemoembolization (TACE) was recommended as the modality of choice.14,15 The present authors hypothesize that the outcomes of resection will be similar in G-HCC and S-HCC and believe that surgery is the sole modality for cure in G-HCC. Hence, it is crucial to evaluate surgical outcomes in these patients in order to establish whether surgery is justified.
This study shows that the survival outcome of surgical resection of G-HCC is comparable with the outcome of surgical resection of S-HCC, despite the fact that significantly more major hepatectomies were performed in the G-HCC group (82.6% versus 31.7%; P < 0.001). Factors affecting OS included the presence of satellite lesions and need for perioperative blood transfusions. Tumour size was not a determinant of OS. These findings are similar to those of other reported series16,17 and strongly suggest that curative surgery is a viable option for patients with G-HCC. In this study, the median OS in patients submitted to resection of G-HCC was 39.0 months. Other studies have reported median survival post-resection of HCC of ≥10 cm of 30–32 months.18–20 In addition, similarly to other reports by Liau et al.20 and Pawlik et al.,21 the current study showed comparable 30-day mortality rates of 8.7% post-resection of G-HCC. These results suggest that surgery should be considered as a first-line therapy in the treatment of G-HCC whenever possible as it can be performed safely and provides for excellent OS in patients in whom only palliative treatment would otherwise be considered.
The current study revealed that the presence of satellite lesions was an independent variable predicting poor OS. The presence of satellite nodules has been shown in previous studies16,22,23 to adversely affect OS as it suggests multicentric carcinogenesis or intrahepatic metastasis. In addition, the finding that perioperative blood transfusion is an independent prognostic factor in OS is consistent with various other studies,19,20,24 including that by Asahara et al., 25 who reported that patients who received perioperative blood transfusions were up to 7.61 times more likely to experience tumour recurrence compared with patients who did not receive perioperative blood transfusions, and that these patients had a significantly reduced rate of 5-year OS of 27.9% compared with 45.9% in the non-transfused group. It has been postulated that the immunomodulating effects of blood transfusions lead to increased HCC recurrence and hence shorter OS.25 However, need for blood transfusion may also reflect a sicker patient or a more extensive tumour and may not be the direct cause of poorer outcomes.
Several studies have shown microvascular invasion to be a significant prognostic factor in OS.15,26,27 In this study, microvascular invasion was found to represent a significant factor in univariate analysis for OS, but was not significant on multivariate analysis. The present authors believe that, in a larger sample size, microvascular invasion might have been identified as a significant prognostic factor on multivariate analysis.
Although many studies have shown cirrhosis to be an important negative prognostic factor,16,22,28 the present study did not identify liver cirrhosis as adversely affecting the survival of patients with HCC. There were significantly fewer patients with cirrhosis in the G-HCC group, which may reflect an inherent characteristic of presentation and selection bias in patients with G-HCC. Patients with cirrhosis are often under regular surveillance and thus any HCC that develops in the cirrhotic liver is often detected when it is still relatively small. Patients with significant liver cirrhosis who develop HCC of >10 cm are very unlikely to be resectable as the remnant liver function is likely to be inadequate after major liver resection. Although techniques such as portal vein embolization29 and the associating of liver partition with portal vein ligation for staged hepatectomy30 may increase the chances of resectability, the regenerative potential of a cirrhotic liver is often unpredictable and is quite certainly inferior to that of a non-cirrhotic liver. The present study showed that patients with G-HCC were less likely to have liver cirrhosis. In fat, their disease was more likely to present in the form of an abdominal mass or to be picked up incidentally on scans. These factors may explain why longterm outcomes in G-HCC patients were comparable with those in S-HCC patients.
This study was limited by the low number of patients who underwent resection of G-HCC. Overall survival was significantly better than rates reported in other similar studies,16,18,19 although this may be secondary to the limited sample size and compounded by the selection of patients with better hepatic reserves for surgery. However, this limitation will have been inherent in similar papers as a result of the small number of G-HCC patients submitted to hepatic resection.
In conclusion, this study shows that tumour size does not influence surgical outcomes in resection of HCC.
Conflict of interest
None to declare.
References
- Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer Incidence in Five Continents. VII. Lyon: IARC; 1997. , eds. (, Vol.. IARC Science Publications No. 143. [Google Scholar]
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- Hassoun Z, Gores GJ. Treatment of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2003;1:10–18. doi: 10.1053/jcgh.2003.50003. [DOI] [PubMed] [Google Scholar]
- Uchiyama K, Mori K, Tabuse K, Ueno M, Ozawa S, Nakase T. Assessment of liver function for successful hepatectomy in patients with hepatocellular carcinoma with impaired hepatic function. J Hepatobiliary Pancreat Surg. 2008;15:596–602. doi: 10.1007/s00534-007-1326-2. [DOI] [PubMed] [Google Scholar]
- Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–1259. [PubMed] [Google Scholar]
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Hemming AW, Gallinger S, Greig PD, Cattrall MS, Langer B, Taylor BR, et al. The hippurate ratio as an indicator of functional hepatic reserve for resection of hepatocellular carcinoma in cirrhotic patients. J Gastrointest Surg. 2001;5:316–321. doi: 10.1016/s1091-255x(01)80054-8. [DOI] [PubMed] [Google Scholar]
- Levy I, Greig PD, Gallinger S, Langer B, Sherman M. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg. 2001;234:206–209. doi: 10.1097/00000658-200108000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terminology Committee of the International Hepato-Pancreato-Biliary Association. IHPBA Brisbane terminology of liver anatomy and resections. HPB. 2000;2:333–339. [Google Scholar]
- Liu CL, Fan ST, Lo CM, Tung-Ping Poon R, Wong J. Anterior approach for major right hepatic resection for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25–31. doi: 10.1097/00000658-200007000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC, Fan ST, Lo CM, Chu KM, Liu CL. Anterior approach for difficult major right hepatectomy. World J Surg. 1996;20:314–317. doi: 10.1007/s002689900050. ; discussion 318. [DOI] [PubMed] [Google Scholar]
- Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Barcelona Liver Cancer Group. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, et al. International Cooperative Study Group on Hepatocellular Carcinoma. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364–373. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592–602. doi: 10.1016/s1072-7515(02)01163-8. [DOI] [PubMed] [Google Scholar]
- Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br J Surg. 2007;94:320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Taketomi A, Shirabe K, Aishima S, Tsuijita E, Morita K, et al. Outcomes of hepatic resection for huge hepatocellular carcinoma (>10 cm in diameter) J Surg Oncol. 2011;104:292–298. doi: 10.1002/jso.21931. [DOI] [PubMed] [Google Scholar]
- Liau KH, Ruo L, Shia J, Padela A, Gonen M, Jarnagin WR, et al. Outcome of partial hepatectomy for large (> 10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948–1955. doi: 10.1002/cncr.21415. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. International Cooperative Study Group on Hepatocellular Carcinoma. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005;140:450–457. doi: 10.1001/archsurg.140.5.450. ; discussion 457–458. [DOI] [PubMed] [Google Scholar]
- Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long-term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14:2817–2823. doi: 10.1245/s10434-007-9518-1. [DOI] [PubMed] [Google Scholar]
- Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81:195–202. doi: 10.1002/jso.10178. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- Asahara T, Katayama K, Itamoto T, Yano M, Hino H, Okamoto Y, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999;23:676–680. doi: 10.1007/pl00012367. [DOI] [PubMed] [Google Scholar]
- Zhou L, Rui JA, Ye DX, Wang SB, Chen SG, Qu Q. Edmondson–Steiner grading increases the predictive efficiency of TNM staging for long-term survival of patients with hepatocellular carcinoma after curative resection. World J Surg. 2008;32:1748–1756. doi: 10.1007/s00268-008-9615-8. [DOI] [PubMed] [Google Scholar]
- Cho YB, Lee KU, Lee HW, Cho EH, Yang SH, Cho JY, et al. Outcomes of hepatic resection for a single large hepatocellular carcinoma. World J Surg. 2007;31:795–801. doi: 10.1007/s00268-006-0359-z. [DOI] [PubMed] [Google Scholar]
- Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. ; discussion 799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandanaboyana S, Bell R, Hidalgo E, Toogood G, Prasad KR, Bartlett A, Lodge JP. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery. 2015;157:690–698. doi: 10.1016/j.surg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510–1519. doi: 10.1007/s00268-014-2513-3. [DOI] [PubMed] [Google Scholar]


