Abstract
Background
Standard lymphadenectomy during pancreaticoduodenectomy (PD) for peri-ampullary cancer does not include the routine removal of para-aortic lymph nodes (PALN) (station 16, according to the JPS staging system). The aim of this study was to report the incidence and the prognostic value of PALN metastases in patients undergoing PD for peri-ampullary cancer.
Materials and methods
One hundred thirty-five consecutive patients who underwent PD and PALN dissection for peri-ampullary cancer were prospectively evaluated. The relationship between clinicopathological factors, including PALN metastases and survival was evaluated at univariate and multivariate analysis.
Results
PALN metastases (N16+) were found in 11.1% of cases. At univariate analysis, R1 resection, metastatic nodes different from para aortic (N1) and N16+ significantly affected patients' prognosis. Compared with N16+, the median overall survival (OS) of N0 patients was significantly longer (32 versus 69 months, respectively; P < 0.05), whereas no difference was found between N16+ and N1 patients (32 versus 34 months, respectively) (P > 0.05). At multivariate analysis, only R1 resection reached statistical significance and was confirmed an independent prognostic factor.
Conclusions
Neoplastic involvement of PALN in peri-ampullary cancer is frequent and, so, their removal during PD could be justified. Moreover, PALN metastases should be not considered an absolute contraindication to radical surgery.
Introduction
Pancreaticoduodenectomy (PD) is the treatment of choice of patients affected by peri-ampullary cancer.1 The extent of the cancer to the regional lymph nodes is a powerful prognostic factor after resection independently from cancer histology.2 For this reason, lymphadenectomy is considered a crucial step of PD for cancer.3,4 In 2014, a consensus meeting of the International Study Group on Pancreatic Surgery (ISGPS) in Verona5 on the definition and the prognostic role of lymphadenectomy during PD for cancer stated that: (i) the use of the nomenclature for nodal stations based on the classification of the Japanese Pancreas Society6 is recommended; (ii) an extended lymphadenectomy does not improve the oncological outcome of patients and should not be associated with PD for cancer; (iii) lymphadenectomy should include the removal of the hepatoduodenal ligament nodes (stations 5, 6, 12b1, 12b2, 12c), nodes along the hepatic artery (station 8a), the posterior surface of the pancreatic head (station 13a and 13b), the superior mesenteric artery (14a right lateral side, 14b right lateral side) and nodes of the anterior surface of the pancreatic head (stations 17a and 17b).5 As no consensus among experts was reached on the role of para-aortic lymph nodes (PALN), the Verona meeting did not point out any statement on this argument. Therefore, a standard lymphadenectomy, as defined by the ISGPS, does not include the removal of para-aortic nodes along the posterior side of the pancreas, between the aorta and the inferior vena cava (station 16).5 Based on the available evidence on this issue, the following questions about para-aortic nodes are still unsolved:
Which is the real incidence of neoplastic involvement of station 16 in peri-ampullary cancers?
Is PALN involvement a prognostic factor after PD for peri-ampullary cancer?
Is the intra-operative evidence of the metastatic para-aortic nodes at frozen section a contraindication in performing PD?
The aim of this study was to report the results of a prospective evaluation on the incidence and the prognostic value of PALN metastases in patients undergoing PD for peri-ampullary cancer.
Patients and methods
Patients affected by peri-ampullary cancer that underwent PD at the Campus Bio-Medico University of Rome between 2006 and 2014 were prospectively evaluated.
All PDs were performed with curative intent by a single expert surgeon. A standard lymphadenectomy including the removal of stations 5, 6, 8a, 12b1, 12b2, 12c, 13a, 13b, 14a right lateral side, 14b right lateral side, 17a and 17 was routinely performed. Para-aortic nodal dissection including the lymph nodes from the upper part of the celiac trunk to the upper part of the origin of the inferior mesenteric artery was routinely performed. In case of vascular neoplastic infiltration, a vascular resection was performed.
One hundred thirty-five consecutive patients underwent PD for peri-ampullary cancer during the study period. The cohort of patients was composed of 80 males (59.3%) and 55 females (40.7%). One-hundred twenty-one patients (90%) underwent surgery as first approach to the disease; in 14 patients (10%) neoadjuvant treatment was performed. Neoadjuvant treatment (radio-chemotherapy) was performed only in case of locally advanced/unresectable disease, confirmed with a pre-operative computed tomography scan. The pylorus was preserved in 72.6% of cases (Table1).
Table 1.
Clinical and pathological data of the 135 patients
| No. of patients (%) | |
|---|---|
| Gender | |
| Male | 80 (59.3) |
| Female | 55 (40.7) |
| Neoadjuvant treatments | 14 (10.4) |
| Type of resection | |
| Whipple | 37 (27.4) |
| Pylorus-preserving PD (PPPD) | 98 (72.6) |
| Tumour histology | |
| PDAC | 86 (63.7) |
| Ampullary adenocarcinoma | 31 (23.0) |
| Distal bile duct carcinoma | 18 (13.3) |
| T status | |
| T1 | 5 (3.7) |
| T2 | 18 (13.3) |
| T3 | 98 (72.6) |
| T4 | 14 (10.4) |
| N status | |
| N0 | 46 (34.1) |
| N1 | 89 (65.9) |
| R status | |
| R0 | 79 (58.5) |
| R1 | 56 (41.5) |
| M+ (para-aortocaval nodes) | 15 (11.1) |
| Harvested lymph nodes (mean, range) | 30 (5–75) |
| LNR (mean) | 0,11 |
| Adjuvant treatments | 85 (63.0) |
PDAC, pancreatic ductal adenocarcinoma; LNR, lymph-node ratio.
The incidence of PALN metastases was evaluated in all cases. We divided the entire cohort into three groups: (i) patients without nodal involvement (N0 group); (ii) patients with lymph nodal neoplastic involvement, other than the para-aortic station (N1 group); and (iii) patients with para-aortic nodal metastases (N16+). The following clinical-pathological factors were evaluated in these groups: patient demographics, operative procedures, access to neoadjuvant/adjuvant treatments, tumour histology, T and N stage, lymph-node ratio (LNR, i.e. the ratio between the number of positive and harvested lymph nodes) and the resection margin (RM) status. According to Royal College of Pathologist guidelines,7 a surgical resection is defined R1 in the presence of a distance between the tumour and each margin of less than 1 mm.
The follow-up was realized according to a standardized schedule at regular intervals up to 5 years after surgery. The median follow-up was 41 months (2–135). No drop out at follow-up was observed. The overall survival (OS) was calculated from the date of surgery to the date of last follow-up/death. Differences in OS between the N0, N1 and N16+ groups were evaluated.
Statistical analysis
Differences in clinical-pathological parameters between negative and positive nodes where assessed using the chi-square test and one-way anova. Survival data was presented using Kaplan–Meier survivor function. Differences in survivals were performed using the log-rank test of equality. The Cox proportional hazards model was used for multivariate analysis of survival to determine the significance of the various predictive variables that were found to be significant in univariate analysis. All analyses were undertaken with Stata Statistical Software (Stata Corporation LP, College Station, Texas, USA) and a P-value < 0.05 was considered significant.
Results
Clinical and pathological data of 135 evaluated patients are reported in Table1. Final histological report described a pancreatic ductal adenocarcinoma (PDAC) in 63.7% of cases, an ampullary adenocarcinoma in 23% of cases and a bile duct carcinoma in 13.3% of cases. In more than 80% of cases, the tumour was locally extended (stage T3 or T4). A microscopic residual tumour (R1) was present in 41,5% of cases. The mean number of harvested lymph nodes was 30 (5–75), and the mean LNR was 0.11. The overall rate of nodal metastases other than para-aortic (N1) was 65.9%. The overall rate of para-aortic nodal metastases was 11.1%. Sensitivity and specificity of the frozen section in the detection of para-aortic nodal metastases were 83.3% and 100%, respectively.
In Table2, clinical-pathological data of N0, N1 and N16+ patients are reported. The three groups did not significantly differ in terms of gender, type of resection and tumour histology. Neoadjuvant chemoradiation was more frequently performed in N16+ patients (27%) compared with N0 (13%) (P < 0.05) and N1 (5%) patients (P < 0.05). A significant correlation between tumour size and N status was also observed; compared with N0, N1 and N16+ patients were more frequently affected by T3/T4 tumours (P < 0.05); in contrast, no differences in terms of T status were found between N1 and N16+ patients (P = NS). Furthermore, R1 resection was more frequently associated with N1 and N16+ compared with N0 cases (R1-N16+: 55% versus R1-N0: 13%, P < 0.05; R1-N1: 60% versus R1-N0: 13%, P < 0.01); no differences in terms of R1 resection were found between N1 and N16+ patients (R1-N16+: 55% versus R1-N1: 60%, P = NS). The number of harvested lymph nodes was significantly related to N status with N0 patients statistically associated with a smaller number of harvested lymph nodes (mean 22.6) compared with N1 (mean 32.3) (P < 0.01) and N16+ patients (mean 36.4) (P < 0.05). No differences were found in terms of the mean number of harvested lymph nodes between N1 and N16+ patients (32.3 and 36.4, respectively; P = NS). However, the mean LNR was significantly higher in N16+ (0.27) compared with N1 patients (0.13) (P < 0.05). Compared with N0, N1 and N16+ patients underwent more frequently adjuvant treatment [N1: 69% versus N0: 44% (P < 0.05); N16+: 87% versus N0: 44%, (P < 0.05)], whereas no differences were found between the N16+ and N1 groups (N16+: 87% versus N1: 69%; P = NS).
Table 2.
Comparison between N16+ and N16- patients (clinical-pathological data; chi-squared from proportions, one-way anova for continuous data)
| N0 Group (45 cases) N (%) | N1 group (75 cases) N (%) | N16+ group (15 cases) N (%) | P | |
|---|---|---|---|---|
| Gender | ||||
| Male | 27 (60) | 48 (64) | 5 (33) | N0 versus N1: NS N0 versus N16+: NS N1 versus N16+: NS |
| Female | 18 (40) | 27 (36) | 10 (67) | |
| Neoadjuvant treatments | 6 (13) | 5 (5) | 4 (27) | N0 versus N1: NS N0 versus N16+: NS N1 versus N16+: < 0.05 |
| Type of resection | ||||
| Whipple | 9 (20) | 24 (32) | 4 (27) | N0 versus N1: NS N0 versus N16+: NS N1 versus N16+: NS |
| PPPD | 36 (80) | 51 (68) | 11 (73) | |
| Tumour histology | ||||
| PDAC | 26 (58) | 48 (64) | 12 (80) | N0 versus N1: NS N0 versus N16+: NS N1 versus N16+: NS |
| Ampullary adenocarcinoma | 13 (29) | 17 (23) | 1 (7) | |
| Distal bile duct carcinoma | 6 (13) | 10 (13) | 2 (13) | |
| T status | ||||
| T1 | 5 (11) | 0 (0) | 0 (0) | N0 versus N1: <0.01 N0 versus N16+: <0.05 N1 versus N16+: NS |
| T2 | 13 (29) | 5 (7) | 0 (0) | |
| T3 | 23 (51) | 61 (81) | 14 (93) | |
| T4 | 4 (9) | 9 (12) | 1 (7) | |
| R status | ||||
| R0 | 39 (87) | 34 (45) | 6 (40) | N0 versus N1: <0.01 N0 versus N16+: <0.01 N1 versus N16+: NS |
| R1 | 6 (13) | 41 (55) | 4 (60) | |
| Harvested lymph nodes (mean, CI 95%) | 22 (19–27) | 32 (29–36) | 36 (24–49) | N0 versus N1: <0.01 N0 versus N16+: <0.01 N1 versus N16+: NS |
| LNR (mean, CI 95%) | 0 | 13 (11–16) | 27 (18–36) | N1 versus N16+: <0.01 |
| Adjuvant treatments | 20 (44) | 52 (69) | 13 (87) | N0 versus N1: <0.01 N0 versus N16+: <0.01 N1 versus N16+: NS |
NS, not significant; PPPD, pylorus-preserving PD; PDAC, pancreatic ductal adenocarcinoma; LNR, lymph-node ratio; CI, confidence interval.
The median OS of the entire cohort was 41 months. Compared with N16+, the median OS of N0 patients was significantly longer (N16+: 32 months; N0: 69 months; P < 0.05) (Fig.1 and Table3). Comparison in terms of the median OS between N1 and N0 patients showed that N1 patients were affected by a shorter median OS (34 versus 69 months; HR 1.82) (P < 0.05) (Table3). Conversely, the comparison of the median OS between N16+ and N1 patients did not show significant differences (32 versus 34 months, respectively) (P = NS) (Fig.2 and Table3).
Figure 1.
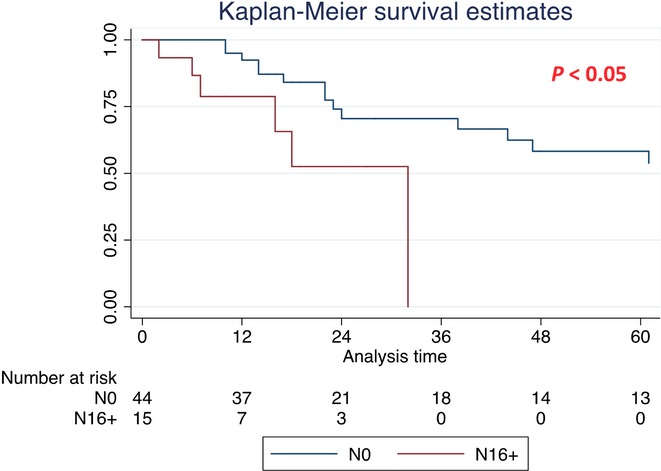
Kaplan–Meier survival curves for the entire cohort [median overall survival (OS) for N0 and N16+ patients]
Table 3.
Differences in the survival median months and hazard ratio in patients without lymph nodes involvement (N0), with lymph nodes involvement different from para-aortic lymph nodes (N1) and with para-aortic lymph-node involvement (N16+)
| Group | N | Median survival (CI 95%) in months | Hazard ratio (CI 95%) | P |
|---|---|---|---|---|
| No lymph-nodes involvement (N0) | 44 | 69 (38–101) | 1 | |
| Para-aortic lymph-nodes involvement (N16+) | 15 | 32 (7–32) | 1.82 (1.08–3.05) | <0.05 |
| No lymph nodes involvement (N0) | 44 | 69 (38–101) | 1 | |
| Lymph nodes involvement different from para-aortic lymph nodes (N1) | 75 | 34 (20–63) | 1.80 (1.02–3.17) | <0.05 |
| Lymph nodes involvement different from para-aortic lymph nodes (N1) | 75 | 34 (20–63) | 1 | |
| Para-aortic lymph nodes involvement (N16+) | 15 | 32 (7–32) | 1.96 (0.79–4.80) | n.s. |
Figure 2.
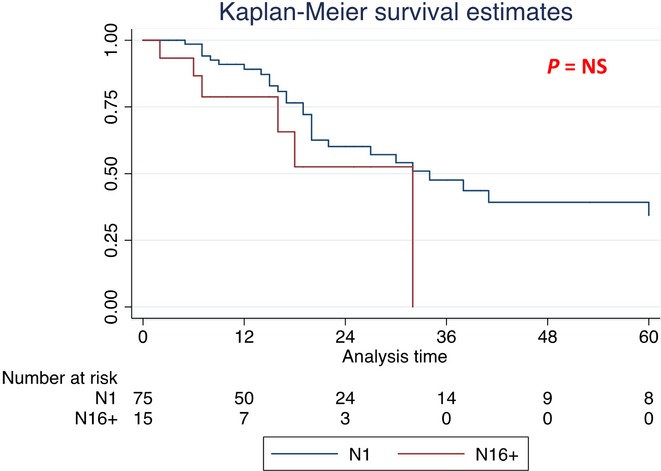
Kaplan–Meier survival curves for the entire cohort [median overall survival (OS) for N1 and N16+ patients]
Survival analysis was also performed in the subgroup of patients affected by PDAC (Table4). The median OS was significantly better in N0 patients (71 months) compared with N1 (38 months, HR 1.8, P < 0.05) and to N16+ (18 months, HR 1.82, P < 0.05) patients (Fig.3 and Table4). No difference in terms of median OS was found between N1 and N16+ patients (38 versus 18 months, P > 0.05) (Fig.4 and Table4).
Table 4.
Differences in survival in subgroup of pancreatic cancer patients median months and hazard ratio in patients without lymph nodes involvement (N0), with lymph nodes involvement different from para-aortic lymph nodes (N1) and with para-aortic lymph nodes involvement (N16+)
| Group | N | Median survival (CI 95%) in months | Hazard ratio (CI 95%) | P |
|---|---|---|---|---|
| No lymph nodes involvement (N0) | 25 | 71 (24–144) | 1 | |
| Lymph nodes involvement different from para-aortic lymph nodes (N1) | 48 | 38 (20–63) | 1.80 (1.02–3.17) | <0.05 |
| No lymph nodes involvement (N0) | 25 | 71 (24–144) | 1 | |
| Para-aortic lymph nodes involvement (N16+) | 12 | 18 (6–18) | 1.82 (1.08–3.05) | <0.05 |
| Lymph nodes involvement different from para-aortic lymph nodes (N1) | 48 | 38 (20–63) | 1 | |
| Para-aortic lymph nodes involvement (N16+) | 12 | 18 (6–18) | 1.96 (0.79–4.80) | n.s. |
CI, confidence interval.
Figure 3.
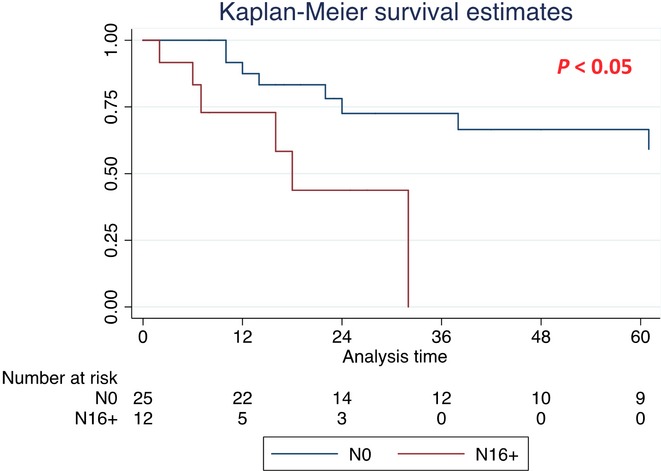
Kaplan–Meier survival curves in subgroup of pancreatic ductal adenocarcinoma (PDAC) patients [median overall survival (OS) for N0 and N16+ patients]
Figure 4.
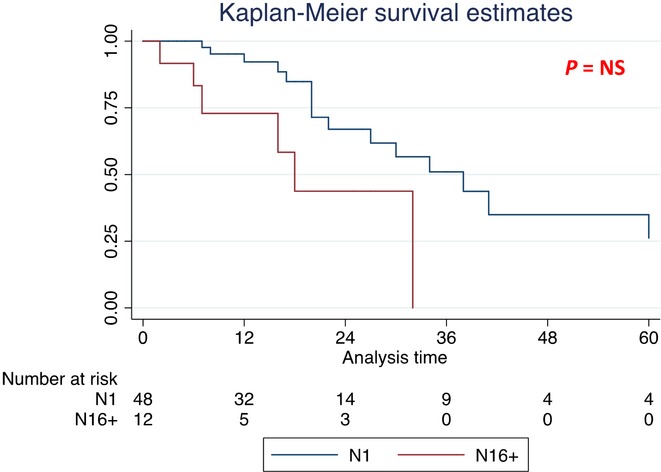
Kaplan–Meier survival curves in subgroup of pancreatic ductal adenocarcinoma (PDAC) patients [median overall survival (OS) for N1 and N16+ patients]
At univariate analysis, an R1 resection, N1 and N16+ significantly affected the prognosis of patients (HR 2.49, 1.79 and 3.47, respectively) (Table5). At multivariate analysis, only an R1 resection reached the statistical significance (Table5).
Table 5.
Univariate and multivariate survival analysis of prognostic factors after resection for peri-ampullary cancer
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| T status | ||||||
| 0 | 1 | |||||
| 1 | 1.18 | 0.82–1.69 | n.s. | |||
| Lymph nodes metastasis | ||||||
| N0 | 1 | |||||
| N1 | 1.79 | 1.01–3.16 | <0.05 | 1.47 | 0.8–2.6 | n.s. |
| N16+ | 3.47 | 1.32–9.09 | <0.01 | 2.41 | 0.8–6.6 | n.s. |
| Surgical margin | ||||||
| R0 | 1 | |||||
| R1 | 2.49 | 1.41–4.38 | <0.01 | 2.07 | 1.1–3.7 | <0.05 |
| Tumour histology | ||||||
| PDAC | 1 | |||||
| Ampullary adenocarcinoma | 1.12 | 0.60–2.00 | n.s. | |||
| Distal bile duct carcinoma | 1.72 | 0.82–3.63 | n.s. | |||
| Adjuvant treatment | ||||||
| No | 1 | |||||
| Yes | 1.17 | 0.69–1.99 | n.s. | |||
HR, hazard ratio; CI, confidence interval; PDAC, pancreatic ductal adenocarcinoma.
Discussion
The 'ideal‘ lymphadenectomy associated with PD for cancer is still an argument of debate. Although the lymph nodal status of resected peri-ampullary cancer patients is considered a relevant predictor of survival, four randomized controlled and a meta-analysis did not support an improved clinical outcome of an 'extended‘ compared with a 'standard‘ nodal dissection.8–12 This evidence was confirmed in 2014 by the final statements of the International Study Group of Pancreatic Surgery (ISGPS) on this topic.5 At the Verona meeting, the role of PALN (stations 16) in surgically resected peri-ampullary cancer patients and the surgical approach to be adopted was also discussed. However, the discussion revealed that only half of the involved surgeons routinely perform a station 16 nodal dissection and that no consensus could be reached between the participating pancreatic surgeons. Consequently, no final recommendations on this issue were subscribed.
Several studies on lymphatic drainage pathways have shown that PALN play a key role in the lymphatic drainage of the pancreatic head.13,14 Lymphatic drainage of peri-ampullary cancers (independently from the histological origin of the tumour) takes place either from the anterior (station 17) as well the posterior (station 13) surface of the pancreatic head. From the pancreatic head, the lymphatic drainage continues towards the lymph nodes along the superior mesenteric artery (station 14) and to the para-aortic station (station 16).12,13 Other anatomical studies showed that in a small percentage of cases, the lymphatic drainage directly merges into the nodal stations along the proper hepatic artery (station 8) before reaching station 16 via the lymph nodes of the celiac axis (station 7).15
In spite of the major role played by the para-aortic stations in lymphatic drainage of peri-ampullary cancers, no clear data regarding the incidence of neoplastic involvement of nodal station 16 in patients undergoing PD for cancer are reported in the literature. This figure was the primary aim of the present study. Our results showed that 11.1% of our cases were affected by para-aortic nodal metastases. This result confirms that PALN are a relevant site in the pathway of peri-ampullary cancer. These results are confirmed by others studies that report a 10–25% incidence of station 16 involvement (Table6).16–24 Remarkably, this incidence is comparable to the rate of lymph nodes metastases of the hepatoduodenal ligament, routinely excised according to the final recommendations of the ISGPS consensus meeting.5 A recent paper from the Memorial Sloan-Kettering including 147 surgically resected pancreatic head cancer patients, reported a 16% rate of neoplastic involvement of the hepatic artery lymph nodes (8a and 8p).25 Therefore, if the incidence of metastases affecting para-aortic, hepato-duodenal ligament and hepatic artery lymph nodes metastases is comparable, we should argue that a lymphadenectomy associated with PD for cancer should be systematically extended to station 16. Moreover, it is necessary to clarify that dissection of the para-aortic nodes is not a complex procedure (it can be easily performed immediately after the Kocher's manoeuvre), and it is not associated with increased morbidity: in our series, no serious adverse event strictly related to the removal of station 16 was observed.
Table 6.
Comparison of studies evaluating the incidence and the prognostic significance of neoplastic involvement of para-aortic lymph nodes
| Author, year of publication | Tumour histology | Type of para-aortic dissection | No. of metastatic para-aortic nodes (%) | Survival | Strategy recommended |
|---|---|---|---|---|---|
| Kayahara M, 199815 | PDAC | Not reported | 18 (18.2) | Not evaluable | Not reported |
| Yoshida T, 199821 | Cholangiocarcinoma | Not reported | 5 (25) | Not reported | Not reported |
| Yoshida T, 200423 | Peri-ampullary tumours | Not reported | 15 (15) | 1-year survival: 33% | Contraindication to PD |
| 2-year survival: 27% | |||||
| 3-year survival: 0% | |||||
| Mean survival 14.7 months | |||||
| Shimada K, 200616 | PDAC | Not reported | 27 (19.8) | Median OS: 13 months | Contraindication to PD |
| Doi R, 200717 | PDAC | 16a2 + 16b1 | 19 (14.3) | Median OS: 5.1 months | Contraindication to PD |
| 1-year survival: 16% | |||||
| Yamada S, 200918 | PDAC | Not reported | 48 (8.9) | Median OS: 8.0 months | Indication to PD |
| Yamada S, 200922 | PDAC | Not reported | 45 (13.4) | Median OS: 7.8 months | Not absolute contraindication to PD |
| Murakami Y, 201120 | Cholangiocarcinoma | 16a2 + 16b1 | 17 (15) | 5-year survival: 24% | Indication to PD |
| Schwarz L, 201419 | PDAC | 16b1 | 17 (15.3) | Median OS: 15.7 months | Contraindication to PD |
| Median DFS: 8.4 months | |||||
| Current study, 2015 | Peri-ampullary tumours and PDAC | 16a2 + 16b1 | 15 (11.1) | Mean OS (periampullary): 32 months | Indication to PD |
| Median OS (PDAC): 18 |
PDAC, pancreatic ductal adenocarcinoma; OS, overall survival.
The kind of para-aortic nodal dissection to be performed is another crucial point. In fact, according to JPS, station 16 can be divided into four subgroups of lymph nodes, from cranial to caudal areas: (i) station 16a1: lymph nodes located in the area of the aortic hiatus (about 4–5 cm in width, surrounded by the medial crus of the diaphragm); (ii) station 16a2: lymph nodes located in the area from the uppermost part of the origin of the celiac trunk to the lower margin of the left renal vein; (iii) station b1: lymph nodes located in the area from the lower margin of the left renal vein to the uppermost part of the origin of the inferior mesenteric artery; and (iv) station 16b2: lymph nodes located in the area from the upper margin of the origin of the inferior mesenteric artery to the aortic bifurcation. Therefore, PALN dissection should include the removal of all four nodal groups. Interestingly, the Verona consensus meeting discussed (without reaching experts' common opinion) the opportunity to remove only station 16b1.5 In contrast, JPS recommendations report that PD should be associated with the removal of sub-stations 16a2 and 16b.6 Unfortunately, most of the studies reporting a para-aortic lymphadenectomy during PD (Table6) do not precisely report what kind of para-aortic nodal dissection is reported15,16,18,21–23 or, if reported, it differs from study to study.19,20 Based on these evidence, it can be concluded that the results in terms of peri-operative and the oncological outcome of a complete station 16 nodal dissection associated with PD for cancer has never been reported. Conversely, based on the description given by Authors in the considered articles, we can affirm that what surgeons performed is a nodal sampling rather than a systematic para-aortic lymphadenectomy. Obviously, these anatomical aspects must be taken into account, as they can significantly affect data regarding the real incidence and the prognostic impact of para-aortic metastases.
The present study showed that each histotype is characterized by a specific attitude to metastasize into PALN. In fact, we found that ampullary cancer is affected by the lowest incidence of para-aortic involvement (3.2%) if compared with pancreatic (13.9%) and distal common bile duct cancer (11.1%) (Table2). This result should be hopefully confirmed by larger series of surgically resected peri-ampullary cancers.
The prognostic impact of the neoplastic involvement of the para-aortic nodes and the surgical strategy to adopt are probably the most demanding problems to be solved. In fact, in the literature some studies showed no difference in terms of survival between patients with or without metastatic PALN undergoing a resection,11,20 whereas others reported poorer survival rates for patients with positive PALN.16,17,19,23 However, to our best knowledge, there is no level I evidence concerning the prognostic impact of metastatic para-aortic nodes after PD for cancer (Table6).15–23 Most of the studies considered only patients affected by pancreatic cancer,15–19,22 others only by distal common bile duct cancer,20,21 and only one study considered patients affected by all peri-ampullary cancers.23 Moreover, the results of the studies in terms of the prognostic impact of para-aortic nodes are discordant. Yoshida et al.23 first retrospectively evaluated in 2004 101 patients that underwent PD with curative intent for peri-ampullary cancer. The rate of metastatic PALN was 15% (26% and 17% of pancreatic and distal common bile duct cancer, respectively). Overall survival resulted significantly longer for PDAC and common bile duct cancer patients without PALN metastasis. According to these results, the authors recommended the systematic execution of para-aortic nodal sampling and, in case of positive frozen section, to abandon radical surgery. These results were confirmed by another three studies that analysed the prognostic impact of metastatic PALN in patients that underwent PD for pancreatic cancer.16,17,19,23 In summary, all the above-reported studies confirmed that metastatic para-aortic nodes significantly affect the prognosis and the authors' conclusion, in case of intra-operative evidence of metastatic station 16+ lymph nodes, was that the surgical excision of the primitive tumour should be abandoned. However, other studies18,20,22 showed different results and stated different conclusions. Yamada et al. 22 showed that the overall survival of patients with para-aortic metastases was significantly shorter if compared with negative nodes patients in a series of 360 PDAC patients undergoing PD. However, the comparison of survival data of resected patients with para-aortic metastatic nodes and non-resected patients for locally advanced and/or metastatic disease showed a survival benefit for N16+ resected patients. For this reason, the authors concluded that an intra-operative positive frozen section of PALN should not be considered a contraindication for radical surgery. A similar conclusion was stated by Murakami et al.20 who reported a shorter overall survival in patients with para-aortic lymph nodes metastases compared with the survival of N0 patients in a series of 113 PD for distal cholangiocarcinoma. However, the authors also showed that the overall survival of patients with PALN metastasis did not differ from UICC N1 cases. The authors' conclusion was that PD should not be abandoned in the case of PALN metastasis. Lastly, similar results was obtained by Schwarz et al.20: the median OS and DFS in patients with regional lymph node involvement only and in patients with both regional and para-aortic node involvement were 21.0 versus 15.1 months (P = 0.110) and 12.7 versus 9.6 months (P = 0.120), respectively. The results of the present study seem to support this conclusion. In our series, patients affected by PALN metastases (N16+) were affected by a worse prognosis if compared with N0 patients (Fig.1). At univariate analysis, both N1 and N16+ patients were found to be significant prognostic factors. More interestingly, similarly to the results of Murakami et al.,20 survival analysis of N1 and N16+ patients did not show significant differences between the two groups (Fig.2). The same results have been obtained considering only the subgroup of patients with PDAC (Figs3 and 4), even if a difference in terms of OS between N16+ and N1 was greater in PDAC patients if compared with the entire cohort. Probably, this result is as a result of a worse biological behaviour of PDAC tumours if compared with other peri-ampullary neoplasms. Larger series considering all peri-ampullary neoplasms and not only PDAC patients are needed to confirm the prognostic role of para-aortic nodal metastases for each histological type of periampullary tumour. These results seem to confirm that the intra-operative evidence of para-aortic nodal metastases should be considered as equivalent to other regional lymph nodes metastases and that it should not be considered an absolute contraindication for radical surgery.
Conclusions
The results of our study clearly demonstrated that the incidence of para-aortic lymph node metastasis during PD for periampullary cancer is relevant (more than 10% of resected cases) and for this reason we think that the removal of para-aortic nodal station (station 16a2+16b1) could be justified.
Regarding the prognostic significance of metastatic para-aortic nodal involvement, we found that the survival of patients with para-aortic metastases seems to be comparable to that of patients with other lymph nodes involvement. For this reason, we concluded that at the moment the presence of para-aortic nodal metastasis should be not considered an absolute contraindication to radical surgery. Further studies with a larger cohort of patients are warranted.
Conflicts of interest
None declared.
References
- Loos M, Kleef J, Friess H, Buchler MW. Surgical treatment of pancreatic cancer. Ann N Y Acad Sci. 2008;1138:169–180. doi: 10.1196/annals.1414.024. [DOI] [PubMed] [Google Scholar]
- Zacharias T, Jaeck D, Oussoultzoglou E, Neuville A, Bachellier P. Impact of lymph node involvement on long term survival after R0 pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas. J Gastrointest Surg. 2007;11:350–356. doi: 10.1007/s11605-007-0113-3. [DOI] [PubMed] [Google Scholar]
- Kim RD, Kundhal PS, McGivray ID, Cattral MS, Taylor B, Langer B, et al. Predictors of failure after pancreaticoduodenectomy for ampullary carcinoma. J Am Coll Surg. 2006;2006:112–119. doi: 10.1016/j.jamcollsurg.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Duffy JP, Hines OJ, Liu JH, Ko CY, Cortina G, Isacoff WH, et al. Improved survival for adenocarcinoma of the ampulla of Vater: fifty-five consecutive resections. Arch Surg. 2003;138:941–950. doi: 10.1001/archsurg.138.9.941. [DOI] [PubMed] [Google Scholar]
- Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, et al. for the International Study Group on Pancreatic Surgery. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japan Pancreatic Society. Classification of Pancreatic Carcinoma. 2nd English edn. Tokyo: Kanehara Pub; 2003. [Google Scholar]
- The Royal College of Pathologists. Standards and Minimun Datasets for Reporting Cancers. Minimum Dataset for the Histopathological Reporting of Pancreatic, Ampulla of Vater and Bile Duct Carcinoma. London: The Royal College of Pathologists; 2002. [Google Scholar]
- Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229:613–614. doi: 10.1097/00000658-199905000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma–part 3: update on 5-year survival. J Gastrointest Surg. 2005;9:1191–1204. doi: 10.1016/j.gassur.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Bugart LJ, Dahl TR, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618–630. doi: 10.1016/j.surg.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19:230–241. doi: 10.1007/s00534-011-0466-6. [DOI] [PubMed] [Google Scholar]
- Hirai I, Muramaki G, Kimura W, Nara T, Dodo Y. Long descending lymphatic pathway from the pancreaticoduodenal region to the para-aortic nodes: its laterality and topographical relationship with the celiac plexus. Okajima Folia Anat Jpn. 2001;77:189–199. doi: 10.2535/ofaj1936.77.6_189. [DOI] [PubMed] [Google Scholar]
- Hirono S, Tani M, Kaway M, Okada K, Miyazawa M, Shimizu A, et al. Identification of the lymphatic drainage pathways from the pancreatic head guided by indocyanine green fluorescence imaging during pancreaticoduodenectomy. Dig Surg. 2012;29:132–139. doi: 10.1159/000337306. [DOI] [PubMed] [Google Scholar]
- Samra JS, Gananadha S, Hugh TJ. Surgical management of carcinoma of the head of the pancreas: extended lymphadenectomy or modified en bloc resection? ANZ J Surg. 2008;78:228–236. doi: 10.1111/j.1445-2197.2008.04426.x. [DOI] [PubMed] [Google Scholar]
- Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Ueno K, Tajima H, et al. Analysis of paraaortic lymph node involvement in pancreatic carcinoma. A significant indication for surgery? Cancer. 1999;85:583–590. doi: 10.1002/(sici)1097-0142(19990201)85:3<583::aid-cncr8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Shimada K, Sakamoto Y, Sano T, Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J Am Coll Surg. 2006;3:345–352. doi: 10.1016/j.jamcollsurg.2006.05.289. [DOI] [PubMed] [Google Scholar]
- Doi R, Kami K, Ito D, Fujimoto K, Kawaguchi Y, Wada M, et al. Prognostic implication of para-aortyc lymph node metastasis in resectable pancreatic cancer. World J Surg. 2007;31:147–154. doi: 10.1007/s00268-005-0730-5. [DOI] [PubMed] [Google Scholar]
- Yamada S, Fuji T, Sugimoto H, Kanazumi N, Kasuya H, Nomoto S, et al. Pancreatic cancer with distant metastases: a contraindication for radical surgery? Hepatogastroenterology. 2009;56:881–885. [PubMed] [Google Scholar]
- Schwarz L, Lupinacci RM, Svrcek M, Lesurtel M, Bubenheim M, Vuarnesson H, et al. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br J Surg. 2014;101:530–538. doi: 10.1002/bjs.9444. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Is para-aortic lymph node metastasis a contraindication for radical resection in biliary carcinoma? World J Surg. 2011;55:1085–1093. doi: 10.1007/s00268-011-1036-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Aramaki M, Bandoh T, Kawano K, Sasaki A, Matsumoto T, et al. Para-aortic lymph node metastasis in carcinoma of the distal bile duct. Hepatogastroenterology. 1998;45:2388–2391. [PubMed] [Google Scholar]
- Yamada S, Nakao A, Fujii T, Sugimoto H, Kanazumi N, Nomoto S, et al. Pancreatic cancer with para-aortic lymph node metastasis. A contraindication for radical surgery. Pancreas. 2009;38:e13–e17. doi: 10.1097/MPA.0b013e3181889e2d. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Matsumoto T, Sasaki A, Shibata K, Aramaki M, Kitano S. Outcome of paraaortic node-positive pancreatic head and bile duct adenocarcinoma. Am J Surg. 2004;187:736–740. doi: 10.1016/j.amjsurg.2003.07.031. [DOI] [PubMed] [Google Scholar]
- LaFemina J, Chou JF, Gönen M, Rocha FG, Correa-Gallego C, Kingham TP, et al. Hepatic arterial nodal metastases in pancreatic cancer: is this the node of importance? J Gastrointest. 2013;17:1092–1097. doi: 10.1007/s11605-012-2071-7. [DOI] [PubMed] [Google Scholar]


