Abstract
Background
Population-based studies historically report underutilization of a resection in patients with colorectal metastases to the liver. Recent data suggest limitations of the methods in the historical analysis. The present study examines trends in a hepatic resection and survival among Medicare recipients with hepatic metastases.
Methods
Medicare recipients with incident colorectal cancer diagnosed between 1991 and 2009 were identified in the SEER(Surveillance, Epidemiology and End Results)-Medicare dataset. Patients were stratified into historical (1991–2001) and current (2002–2009) cohorts. Analyses compared treatment, peri-operative outcomes and survival.
Results
Of 31 574 patients with metastatic colorectal cancer to the liver, 14 859 were in the current cohort treated after 2002 and 16 715 comprised the historical control group. The overall proportion treated with a hepatic resection increased significantly during the study period (P < 0.001) with pre/post change from 6.5% pre-2002 to 7.5% currently (P < 0.001). Over time, haemorrhagic and infectious complications declined (both P ≤ 0.047), but 30-day mortality was similar (3.5% versus 3.9%, P = 0.660). After adjusting for predictors of survival, the use of a hepatic resection [hazard ratio (HR) = 0.40, 95% confidence interval (CI): 0.38–0.42, P < 0.001] and treatment after 2002 (HR = 0.88, 95% CI: 0.86–0.90, P < 0.001) were associated with a reduced risk of death.
Conclusions
Case identification using International Classification of Diseases, 9th Revision (ICD-9) codes is imperfect; however, comparison of trends over time suggests an improvement in multimodality therapy and survival in patients with colorectal metastases to the liver.
Introduction
Colorectal cancer (CRC) is the most common gastrointestinal malignancy, most common potentially resectable metastatic tumour to the liver and the most common indication for a liver resection in the United States. Up to 50% of patients with primary CRC will develop liver metastases in their lifetime with up to 20% of these patients eligible for a hepatic metastasectomy at high-volume centres.1–3 With advances in operative techniques, peri-operative management and multi-modality therapy, nationwide post-operative mortality after a liver resection approaches 2–5%.4–6 From a population-based perspective, only a minority of patients with hepatic metastases from CRC complete a metastasectomy.
The differences between institutional and population-level data have been difficult to reconcile. Unlike the 20% resection rate reported at select high-volume referral centres, a hepatic metastasectomy was performed in 6% of Medicare beneficiaries with the diagnosis of hepatic metastases from colorectal cancer prior to 2002.3,7 This discrepancy is likely multifactorial and attributable to both patient and physician-specific factors. Recent nationwide inpatient data suggests increased utilization of a hepatic metastasectomy for patients with colorectal cancer; however, current resection rates have not been established.8
The linked SEER (Surveillance, Epidemiology and End Results)-Medicare database allows for the population-based identification of incident cancer cases and the follow-up of patient care through time. Previously published SEER-Medicare data summarizing patient data between 1991 and 2001 identified a potential underutilization of operative treatment in patients with hepatic metastases from colorectal cancer.7 This study has been cited extensively in both hepatobiliary surgery and public health and outcomes literature as evidence of underutilization of hepatic resection among patients with metastatic colorectal cancer. However, since its publication in 2007, the methodology used to assemble the study cohort has been debated. Recent studies have emphasized limitations in the detail of International Classification of Diseases, 9th Revision (ICD-9) codes in hospital claims data, with aggregated accuracy of ICD-9 codes in diagnosis of metastatic colorectal cancer estimated to approximate 80%.9–11
Despite the limitations of ICD-9 codes, SEER-Medicare is the only United States national database to report both estimates of patients treated (operation and/or chemotherapy) for metastatic colorectal cancer to the liver and estimates of all patients with this diagnosis. As such, comparison of historic (pre-2002) and current hepatic resection rates using similar data acquisition methods may provide helpful estimates in the use of treatment for patients with metastatic CRC. Given ongoing advances in multimodality management and peri-operative treatment of patients with metastatic colorectal cancer to the liver, we hypothesized a significant increase in the hepatic metastasectomy rate for this patient population in the past decade.
Patients and methods
SEER and SEER-Medicare
The SEER programme is a the United States population-based cancer registry supported by the National Cancer Institute (NCI) and Centers for Disease Control and Prevention. The current registry includes data collected from multiple localities and states from 1973 to 2011. The most current reporting period includes data from 18 cancer registries drawn from approximately 28% of the population of the United States.
The SEER-Medicare linked database is a collaboration between NCI, SEER, and the Centers for Medicare and Medicaid Services. The SEER-Medicare database provides a combination of incident cancer cases and cancer-specific data collected by SEER with Medicare claims data for covered health services for Medicare beneficiaries identified from the SEER registry. Specific Medicare claim files include Part A: Medicare Provider Analysis and Review (MedPAR) hospital inpatient claims, and Part B: carrier claims (physician/supplier claims) and outpatient claims. The latest available data linkage at the time of data collection was performed in 2012 and includes SEER incident data through 2009 and Medicare claims through 2011.
Data collection and management
The data collection and study design were reviewed and approved by the University of Virginia Institutional Review Board. We conducted a retrospective, population-based cohort study of all patients diagnosed with colorectal adenocarcinoma using the SEER registry coding system. Patients included in the study had the following combination of characteristics: ICD-0-3 histology code adenocarcinoma (8140/1-3) and primary pathology sites colon (C15-23) and anorectum (C25-27) between 1991 and 2009, who also had a Medicare diagnosis of malignant neoplasm of the liver, secondary (ICD-9-CM 197.7) between 1991 and 2011 using the MedPAR, carrier claims and outpatient claims data. The study population was dichotomized into historical and current cohorts, based on data published previously summarizing outcomes for 1991 to 2001 SEER-Medicare patients.7 The historical cohort included patients diagnosed with colorectal cancer between 1991 and 2001. The current cohort included patients diagnosed with colorectal cancer between 2002 and 2009.
Patients who were enrolled in a Medicare HMO or not enrolled in Medicare Part B in the 2 years from the date of colon cancer diagnosis were excluded, as were patients with the previous metastatic disease. Patients less than 66 years of age at the time of diagnosis were also excluded in order to ensure all patients had at least 1 year of Medicare claims preceding diagnosis. Patients who did not complete a colorectal resection were also excluded.
Demographic data included age, gender and race. Race was classified as Caucasian, African–American or Other (Asian, Hispanic, Unknown). Patient-specific comorbidities included within 1 year of cancer diagnosis and present at least twice within claims data were abstracted from MedPAR, carrier claims, and outpatient claims and summarized as a Charlson/Deyo comorbidity index as described previously.12,13 Patients receiving systemic chemotherapy were identified using outpatient claims for chemotherapy administration within 6 months of the diagnosis of hepatic metastases. Chemotherapy administration was also identified for hepatic resection patients, including both administration of chemotherapy 6 months prior and/or 6 months after the liver resection.
Hepatic resections were identified using the ICD-9-CM diagnosis codes for a partial hepatectomy or wedge resection (50.22), lobectomy of the liver (50.3), and total hepatectomy (50.4), as well as, using Current Procedural Terminology (CPT) procedure codes for a partial lobectomy (47120), trisegmentectomy (47122), total left lobectomy (47125) and total right lobectomy (47130). The extent of a hepatic resection was categorized as a partial hepatectomy (50.22 and/or 47120) and a major (≥3 segments) anatomic hepatectomy (50.3, 50.4 and/or 47122, 47125, 47130). Post-operative complications were abstracted from secondary diagnoses reported in MedPAR hospital claims and were categorized into three groups as follows (i) haemorrhagic complications: accidental laceration, post-operative haemorrhage and post-haemorrhagic anaemia; (ii) gastrointestinal complications: paralytic ileus, gastrointestinal complications, gastrointestinal haemorrhage and intestinal fistula; and (iii) infectious complications: post-operative infection, wound dehiscence, peritonitis and liver abscess. For patients with more than one liver operation, comorbidities and complications associated with the first liver resection were abstracted. Thirty-day post-operative mortality was abstracted from Medicare data. The overall survival was calculated as months between the diagnosis of hepatic metastases and death.
Statistical analysis
Distributional characteristics of data for continuous variables are reported as medians with an interquartile range (IQR), and as percentages for categorical data. The statistical significance of differences between cohorts in the proportional distribution of categorical variables was assessed using Pearson's chi-square test statistic. Differences in the median and mean values of continuous variables between groups were compared with Wilcoxon's rank–sum test. A weighted linear regression was used to represent graphically the change in hepatic resection rates over time (weighted linear R2 = 0.673). Logistic regression using a hepatic resection as the outcome and liver metastasis diagnosis year as the predictor was used to estimate the change in the hepatic resection rate over time. Kaplan–Meier survival analysis, with the log–rank test for between-group comparisons, was used to test the bivariable effect of hepatic resection on survival. Cox's proportional hazards regression was used to develop a multivariable model adjusting for the combined effects of age, gender, race, incident AJCC stage at the time of diagnosis, the Charlson/Deyo comorbidity index, hepatic resection, systemic chemotherapy and treatment time period on survival in patients with colorectal cancer hepatic metastases.
SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) software was used to conduct data management, statistical analysis and to produce plots of results. A P-value of <0.05 was considered to indicate statistically significant differences for all comparisons.
Results
Patient demographics and staging
Of 31 574 patients in the study cohort, 16 715 patients comprised the historical control group (1991–2001) and 14 859 patients comprised the current cohort (2002–2009). Demographic and clinical data are summarized in Table1. Patients in the current cohort had a higher median age, 78 (72–83) versus 77 (73–82) years, P = 0.040. Sex and race proportions were not significantly different between the two groups. Patients in the current cohort presented with higher AJCC stage (P < 0.001), and more patients in the current cohort had Stage III and IV disease at the time of incident diagnosis. There was no difference in the use of systemic chemotherapy within 6 months of the diagnosis of hepatic metastases (current cohort: 33.0% versus historical cohort: 32.1%, P = 0.077).
Table 1.
Demographic and clinical data of patients with colorectal cancer hepatic metastases
| Historical cohort (1991–2001) 16 715 patients | Current cohort (2002–2009) 14 859 patients | P-value | |
|---|---|---|---|
| Age | 77 (73–82) | 78 (72–83) | 0.040 |
| Female | 8671 (51.9) | 7651 (51.5) | 0.495 |
| Race | |||
| Caucasian | 13 788 (82.5) | 12 126 (81.6) | 0.052 |
| African–American | 1639 (9.8) | 1578 (10.6) | |
| Other | 1288 (7.7) | 1155 (7.8) | |
| AJCC Stage at diagnosis | |||
| Stage I | 1286 (7.7) | 907 (6.1) | <0.001 |
| Stage II | 3303 (19.8) | 2291 (15.4) | |
| Stage III | 4686 (28.0) | 4393 (29.6) | |
| Stage IV | 7440 (44.5) | 7268 (48.9) | |
| Chemotherapy | 5357 (32.1) | 4901 (33.0) | 0.077 |
Data reported as n (%) except for the Age which is reported as median (interquartile range).
Liver resection patients
Of 31 574 patients included in the study, 2190 (6.9%) underwent a liver resection. The proportion of patients treated with a liver resection increased during the entire study period with a statistically significant yearly increase in odds each year: odds ratio (OR) 1.03; 95% confidence interval (CI): 1.02–1.04, P < 0.001 (Fig.1). The use of a liver resection increased from 1080 patients (6.5%) in the historical cohort to 1110 patients (7.5%) in the current cohort (P < 0.001).
Figure 1.
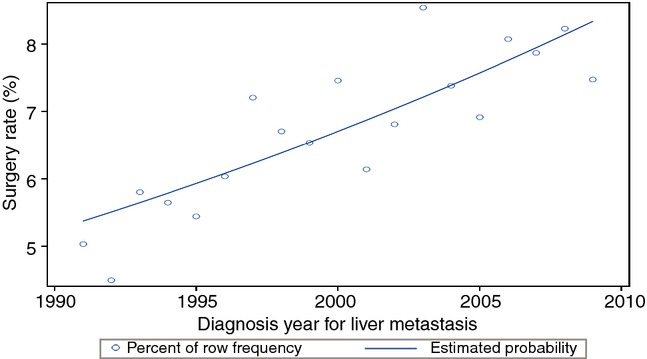
Logistic regression model demonstrating an improvement in resection rates during the study period with a fitted estimated resection rate (P < 0.001)
Peri-operative chemotherapy, operative extent, comorbidities and post-operative complications are summarized in Table2. Patients in the current cohort had higher Charlson/Deyo comorbidity index values (P < 0.001). Both the use of pre-operative chemotherapy (22.3% versus 10.9%) and post-operative chemotherapy (39.6% versus 31.5%) was higher in patients treated after 2002 (both P < 0.001). The use of a major (≥3 segments) hepatectomy did not differ between the two time periods (P = 0.974). The proportion of patients with post-hepatectomy complications was lower in the current cohort, with fewer patients developing ≥ 1 complication after a resection (25.5% versus 28.1%, P = 0.021). Both haemorrhagic and infectious complications were significantly lower in the current cohort (both P ≤ 0.047); gastrointestinal complications did not differ between the two groups (P = 0.520). Post-operative 30-day mortality was 3.5% among the historical controls and 3.9% in the current cohort (P = 0.660).
Table 2.
Peri-operative data for a hepatic metastasectomy
| Historical cohort (1991–2001) 1080 patients | Current cohort (2002–2009) 1110 patients | P-value | |
|---|---|---|---|
| Age | 74 (IQR: 71–78) | 74 (IQR: 70–78) | 0.056 |
| Charlson/Deyo comorbidity index | 0 (IQR: 0–1) (range: 0–6) | 0 (IQR: 0–1) (range: 0–7) | <0.001 |
| Pre-metastasectomy chemotherapy | 118 (10.9) | 248 (22.3) | <0.001 |
| Post-metastasectomy chemotherapy | 340 (31.5) | 439 (39.6) | <0.001 |
| Operative extent | |||
| Partial hepatectomy | 767 (71.0) | 789 (71.1) | 0.974 |
| ≥3 segments hepatectomy | 313 (29.0) | 321 (28.9) | |
| ≥1 complication | 304 (28.1) | 283 (25.5) | 0.021 |
| Haemorrhagic complicationsa | 151 (14.0) | 124 (11.2) | 0.047 |
| Gastrointestinal complicationsb | 152 (14.1) | 167 (15.1) | 0.520 |
| Infectious complicationsc | 35 (3.2) | 17(1.5) | 0.009 |
| 30-day mortality | 38 (3.5) | 43 (3.9) | 0.660 |
Accidental laceration, post-operative hemorrhage and post-hAemorrhagic anaemia.
Paralytic ileus, gastrointestinal complications, gastrointestinal haemorrhage and intestinal fistula.
Post-operative infection, wound dehiscence, peritonitis and liver abscess.
Data are reported as n (%) except for age which is reported as the median (interquartile range) and Charlson/Deyo comorbidity index which is reported as the median (interquartile range and overall range).
Survival analyses
Patients treated with a hepatic resection had a better overall survival compared with patients who did not complete a hepatic metastasectomy in both cohorts (Fig.2a, b), log–rank P < 0.001. In the historical cohort, the median survival was significantly better in patients completing a hepatic metastasectomy (32 versus 7 months, log–rank P < 0.001). Similarly, the median survival was significantly better among hepatic metastasectomy patients in the current cohort (39 versus 8 months, log–rank P < 0.001). The 5-year survival was significantly better for patients selected for a metastasectomy during both study periods (historical cohort: 26% vs. 6%; current cohort: 33% versus 7%; both P < 0.001).
Figure 2.
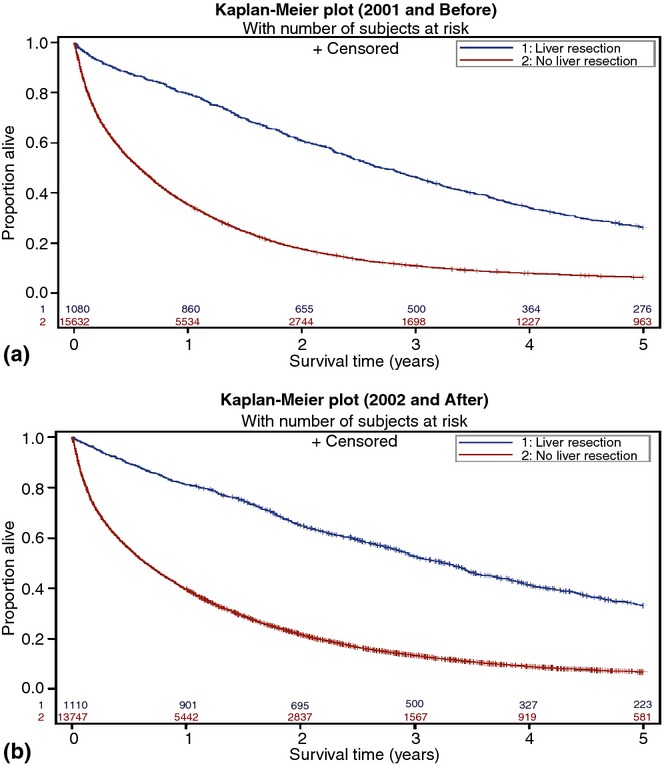
(a) Survival by a hepatic metastasectomy in the historic cohort (1991–2001). Patients completing a hepatic metastasectomy had an improved overall survival, P < 0.001. (b) Survival by a hepatic metastasectomy in the current cohort (2002–2009). Patients completing a hepatic metastasectomy had an improved overall survival, P < 0.001
Multivariable Cox's proportional hazards regression analysis was used to assess the adjusted effects of demographic and clinical covariates on overall survival. The multivariable model is summarized in Table3. Age, Charlson/Deyo comorbidity index and incident AJCC stage had independent negative effects on survival (all P < 0.001). The use of systemic chemotherapy was associated with a lower risk of death (HR = 0.64; 95% CI: 0.63–0.66; P < 0.001). Both the use of a hepatic resection (HR = 0.40; 95% CI: 0.38–0.42, P < 0.001) and treatment in the current study cohort (HR = 0.88; 95% CI: 0.86–0.90, P < 0.001) were also associated with a lower risk of death.
Table 3.
Multivariable Cox's proportional hazards model for overall survival
| HR | 95% CI | P-value | |
|---|---|---|---|
| Age at diagnosisa | 1.03 | 1.03–1.03 | <0.001 |
| Female sex | 0.99 | 0.97–1.01 | 0.441 |
| Race | |||
| African American | 0.98 | 0.94–1.02 | 0.329 |
| Other | 0.82 | 0.79–0.86 | <0.001 |
| Charlson/Deyo comorbidity index | 1.07 | 1.06–1.08 | <0.001 |
| AJCC initial diagnosis stage II vs. I | 1.07 | 1.01–1.12 | 0.023 |
| AJCC initial diagnosis stage III vs. I | 1.39 | 1.32–1.46 | <0.001 |
| AJCC initial diagnosis stage IV vs. I | 1.63 | 1.56–1.72 | <0.001 |
| Hepatic resection | 0.40 | 0.38–0.42 | <0.001 |
| Chemotherapy | 0.64 | 0.63–0.66 | <0.001 |
| Current study cohort | 0.88 | 0.86–0.90 | <0.001 |
Estimates of effects of age to one thousandth: HR = 1.029, 95% CI: 1.028–1.031, P < 0.001.
Discussion
Rates of hepatic metastasectomy for Stage IV colorectal cancer continue to improve in Medicare recipients. Previously published studies using SEER-Medicare registry reported 3.9% to 6.1% hepatic resection rates among patients with metastatic colorectal cancer to the liver.7,14 The overall use of a hepatic resection increased during the past decade with a current aggregated resection rate of 7.5% for patients treated between 2002 and 2009. The use of peri-operative systemic chemotherapy also increased between pre-2002 and current cohorts. While the overall administration of chemotherapy in all patients with hepatic metastases did not change with time and is consistent with previously published SEER-Medicare data,15 the use of chemotherapy in patients selected for a metastasectomy within 6 months prior to a liver resection increased from 10.9% to 22.3%, and the use of post-hepatectomy chemotherapy increased from 31.5% to 39.6%. Temporal treatment after 2002, a hepatic resection and chemotherapy were all independently associated with an improved overall survival in patients with hepatic metastases from colorectal cancer.
The use of resection and peri-operative chemotherapy in Medicare recipients is increasing despite an aging patient population with more comorbidities. The median age of patients selected for a hepatic metastasectomy in this SEER-Medicare study population of patients over 66 years at the time of diagnosis was 74 years; Charlson/Deyo comorbidity index values were higher in patients treated between 2002 and 2009 than patients treated prior to 2002. Despite a seemingly more aggressive treatment approach, the frequency of overall, haemorrhagic and infectious complications has declined. The 30-day post-operative mortality remains less than 4% and is comparable to nationwide National Surgical Quality Improvement Program data.4,6 Similarly, long-term survival rates in patients selected for a metastasectomy post-2002 are comparable to 30–40% 5-year overall survival described in multi-institutional and nationwide data.2,16,17
Unfortunately, identification of specific tumour features affecting organ-specific or the overall metastatic burden and/or disease biology is not possible with SEER-Medicare data. Definition of metastatic sites and/or disease recurrence using ICD-9 codes is limited. Sensitivity and the positive predictive value (PPV) of distant CRC metastases using ICD-9 code definitions are 80% and 70%, respectively, in historically reviewed SEER-Medicare data.9 A corresponding diagnosis of CRC metastases using ICD-9 data in proprietary claims databases achieves a PPV between 73% and 80% and an overall accuracy of 80%.10,11 Also, identification of colorectal cancer recurrence and/or presence of metachronous metastases using ICD-9 data has a sensitivity of 71% and specificity of 91% among Medicare recipients.18 With an overall accuracy approaching 80%, ICD-9 code definitions alone are limited in defining patients with hepatic metastases. However, despite this limitation, data acquisition methods are believed to have been consistent between historic and current study cohorts. Thus, even considering these limitations, our results suggest an improvement of hepatic resection rates and in the use of pre- and post-operative chemotherapy in Medicare recipients with metastatic colorectal cancer.
Population-based analyses have other significant limitations. The exact burden of disease and suitability of the operative approach cannot be determined. To this extent, it is difficult to compare 20% resection rates reported by institutional data and a 7.5% resection rate in this population-based study. The higher rates of resection in institutional data can reflect a referral bias of tertiary and quaternary care centres.19,20 In addition, differences in the burden of disease and/or the significantly older age of Medicare recipients can explain variability in the resection rates between institutional and registry data. Interestingly, a similar analysis among the patients enrolled in the English National Health Service demonstrated improvement in the hepatic metastasectomy rate from 1.7% in 1998 to 3.8% in 2004.20
Proposed barriers to the delivery of population-level care include existing or perceived burden of disease, demographic or socioeconomic disparities, perceived morbidity of treatment and patient comorbidity, and a lack of education about the efficacy of a resection and/or referral patterns. Unlike recent data suggesting disparities in treatment associated with insurance disparities,21 all patients in this study were insured. With advances in chemotherapy regimens, targeted therapy options, as well as, operative and re-operative techniques, metastatic colorectal cancer has been described as a chronic disease.22–25 On-going efforts should focus on the emphasis of multidisciplinary approaches and the multimodality treatment of patients with colorectal cancer hepatic metastases.
Conclusion
Hepatic resection rates and the use of peri-operative chemotherapy for patients with hepatic metastases from colorectal cancer continue to improve among Medicare recipients. Despite advanced age and comorbidities, overall survival is improved in patients selected for metastasectomy. Both a hepatic resection and systemic chemotherapy are associated with improved survival.
Acknowledgments
The authors sincerely thank Craig L. Slingluff, MD, for editorial review and mentorship during manuscript preparation. The contents of this study including the interpretation and reporting of the SEER-Medicare data are the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Conflicts of interest
None declared.
References
- Mayo SC, Pawlik TM. Current management of colorectal hepatic metastasis. Expert Rev Gastroenterol Hepatol. 2009;3:131–144. doi: 10.1586/egh.09.8. [DOI] [PubMed] [Google Scholar]
- Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–1239. doi: 10.1634/theoncologist.2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB. 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JP, Ng SC, Hill JS, Shah SA, Zhou Z, Tseng JF. In-hospital mortality for liver resection for metastases: a simple risk score. J Surg Res. 2009;156:21–25. doi: 10.1016/j.jss.2009.03.073. [DOI] [PubMed] [Google Scholar]
- Tzeng CW, Cooper AB, Vauthey JN, Curley SA, Aloia TA. Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB. 2014;16:459–468. doi: 10.1111/hpb.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- Bartlett EK, Simmons KD, Wachtel H, Roses RE, Fraker DL, Kelz RR, et al. The rise in metastasectomy across cancer types over the past decade. Cancer. 2015;121:747–757. doi: 10.1002/cncr.29134. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Yuan Z, Stange KC, Amini SB, Dennis LK, Rimm AA. The utility of Medicare claims data for measuring cancer stage. Med Care. 1999;37:706–711. doi: 10.1097/00005650-199907000-00010. [DOI] [PubMed] [Google Scholar]
- Whyte JL, Engel-Nitz NM, Teitelbaum A, Gomez Rey G, Kallich JD. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative Claims data. Med Care. 2015;53:e49–e57. doi: 10.1097/MLR.0b013e318289c3fb. [DOI] [PubMed] [Google Scholar]
- Nordstrom BL, Whyte JL, Stolar M, Mercaldi C, Kallich JD. Identification of metastatic cancer in claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl. 2):21–28. doi: 10.1002/pds.3247. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40 doi: 10.1097/00005650-200208001-00004. :IV-26-35. [DOI] [PubMed] [Google Scholar]
- Temple LK, Hsieh L, Wong WD, Saltz L, Schrag D. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22:3475–3484. doi: 10.1200/JCO.2004.10.218. [DOI] [PubMed] [Google Scholar]
- Parikh AA, Ni S, Koyama T, Pawlik TM, Penson D. Trends in the multimodality treatment of resectable colorectal liver metastases: an underutilized strategy. J Gastrointest Surg. 2013;17:1938–1946. doi: 10.1007/s11605-013-2325-z. [DOI] [PubMed] [Google Scholar]
- Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas GM, Parmar AD, Sheffield KM, Tamirisa NP, Brown KM, Riall TS. Impact of liver-directed therapy in colorectal cancer liver metastases. J Surg Res. 2014;191:42–50. doi: 10.1016/j.jss.2014.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett MJ, Ritzwoller DP, Taback N, Carroll N, Cronin AM, Ting GV, et al. Validating billing/encounter codes as indicators of lung, colorectal, breast, and prostate cancer recurrence using 2 large contemporary cohorts. Med Care. 2014;52:e65–e73. doi: 10.1097/MLR.0b013e318277eb6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97:1110–1118. doi: 10.1002/bjs.7032. [DOI] [PubMed] [Google Scholar]
- Parikh AA, Robinson J, Zaydfudim VM, Penson D, Whiteside MA. The effect of health insurance status on the treatment and outcomes of patients with colorectal cancer. J Surg Oncol. 2014;110:227–232. doi: 10.1002/jso.23627. [DOI] [PubMed] [Google Scholar]
- Andreou A, Brouquet A, Abdalla EK, Aloia TA, Curley SA, Vauthey JN. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB. 2011;13:774–782. doi: 10.1111/j.1477-2574.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DL, Chu E. Can metastatic colorectal cancer be cured? Oncology (Williston Park) 2012;26:266–275. [PubMed] [Google Scholar]
- Chua TC, Liauw W, Chu F, Morris DL. Viewing metastatic colorectal cancer as a curable chronic disease. Am J Clin Oncol. 2012;35:77–80. doi: 10.1097/COC.0b013e3181fe4444. [DOI] [PubMed] [Google Scholar]
- Abdalla EK, Bauer TW, Chun YS, D'Angelica M, Kooby DA, Jarnagin WR. Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements. HPB. 2013;15:119–130. doi: 10.1111/j.1477-2574.2012.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


