Abstract
Objective
To compare surgical outcomes between matched central hepatectomy (CH) and extended hepatectomy (EH) groups.
Background
Surgical choices for centrally located liver tumours are limited. The traditional EH harbours substantial risks, whereas CH is an alternative parenchymal-sparing resection that may improve peri-operative morbidity.
Methods
A review of 4661 liver resections at a single institution was performed. The cases (CH) were matched in a 1:1 ratio with EH controls.
Results
The CH group was matched for demographic, tumour and laboratory factors with either right EH or combined (right/left) EH groups (n = 63 per group). Colorectal liver metastases were the most common diagnosis occurring in 70% of the patients. Higher intra-operative blood loss was observed in the right EH(P = 0.01) and combined EH groups (P < 0.01) compared with the CH group. There was a trend towards lower 90-day morbidity in the CH group (43%) compared with the right EH(59%, P = 0.1) and combined EH groups (56%, P = 0.2). The length of hospital stay was significantly longer in the control groups (P < 0.01 for both). The control groups had significantly higher post-operative bilirubin and International Normalized Ratio (INR) levels compared with the CH group. A post-operative bilirubin higher than 4 mg/dl was observed in 2% of the CH group compared with 39% of the right EH group (P < 0.01) and 52% of the combined EH group (P < 0.01). No differences in the rates of bile leak/biloma, post-hepatectomy liver failure or 90-day mortality were found.
Conclusions
CH, as compared with EH, was safe and associated with a shorter hospital stay and less post-operative liver dysfunction. CH should be considered in patients with centrally located tumours amenable to such a resection.
Introduction
A hepatic resection is an effective treatment option for selected patients with malignant liver tumours.1,2 Centrally located tumours of the liver involving segments 4, 5 and 8 may require extended resections (right or left hepatectomy extended to the contralateral liver) because of their proximity to major central vascular and biliary structures.3,4 As it is well documented that the morbidity of a liver resection is related to the extent of resection, such extended resections carry a significant risk of post-operative morbidity, liver dysfunction and mortality.5–7 These considerations are especially relevant for patients with a compromised functional liver reserve.
A central hepatectomy (CH) was first reported for gallbladder cancer in 1972 and is used to describe the procedure to resect segments 4, 5 and 8 (Fig.1).8–14 The potential risks of CH compared with traditional major liver resections such as an extended- or hemi-hepatectomy include a longer operating time, greater intra-operative blood loss and a higher risk of biliary and vascular complications; all potentially attributed to the presence of two parenchymal transection planes instead of one. Another potential disadvantage of CH is compromise of surgical margins. The potential advantages of CH include preservation of liver parenchyma with the potential for decreasing the risk of post-operative liver dysfunction or failure. A central hepatectomy may also increase the chance for a future repeat resection.3,4,15–17
Figure 1.
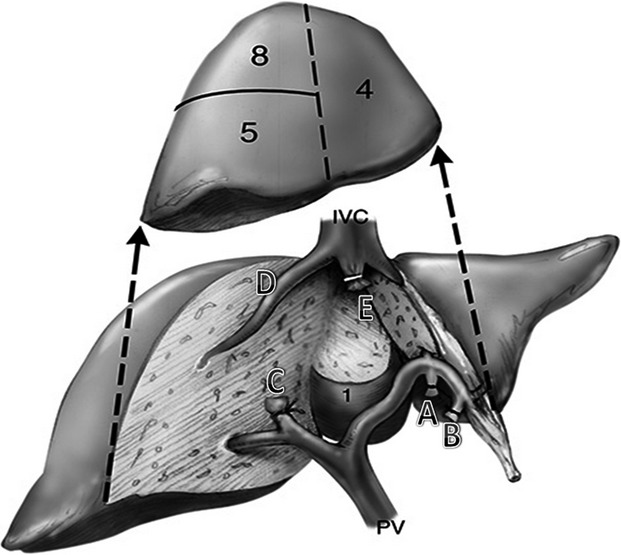
Central hepatectomy–segment-orientated resection.14 Central segments labelled 4, 5, 8 ± 1 (caudate lobe) are the segments resected in a central hepatectomy (CH). The inflow pedicles to segment 4 (A and B) are ligated and divided along the umbilical fissure, the right anterior sectoral pedicle (C) is then divided. The parenchymal transection proceeds from the left side and then the right side until the two planes meet just anterior to the inferior vena cava (IVC). The right hepatic vein (D) is preserved. The middle hepatic vein (E) can be divided with a stapler. Segments 4, 5, 8 ± 1 are removed en masse. (Adapted from Liau, et al.14)
Recent reports, albeit few and small in sample size, have demonstrated that CH is safe and is associated with comparable complication and overall survival rates as compared with conventional major hepatectomies.18 This current literature comparing patients undergoing an extended hepatectomy (EH) and CH consists of small retrospective studies largely involving patients with hepatocellular carcinoma (HCC). These are mostly Asian studies performed more than a decade ago and their CH and EH cohorts are not matched (e.g. the CH cohort in most of these studies was compared with a combined group that included both lobar and extended hepatectomies and patients' clinicopathological factors were not comparable), thus outcomes may not be fairly comparable as well.5,10,18 The aim of this study was to analyse and compare the short- and long-term outcomes for centrally located liver malignancies treated with either CH or EH in matched cohorts.
Patients and methods
Patients undergoing liver resections at Memorial Sloan Kettering Cancer Center (MSKCC) were identified from a prospectively maintained Hepatobiliary database between 1991 and 2013. Sixty-three patients comprised the cases (CH) and were matched in a 1:1 ratio with EH controls selected from the larger group of patients who had undergone EH at the same period. Matching was initially performed with the following variables: patient demographics [age at surgery (±5 years), body mass index (BMI) (< 25, 25–29, ≥30 kg/m2)], tumour factors [disease diagnosis, tumour size (±3 cm)] and pre-operative platelets count (±50 × 109/l). These variables were selected based on their known confounding effects on clinical outcomes.19–21 The continuous matching criteria were widened to age (±10 years) and pre-operative platelets (±100 × 109/l) for 16 patients with CH in order to find an EH control; clinically meaningful matching for tumor size was not possible for these 16 patients. Finally, platelets count was further widened in order to find controls for two cases. We performed the comparison between the CH group and either a matched combined EH group (patients who had undergone either right or left EH) or a matched right EH group (patients who had undergone right extended hepatectomy), as there were not enough similarly matched patients for a separate cohort of extended left hepatectomy patients. Wilcoxon's matched-pairs signed ranks test and exact McNemar's test were used to examine the difference in covariates between the paired groups on outcomes.
Patients' demographics and clinicopathological variables were analysed to compare short-term outcomes. Patients whose tumours were either treated with a liver resection and intra-operative ablation or patients who had undergone a prior liver resection were excluded. Only patients with a confirmed pathological diagnosis of malignancy were included. Cholangiocarcinoma requiring bilio-enteric anastomosis was excluded given the high risk of bile leak. All pathological specimens were reviewed and confirmed by MSKCC pathologists. Definitions and measurements of prognostic factors and clinical risk score calculation (CLM patients only) were previously reported.22 Survival distributions were estimated from the date of surgery using the Kaplan–Meier method (CLM patients only) and compared with the stratified log-rank test. Overall survival (OS) was defined as death from any cause. An event for recurrence-free survival (RFS) was defined as either death or recurrence. Patients without events were censored at last follow-up. All P-values were based on two-tailed statistical analyses and P < 0.05 was considered to indicate statistical significance. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA). Approval for the study was obtained from the MSKCC institutional review board.
Pre-operative assessment
Complete staging and evaluation was performed prior to surgery and included a history and physical examination, laboratory investigations and cross-sectional contrast-enhanced imaging [computed tomography (CT) and/or Magnetic Resonance Imaging (MRI)] of the chest, abdomen and pelvis (including liver with liver-specific protocols). Other radiological and diagnostic procedures were used at the discretion of the treating physicians. Suitability for a liver resection and the type of resection (CH or EH) was based on fitness for surgery, an adequate future liver remnant (FLR) with adequate inflow, outflow and biliary drainage; and the likelihood to achieve an R0 resection with adequate margins. This decision is made by the primary surgeon and discussed at a multidisciplinary conference for radiological review, opinions and consensus if necessary.
Surgery
All liver resections were performed using a standard technique as reported previously.23–25 Intra-operative ultrasonography was routinely performed to detect additional tumours in the liver, delineate the relationship of the tumour to major vascular and biliary structures, as well as to guide the plane of parenchymal transection. Similar to the Brisbane classification, a right EH was defined as a liver resection of segments 5 to 8, if it included a significant portion of segment 4 (with or without segment 1). A left EH was defined as a liver resection of segments 2 to 4 and segment 8, if it included a significant portion of segment 5 or 8 (with or without segment 1).8 A central hepatectomy was defined as a liver resection of segments 4, 5 and 8; procedures were considered as CH in this study if the majority of segments 4a and 4b and the majority of segments 5 and 8 were removed, with a significant bi-planar resection (Fig.1).14,24,25
Post-operative morbidity and mortality were defined as complications or deaths within 90 days after surgery, respectively. Events were recorded prospectively in the department of surgery complication database (MSKCC Surgical Secondary Events Program) and graded in severity with a score of 1 to 5. Grade 1 complications require only oral medication or bedside medical care. Grade 2 events require intravenous medical therapy, antibiotics or total parenteral nutrition with resolution. Grade 3 events require radiological, endoscopic or operative intervention. Grade 4 complications are associated with chronic deficit or disability, and grade 5 is defined as mortality associated with sequelae of the event.26
Post-hepatectomy liver failure (PHLF) was defined according to a proposed consensus by the International Study Group of Liver Surgery (ISGLS) in 2011.27 In essence, the ISGLS defined PHLF as an increased INR and hyperbilirubinaemia (according to the normal levels defined by the local laboratory) on or after post-operative day (POD) 5. In addition, we also used the ‘50–50’ criteria as an alternative marker of liver dysfunction, which is based on prothrombin time (PT) <50% and serum bilirubin >3 mg/dl on POD 5.28
Results
Patients' characteristics
A total of 4661 liver resections were performed at MSKCC during the study period (1991 to 2013). After review of operative details and diagnoses, 63 patients who had undergone CH were identified and another 886 patients who had undergone EH were identified (724 right EH and 162 left EH). The median age of patients who underwent CH was 61 years (range: 36–86) and the majority were being treated for CLM (n = 44; 70%). After matching, the right EH group included 63 patients, and the combined EH group included 11 left EH and 52 right EH patients. Table1 details the clinicopathological characteristics. No differences were noted between the cases and controls with regard to the matching criteria except for tumour size, which was 0.5 cm (median) smaller in the CH group compared with the control groups (P < 0.01 for both). Portal vein embolization (PVE) was not performed in the CH group, whereas it was performed in 19% and 16% of the right EH and combined EH groups, respectively.
Table 1.
Patients' and tumours' characteristics stratified by cases (CH) and extended hepatectomy (EH) groups
| CH group (n = 63) | Right EH group (n = 63) | P-valuea | Combined EH group (N = 63) | P-valueb | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at surgery, years | 61 (36–86) | 60 (35–81) | 0.9 | 61 (31–81) | 0.7 |
| Male | 44 (70%) | 39 (62%) | 0.4 | 40 (63%) | 0.5 |
| Body mass index, kg/mb | |||||
| <25 | 15 (24%) | 15 (24%) | 1.0 | 15 (24%) | 1.0 |
| 25–29 | 29 (46%) | 29 (46%) | 29 (46%) | ||
| ≥30 | 19 (30%) | 19 (30%) | 19 (30%) | ||
| Tumour characteristics | |||||
| Indication for surgery disease | |||||
| Hepatocellular cancer | 5 (8%) | 5 (8%) | 1.0 | 5 (8%) | 1.0 |
| Metastases – Colorectal | 44 (70%) | 44 (70%) | 44 (70%) | ||
| Metastases – Others | 14 (22%) | 14 (22%) | 14 (22%) | ||
| Largest tumour size, cm | 3.6 (1–21) | 4.1 (2–14) | <0.01 | 4.1 (1–14) | <0.01 |
| Pre-operative investigations | |||||
| Platelets, ×109/l | 214 (95–453) | 207 (112–467) | 0.3 | 210 (112–467) | 0.09 |
| Creatinine, mg/dl | 1.0 (0.6–1.6) | 1.0 (0.6–1.7) | 0.8 | 1.0 (0.6–1.90) | 0.8 |
| International Normalized Ratio | 1.1 (0.9–1.7) | 1.1 (0.9–1.7) | 0.3 | 1.1 (0.88–1.67) | 0.3 |
| Bilirubin, mg/dl | 0.6 (0.3–1.3) | 0.7 (0.2–10.1) | 0.3 | 0.6 (0.2–10.1) | 0.4 |
| Carcinoembryonic antigen, ng/ml [CLM patients only (n = 44)] | 13.7 (1.4–792) | 12.3 (1.1–476) | 0.9 | 20.6 (1.1–475.6) | 0.9 |
| Pre-operative portal vein embolization | 0 | 12 (19%) | <0.001 | 10 (16%) | <0.01 |
CLM, colorectal liver metastasis; Continuous variables are expressed as median (range); categorical variables are expressed as n (%).
Comparison of the CH and right EH groups.
Comparison of the CH and combined EH groups.
Bold values denote P-values < 0.05.
Peri-operative outcomes
Shorter Pringle time and higher intra-operative blood loss were observed in the right EH (P = 0.04 and P = 0.01, respectively) and the combined EH group (P = 0.03 and P < 0.01, respectively) compared with the CH group (Table2). There was a trend towards a lower 90-day morbidity in the CH group (43%) compared with the right EH (59%, P = 0.1) and combined EH groups (56%, P = 0.2). The length of hospital stay was significantly longer in the control groups (P < 0.01 for both). Compared with the CH group, the control groups demonstrated significantly higher post-operative peak serum total bilirubin (right EH versus CH: 3.2 versus 1.8 mg/dl, P = 0.001; combined EH versus CH: 3 versus 1.8 mg/dl, P = 0.001) and International Normalized Ratio (INR) levels (right EH versus CH: 1.5 versus 1.3, P = 0.02; combined EH versus CH: 1.5 versus 1.3, P = 0.02). A post-operative serum bilirubin higher than 4 mg/dl was observed in 2% of the CH group as compared with 39% in the right EH group (P < 0.01) and 52% in the combined EH group (P < 0.01). The ‘50–50 criteria’ were not satisfied by any of the patients in any group. In addition, no differences were noted with regard to the ISGLS PHLF rates (CH: 62%, right EH: 74%, combined EH: 68%; P = 1.0 for both) or 90-day mortality rates (CH: 1.6%, right EH: 4.7%, combined EH: 4.7%; P = 0.6 for both). Symptomatic bile leak/biloma occurred in two patients (3%) in the CH group, which resolved after percutaneous drainage. There were no bile leaks/biloma in the EH groups (P = 0.5 for both).
Table 2.
Short- and long-term outcomes stratified by the cases (CH) and extended hepatectomy (EH) groups
| CH group (n = 63) | Right EH group (n = 63) | P-valuea | Combined EH group (n = 63) | P-valueb | |
|---|---|---|---|---|---|
| Operative details | |||||
| Operative time, min | 244 (110–539) | 255 (112–557) | 0.4 | 258 (5–557) | 0.09 |
| Blood loss, ml | 500 (50–4500) | 800 (100–5000) | 0.01 | 850 (100–5000) | <0.01 |
| Total Pringle time, min | 50 (0–136) | 36 (0–151) | 0.04 | 36 (0–151) | 0.03 |
| Resection status (CLM patients only) | |||||
| R0 resection | 34 (92%) | 33 (89%) | 0.7 | 32 (86.4%) | 0.7 |
| Margins width, mm | 0.3 (0–2.4) | 0.5 (0–3.5) | 0.3 | 0.4 (0–3.5) | 0.9 |
| Morbidity and mortality | |||||
| Length of stay, days | 8 (5–42) | 10 (5–62) | <0.01 | 9 (5–62) | <0.01 |
| 90-day mortality | 1 (1.6%) | 3 (4.7%) | 0.6 | 3 (4.7%) | 0.6 |
| 90-day morbidity | 27 (43%) | 37 (59%) | 0.1 | 35 (56%) | 0.2 |
| Bile leak/biloma | 2 (3%) | 0 | 0.5 | 0 | 0.5 |
| Major complications (≥Grade 3) | 8 (19%) | 8 (19%) | 1.0 | 7 (17%) | 0.7 |
| Bilirubin, mg/dl | |||||
| POD1 | 1.5 (0.6–3.9) | 2.2 (0.6–7.6) | <0.01 | 2 (0.4–7.6) | <0.01 |
| POD3 | 1.5 (0.6–3.8) | 2.1 (0.4–13) | <0.01 | 2 (0.4–13) | 0.01 |
| POD5 | 1.5 (0.5–3.0) | 2.3 (0.4–14.9) | <0.01 | 2.2 (0.4–14.9) | 0.01 |
| Maxc | 1.8 (0.7–4.1) | 3.2 (0.6–16.7) | 0.001 | 3 (0.6–16.7) | 0.001 |
| Bilirubinc>4 mg/dl | 1 (2%) | 17 (39%) | <0.01 | 12 (52%) | <0.01 |
| INR | |||||
| POD1 | 1.2 (1.0–1.8) | 1.4 (1.2–2.1) | 0.17 | 1.4 (1.2–2.1) | 0.1 |
| POD3 | 1.2 (1.0–1.4) | 1.3 (1.0–1.8) | 0.16 | 1.3 (1.0–1.8) | 0.04 |
| POD5 | 1.1 (0.9–1.4) | 1.3 (1.0–1.7) | 0.2 | 1.2 (1.0–1.7) | 0.02 |
| Maxc | 1.3 (1.1–1.8) | 1.5 (1.2–6.0) | 0.02 | 1.5 (1.2–6.0) | 0.02 |
| ‘50–50’ criteria | 0 | 0 | NA | 0 | NA |
| ISGLS criteria (any grade) | 13 (62%) | 23 (74%) | 1.0 | 23 (68%) | 1.0 |
| CH group (n = 44) | Right EH group (n = 44) | Combined EH group (n = 44) | |||
|---|---|---|---|---|---|
| Oncological characteristics (CLM patients only) | |||||
| Clinical risk score | |||||
| 0–2 | 31 (72%) | 27 (61%) | 0.3 | 25 (57%) | 0.2 |
| 3–5 | 12 (27%) | 17 (39%) | 19 (43%) | ||
| Disease-free interval | |||||
| <12 months | 22 (55%) | 19 (45%) | 0.5 | 22 (53%) | 1.0 |
| >12 months | 18 (45%) | 23 (55%) | 20 (48%) | ||
| Nodal status of the primary | |||||
| Negative | 17 (45%) | 17 (40%) | 0.8 | 13 (31%) | 0.3 |
| Positive | 21 (55%) | 25 (60%) | 29 (69%) | ||
| Pre-operative CEA | |||||
| <200 μg/l | 33 (94%) | 32 (87%) | 0.7 | 33 (87%) | 0.7 |
| ≥200 μg/l | 2 (6%) | 5 (13%) | 5 (13%) | ||
| Tumor size | |||||
| <5 cm | 30 (73%) | 28 (64%) | 0.4 | 27 (61%) | 0.8 |
| ≥5 cm | 11 (27%) | 16 (36%) | 17 (39%) | ||
| Solitary tumour | |||||
| No | 19 (46%) | 29 (66%) | 0.1 | 30 (68%) | 0.06 |
| Yes | 22 (54%) | 15 (34%) | 14 (32%) | ||
| Median follow-upd | 52 months | 96 months | NA | 84 months | NA |
| Median overall survival, months (95% CI) | 59 (40–92) | 35 (17–43) | <0.01 | 35 (18–43) | 0.04 |
| Median recurrence-free survival, months (95% CI) | 17 (14–30) | 14 (9–25) | 0.1 | 11 (8–26) | 0.07 |
CLM, colorectal liver metastasis; Continuous variables are expressed as median (range); categorical variables are expressed as n (%); NA, not analysed; POD, post-operative day. PT, prothrombin time; INR, International Normalized Ratio; CI, confidence interval; CEA, carcinoembryonic antigen.
Comparison of the CH and right EH groups.
Comparison of the CH and combined EH groups.
Max – maximal bilirubin or INR levels from post-operative day 1 through to day 7.
Follow-up for survivors.
Oncological outcome for patients with CLM
CLM was the most common diagnosis with 70% of the patients in the cases and controls being treated for this diagnosis (44 patients in each group, Table1). Among the patients with CLM, no differences were noted in the R0 resection rates, the actual margin width, or other prognostic factors between the CH group and the EH groups (Table2). The median OS was shorter in the right EH group (35 months, P < 0.01) and combined EH group (35 months, P = 0.04) compared with the CH group (59 months). A similar trend was observed with the median RFS in the CH group (17 months) compared with the right EH group (14 months, P = 0.1) and the combined EH groups (11 months, P = 0.07). The better OS in the CH group may be explained in part by the non-statistical significant trend towards smaller and more often solitary tumours in the CH group although the Clinical risk score and other prognostic factors such as nodal status of primary cancer and CEA levels were comparable between the groups.
Surgical volume
In analysing our CH volume with respect to EH cases, three time periods were analysed (1992–1998, 1999–2004 and 2005–2013). There was a gradual increase in the number of CH performed with 44% of all the CH being performed in the latest period whereas the number of right EH performed decreased (Fig.2). The CH group and the EH groups did not differ in their 90-day morbidity rates when stratified by time periods (Table3).
Figure 2.
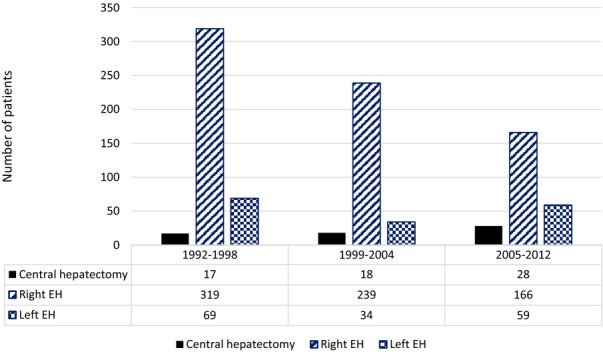
Volume and period of surgery of the cases (CH) and extended hepatectomy (EH) groups
Table 3.
90-day morbidity of the case (CH) and extended hepatectomy (EH) groups stratified by time periods
| Time period | CH group (n = 63) | Right EH group (n = 63) | P-value | Combined EH group (n = 63) | P-value |
|---|---|---|---|---|---|
| 1992–1998 (n = 68) | 11 (65%) | 18 (72%) | 0.7 | 16 (62%) | 0.8 |
| 1999–2004 (n = 79) | 9 (50%) | 17 (53%) | 1.0 | 15 (52%) | 0.9 |
| 2005–2012 (n = 42) | 7 (25%) | 2 (33%) | 0.6 | 4 (50%) | 0.2 |
Discussion
Surgical choices for centrally located liver tumours are limited. The traditional right and left EH, which sacrifices the greater part of the liver parenchyma, harbours substantial risks of morbidity, liver failure and mortality.6 CH is an attractive procedure because it removes the tumour-bearing segments in entirety while preserving the non-tumour liver.5,29 This is especially relevant for patients with limited functional liver reserve such as those with cirrhosis or those with chemotherapy-associated hepatotoxicity. However, CH can be more technically challenging than the conventional EH. As a CH involves two transection planes there is a need for preservation of the bilateral peripheral segments and its vasculature which can be technically challenging especially in the presence of a large and/or a deep-seated tumour in the central portion of the liver. This study, which encompasses 23 years of experience at Memorial Sloan Kettering Cancer Center, is the first matched case-control comparison of CH and EH.
In the present study, the control groups were selected through a comprehensive matching algorithm based on pre-operative clinical characteristics pertaining to clinical outcomes. No differences in baseline characteristics were observed between the CH and the control EH groups, except for a minor and clinically irrelevant (0.5 cm) difference in tumour size. However, a trend was noted towards a lower 90-day morbidity rate in the CH group, which was accompanied by a significantly lower intra-operative blood loss and a shorter length of hospital stay. Our findings are in line with the results reported by Qiu et al.30 who observed that CH was associated with lower morbidity rates compared with non-matched EH cases.
For patients with liver disease considered for a major resection, pre-operative PVE is an option to induce hypertrophy of the FLR in an effort to minimize the risk of PHLF, it also serves as a dynamic test of the liver's regeneration capacity.31–33 Ipsilateral PVE is a feasible pre-operative strategy facilitating extended or staged resections. However, PVE is associated with some risk of morbidity and livers with limited functional reserve may have a lower than expected response to PVE.34 Thus, CH, as a parenchymal-preserving technique, may obviate the need for PVE and avoid its associated morbidity. These considerations are reflected by our findings that none of the CH group patients had undergone pre-operative PVE, whereas 18% of the EH groups required a pre-operative PVE.
Post-hepatectomy liver failure is a major concern and a leading cause of morbidity and mortality in major liver resections especially in patients with prior liver dysfunction (e.g. cirrhosis in HCC patients or steatohepatitis in post-chemotherapy CLM patients).31,33 The present study did not demonstrate mortality differences between the matched groups of CH and EH patients. However, differences were observed with markers of liver dysfunction. Compared with the EH groups, the CH group demonstrated significantly lower bilirubin levels throughout the hospitalization and lower peak INR levels. These data suggest that patients undergoing CH may be less susceptible to liver dysfunction compared with EH.
As an observational and retrospective study, this analysis has inherent selection limitations and the generalizability of these results might be restricted to specialized high-volume centres. However, this is the first matched study to compare CH and EH groups with a separate subgroup analysis on resection for CLM. The previous studies in the literature are mostly small retrospective Asian single-centre studies performed more than a decade ago, focusing mainly on HCC without a comparable or matched control group, as the CH cohort in most of these studies was compared to a combined group that included both lobar and extended hepatectomies and patients' clinicopathological factors were not comparable.5,10,18 This study is limited by its modest sample size as CH was not previously a common procedure in our practice. Admittedly, some but not all of tumours in the EH control groups were likely amenable to CH. Furthermore, the volumetric assessment was not performed and, therefore, comparisons may have been biased. This unique study design was enabled by the statistical power of our prospectively maintained database that included 4661 liver resections. The matching algorithm reduced selection factors and strengthened our findings. An additional exclusive feature of the present study is the predominance of CLM in our patients, as opposed to previous reports, which focused on HCC patients.18
In conclusion, CH is safe and associated with a shorter hospital stay and less post-operative liver dysfunction in a subset of carefully selected patients. This approach does not seem to compromise survival outcomes in patients with colorectal liver metastases. CH should be considered in all patients with centrally located tumours amenable to such a resection.
Conflicts of interest
None declared.
Funding sources
This study was funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg. 2011;253:656–665. doi: 10.1097/SLA.0b013e3181fc08ca. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XP, Qiu FZ, Lau WY, Zhang BX, Chen YF, Zhang WG, et al. Mesohepatectomy for hepatocellular carcinoma: a study of 256 patients. Int J Colorectal Dis. 2008;23:543–546. doi: 10.1007/s00384-007-0411-y. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Yu MC, Wu TH, Lee CF, Chan KM, Chou HS, et al. Surgical resection of centrally located large hepatocellular carcinoma. Chang Gung Med J. 2012;35:178–191. doi: 10.4103/2319-4170.106153. [DOI] [PubMed] [Google Scholar]
- Stratopoulos C, Soonawalla Z, Brockmann J, Hoffmann K, Friend PJ. Central hepatectomy: the golden mean for treating central liver tumors? Surg Oncol. 2007;16:99–106. doi: 10.1016/j.suronc.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Baer HU, Guastella T, Blumgart LH. Comparison of outcome between extended and nonextended liver resections for neoplasms. Surgery. 1993;114:968–975. [PubMed] [Google Scholar]
- Wanebo HJ, Chu QD, Vezeridis MP, Soderberg C. Patient selection for hepatic resection of colorectal metastases. Arch Surg. 1996;131:322–329. doi: 10.1001/archsurg.1996.01430150100019. [DOI] [PubMed] [Google Scholar]
- Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;3:333–339. [Google Scholar]
- Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257:377–382. doi: 10.1097/SLA.0b013e31825a01f6. [DOI] [PubMed] [Google Scholar]
- Wu CC, Ho WL, Chen JT, Tang CS, Yeh DC, Liu TJ, et al. Mesohepatectomy for centrally located hepatocellular carcinoma: an appraisal of a rare procedure. J Am Coll Surg. 1999;188:508–515. doi: 10.1016/s1072-7515(99)00026-5. [DOI] [PubMed] [Google Scholar]
- Chen XP, Zhang ZW, Zhang BX, Chen YF, Huang ZY, Zhang WG, et al. Modified technique of hepatic vascular exclusion: effect on blood loss during complex mesohepatectomy in hepatocellular carcinoma patients with cirrhosis. Langenbecks Arch Surg. 2006;391:209–215. doi: 10.1007/s00423-006-0043-7. [DOI] [PubMed] [Google Scholar]
- Scudamore CH, Buczkowski AK, Shayan H, Ho SG, Legiehn GM, Chung SW, et al. Mesohepatectomy. Am J Surg. 2000;179:356–360. doi: 10.1016/s0002-9610(00)00374-3. [DOI] [PubMed] [Google Scholar]
- Lee JG, Choi SB, Kim KS, Choi JS, Lee WJ, Kim BR. Central bisectionectomy for centrally located hepatocellular carcinoma. Br J Surg. 2008;95:990–995. doi: 10.1002/bjs.6130. [DOI] [PubMed] [Google Scholar]
- Liau KH, Blumgart LH, DeMatteo RP. Segment-oriented approach to liver resection. Surg Clin North Am. 2004;84:543–561. doi: 10.1016/j.suc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Wu CC, Yeh DC, Ho WM, Yu CL, Cheng SB, Liu TJ, et al. Occlusion of hepatic blood inflow for complex central liver resections in cirrhotic patients: a randomized comparison of hemihepatic and total hepatic occlusion techniques. Arch Surg. 2002;137:1369–1376. doi: 10.1001/archsurg.137.12.1369. [DOI] [PubMed] [Google Scholar]
- Hu RH, Lee PH, Chang YC, Ho MC, Yu SC. Treatment of centrally located hepatocellular carcinoma with central hepatectomy. Surgery. 2003;133:251–256. doi: 10.1067/msy.2003.102. [DOI] [PubMed] [Google Scholar]
- Chouillard E, Cherqui D, Tayar C, Brunetti F, Fagniez PL. Anatomical bi- and trisegmentectomies as alternatives to extensive liver resections. Ann Surg. 2003;238:29–34. doi: 10.1097/01.sla.0000075058.37052.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY. Central hepatectomy for malignant liver tumors: a systematic review. World J Hepatol. 2014;6:347–357. doi: 10.4254/wjh.v6.i5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Laan MJ. Why match? Investigating matched case–control study designs with causal effect estimation. Int J Biostat. 2009;5:Article 1. doi: 10.2202/1557-4679.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenjo A, Miyata H, Gotoh M, Kitagawa Y, Shimada M, Baba H, et al. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg. 2014;218:412–422. doi: 10.1016/j.jamcollsurg.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB. 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Ito H, Are C, Allen PJ, Fong Y, DeMatteo RP, et al. Laparoscopic versus open liver resection: a matched-pair case control study. J Gastrointest Surg. 2009;13:2276–2283. doi: 10.1007/s11605-009-0993-5. [DOI] [PubMed] [Google Scholar]
- Billingsley KG, Jarnagin WR, Fong Y, Blumgart LH. Segment-oriented hepatic resection in the management of malignant neoplasms of the liver. J Am Coll Surg. 1998;187:471–481. doi: 10.1016/s1072-7515(98)00231-2. [DOI] [PubMed] [Google Scholar]
- DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4:178–184. doi: 10.1016/s1091-255x(00)80054-2. [DOI] [PubMed] [Google Scholar]
- Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–364. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. , discussion 828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng KS, Jeng WJ, Sheen IS, Lin CC, Lin CK. Is less than 5 mm as the narrowest surgical margin width in central resections of hepatocellular carcinoma justified? Am J Surg. 2013;206:64–71. doi: 10.1016/j.amjsurg.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Qiu J, Wu H, Bai Y, Xu Y, Zhou J, Yuan H, et al. Mesohepatectomy for centrally located liver tumours. Br J Surg. 2013;100:1620–1626. doi: 10.1002/bjs.9286. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kluger MD. Cherqui D. Surgical management of hepatocellular carcinoma. In: Kee S, Murthy R, Madoff D, editors; Clinical Interventional Oncology: Management and Practice. Vol. 1. Philadelphia, PA: Elsevier; 2014. pp. 66–76. [Google Scholar]
- Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golse N, Bucur PO, Adam R, Castaing D, Sa Cunha A, Vibert E. New paradigms in post-hepatectomy liver failure. J Gastrointest Surg. 2013;17:593–605. doi: 10.1007/s11605-012-2048-6. [DOI] [PubMed] [Google Scholar]
- van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013;36:25–34. doi: 10.1007/s00270-012-0440-y. . [DOI] [PMC free article] [PubMed] [Google Scholar]


