Abstract
Background
A post-operative pancreatic fistula (POPF) is a major cause of morbidity and mortality after a pancreaticoduodenectomy (PD). This systematic review aimed to identify all scoring systems to predict POPF after a PD, consider their clinical applicability and assess the study quality.
Method
An electronic search was performed of Medline (1946–2014) and EMBASE (1996–2014) databases. Results were screened according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and quality assessed according to the QUIPS (quality in prognostic studies) tool.
Results
Six eligible scoring systems were identified. Five studies used the International Study Group on Pancreatic Fistula (ISGPF) definition. The proposed scores feature between two and five variables and of the 16 total variables, the majority (12) featured in only one score. Three scores could be fully completed pre-operatively whereas 1 score included intra-operative and two studies post-operative variables. Four scores were internally validated and of these, two scores have been subject to subsequent multicentre review. The median QUIPS score was 38 out of 50 (range 16–50).
Conclusion
These scores show potential in calculating the individualized patient risk of POPF. There is, however, much variation in current scoring systems and further validation in large multicentre cohorts is now needed.
Introduction
A pancreaticoduodenectomy (PD) remains the operation of choice for the majority of malignant and benign neoplasms of the head of the pancreas and periampullary region. Despite the centralization of PD to high-volume tertiary centres resulting in a reduction in mortality from 14% to 2%,1–4 post-operative morbidity remains significant at 30–50%.5
A post-operative pancreatic fistula (POPF) occurs in 5–30% of patients.6 This is associated with serious sequelae including sepsis, haemorrhage and death, with resulting prolonged hospital stays and increased healthcare costs.1,6 The incidence of POPF has remained fairly constant over the past 30 years even in experienced centres.7,8
The risk factors for POPF have been extensively analysed and published,2,5,6,9–14 more recently abetted by the wide acceptance of the POPF definition from the International Study Group on Pancreatic Fistula (ISGPF).5 Based on these risk factors, a number of groups have now proposed the use of predictive scores to stratify a patient's risk of developing a POPF. A predictive score would allow clinicians to consent accurately patients on their individual risk of developing a POPF, as well as directing post-operative management such as early drain removal and, therefore, promoting enhanced recovery.
The aim of this systemic review was to identify all scoring systems proposed to predict the likelihood of a POPF after a PD, consider their clinical applicability and assess the study quality.
Methods
Study design and identification
A search strategy was designed to identify all clinical studies proposing a score to predict the likelihood of a POPF after a PD. An electronic search was performed of the MEDLINE and EMBASE database for the period 1946 (MEDLINE) and 1996 (EMBASE) to December 2014. The references from the included studies were searched to identify additional studies.
Records extracted by the initial search were screened, and potentially relevant papers were retrieved and assessed in more detail according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance.15 Eligibility assessment was performed by two authors (A.E.V. and A.L.Y.) independently. Any disagreement over study inclusion was resolved by discussion with the senior author (A.M.S.).
The following search strategy was used (pylorus preserv* OR Whipple* OR pancreat* resection, pancreatojejunostom* OR pancreatoduodenectomy* OR pancreaticoduodenectom* OR duodenopancreatectom*, pancreatectom*) AND [(risk assessment OR scoring system OR prognostic score OR decision support techniques) OR (pancrea* fistula OR pancrea* leak OR anastomotic leak)].
Inclusion criteria
Studies were included if they proposed a novel scoring system to predict the development of POPF. The search was limited to English-language publications in human subjects. Only studies exclusively investigating PD in adult patients (aged ≥18 years) were included. The minimum data necessary for inclusion was a description of POPF definition and the proposed scoring system.
Exclusion criteria
Abstracts (such as those published as part of conference proceedings), reviews and case reports were excluded as were studies including additional types of pancreatic resection in the modelling database.
Quality assessment and data extraction
The quality of each study was assessed using the QUIPS (quality in prognosis studies) tool.16 This involves the scoring of five domains of potential bias (study participation, prognostic factor measurement, outcome measurement, confounding factor measurement and analysis) according to whether it is quality limits potential bias. A score of 2 indicates that it does; a score of 1 indicates that it does so ‘partly’, and a score of 0 indicates that it does not. Data were extracted independently by the authors and disagreements resolved by discussion with the senior author.
Results
Search results
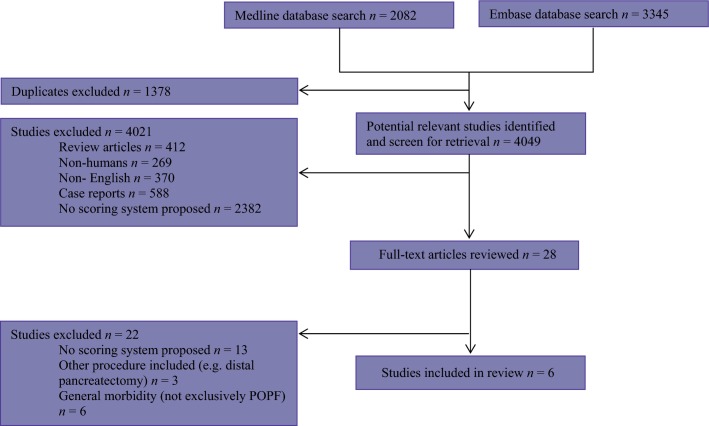
The electronic search yielded 2082 hits in MEDLINE and 3345 hits in EMBASE and is detailed in Fig.1. A total of six studies met the eligibility criteria and were included in the review.
Figure 1.
Search strategy used to identify included studies
Summary of selected study characteristics
All six studies were published since 2009. These studies (3 from Europe, 1 Asia and 2 USA) utilized retrospective data from a single institution over a period of 1–5 years and included between two and five variables in the eventual proposed score. Sixteen variables in total were proposed with one variable (age) present in two scores and three variables [body mass index (BMI), pancreatic duct width and pathological diagnosis] present in three scores. Three scores could be calculated from pre-operative variables alone whereas one required intra-operative variables and two included post-operative variables.
The median number of patients included in the score modelling group in each study was 172 (range 62–279) and the median rate of POPF in the modelling databases was 31% (range 22–53%). Five studies used the ISGPF definition of POPF of which three included all grades whereas the remaining two included only Grades B and C. There was no validation performed in two studies, whereas in four studies the dataset was divided into a derivation and validation set to perform internal validation. In two studies, the scoring system has subsequently been applied to an external patient cohort. Table1 summarizes the scoring systems predicting a POPF after a PD and Table2 details the variables included in each of these studies.
Table 1.
Current published scoring systems predicting POPF following PD
| Author and year of publication (Time period of data collection) | Patients in modelling cohort | Outcome of interest | Proportion of modelling cohort developing outcome (%) | Proposed score scale | Predicative accuracy | Internal validation – predictive accuracy (N) | External validation – predictive accuracy (N) |
|---|---|---|---|---|---|---|---|
| Gaujoux et al.25 2004–2005 | 100 | ISGPF grade A–C and B–C | 31 (31) A–C 27 (27) B–C | 0–3 | AUC 0.78 (A–C) AUC 0.81 (B–C) | Not done | Not done |
| Wellner et al.17 2006–2008 | 62 | ISGPF grade A–C | 19 (31) | −3 to 2 | SR correlation coefficient = 0.47 | SR correlation coefficient = 0.35 (279) | Not done |
| Yamamoto et al.18 2004–2009 | 279 | ISGPF grade B–C | 103 (37) | 0–7 | AUC 0.810 | AUC 0.808 (108) | Not done |
| Callery et al.22 2002–2007 | 233 | ISGPF grade A–C | 58 (24.7) A–C | 0–10 | Not stated | AUC 0.942 (212) | AUC 0.716 (594) AUC 0.763 (265) |
| Graham et al.26 2007–2012 | 146 | Drain amylase >3× normal serum amylase on or after POD 4 | 50 (34) | Continuous 0–100% | 72% sensitivity 81.3% specificity | Not done | Not done |
| Roberts et al.20 2007–2012 | 217 | ISGPF grade A–C | 48 (22.1) | Continuous 0–100% | AUC 0.832 | AUC 0.751 (108) | Score predictive (P < 0.001) (630) |
SR, Spearman rank; AUC, area under curve.
Table 2.
Variables included in each score with the total number of appearances in the right column. White background denotes pre-operative variables, light grey intra-operative variables and dark grey post-operative variables
| Wellner et al. (2010) 17 | Callery et al. (2013) 22 | Roberts et al. (2014) 20 | Gaujoux et al. (2009) 25 | Yamamoto et al. (2011) 18 | Graham et al. (2013) 26 | Total | |
|---|---|---|---|---|---|---|---|
| Age | • | • | 2 | ||||
| Gender | • | 1 | |||||
| Smoking history | • | 1 | |||||
| BMI | • | • | • | 3 | |||
| Weight loss | • | 1 | |||||
| IA fat thickness | • | 1 | |||||
| Pancreatitis history | • | 1 | |||||
| Relation of PV to tumour | • | 1 | |||||
| Diagnosis | • | • | • | 3 | |||
| PD width (radiological) | • | • | • | 3 | |||
| Blood loss | • | 1 | |||||
| Pancreatic texture | • | 1 | |||||
| PD width (intra-operative) | • | 1 | |||||
| Pancreatic fat | • | 1 | |||||
| Pancreatic fibrosis | • | 1 | |||||
| Drain amylase (POD2) | • | 1 |
BMI, body mass index; IA, intra-abdominal; PV, portal vein; PD, pancreaticoduodenectomy; POD, post-operative day.
Pre-operative models
Wellner (2010)
The score from Wellner et al. was formed by first establishing peri-operative predictors of POPF and extrapolating these to devise a score that may be completed pre-operatively.17 A history of weight loss and soft pancreatic texture (as measured intra-operatively) were found to predict strongly a POPF. Subsequent univariate analysis demonstrated age less than 66 years and a history of acute pancreatitis or smoking independently predicted a firm pancreatic texture. The final POPF score, therefore, included these pre-operative predictors of texture. The final score allowed patients to be subdivided into low-, moderate- or high-risk groups for POPF. When the score was internally validated, the incidence of POPF significantly correlated with each of the three patient risk groups (correlation coefficient r = 0.35).
Yamamoto (2011)
The score proposed by Yamamoto et al. included patient gender and four measurements from pre-operative CT images: the main pancreatic duct (MPD) index (defined as the ratio of the diameter of MPD to the diameter of the short axis of the pancreatic body), relation of the portal vein to the tumour (attached/ compressed/ involved), intra-abdominal fat thickness and a diagnosis other than pancreatic cancer.18 Variables were assigned a value based on the magnitude of the beta-coefficients identified from the regression analysis. A stratum-specific likelihood ratio (SSLR), which indicates to what degree a diagnostic test result increases or decreases the pre-test probability of POPF,19 showed that the SSLR values at the score extremes indicated moderate to large changes in the likelihood of POPF.
Roberts (2014)
Roberts et al. constructed a score based on only two variables, body mass index (BMI) and the pre-operative radiological measurement of the pancreatic duct width.20 The score has been made available online (http://www.uhb.nhs.uk/preoperative-prediction-of-pancreatic-fistula-calculator.htm). The authors found that patients with a pancreatic duct of >10 mm regardless of BMI, have a risk of <5% and patients with a BMI of 30–35 kg/m2 with a pancreatic duct <3 mm have a 30–55% risk of POPF. This score was subsequently externally validated upon a multicentre cohort.21 Attempts to improve the score were not possible, despite the inclusion of intra- or post-operative variables. The score demonstrated a significant stepwise increase with more severe types of POPF.
Intra-operative models
Callery (2013)
Callery et al. devised a score selected from three potential models, each examined prospectively on a validation cohort.22 The intra-operative assessment of gland texture (firm/ soft) and pancreatic duct diameter (as measured with a scale at the cut surface), pre-operative diagnosis and blood loss were included as score components. Variables in Model I were weighted equally which was felt to be overly simplistic and, therefore, Model II assigned points according to the magnitude of the beta-coefficients identified from the regression analysis. This model allowed pancreatic duct diameter and intra-operative blood loss to be analysed as continuous variables although the complexity was felt to act potentially as a barrier to widespread use. Model III, resembling model II but designed to be more practical with a scale of 0–10, also demonstrated the best performance in internal validation. No patient with a score of 0 developed POPF whereas all patients with scores of 9–10 developed clinically relevant POPF. This score has since been applied to two external patient cohorts. Millers group (2014) scored 594 patients from three institutions, finding the fistula risk score to correlate with grade B and C POPF development (P < 0.001) with an area under the curve (AUC) of 0.716.23 Kunstman et al. calculated this fistula risk score for 265 PD patients and demonstrated a 1.6-fold increase in grade B and C POPF per 1-point increase in risk score, a negative predictive value of 100% in patients with a low score (<3) and a similar AUC of 0.763.24 However, of the patients with a high score7–10 only 29% (Miller) and 16.7% (Kunstman) developed a POPF.
Post-operative models
Gaujoux (2009)
Gaujoux et al. were the first group to propose a predictive scoring system.25 They identified that a BMI ≥25 kg/m2, the absence of pancreatic fibrosis and the presence of fatty pancreas were significant predictors on multivariable logistic regression analysis of POPF.25 Pancreatic steatosis and fibrosis were examined histologically by independent and blinded assessors. The proposed score was based on the number of risk factors present (0–3). No further validation was performed.
Graham (2013)
The score devised by Graham et al. is another available to use online (http://georgetowncriteria.tumblr.com/).26 Beta-coefficients from the covariates (age, BMI, post-operative day 2 drain amylase and the intra-operative measure of pancreatic duct diameter) were formed to devise a formula to predict the likelihood of POPF. This model was optimized to give an optimum balance of sensitivity to specificity on the author's original dataset but no further validation occurred.
Quality assessment
The median QUIPS score for the included studies was 38.5 (range 16–45) (Table3). The domain at the highest risk of bias was confounding factor measurement with a median score of 8 out of a possible 14 (range 1–12).
Table 3.
Quality assessment of included studies according to the quality in prognosis studies (QUIPS) tool
| Median quality score (range) | Maximum score | |
|---|---|---|
| Study participation | 9.5 (2–10) | 10 |
| Population described for key characteristics | 2 | 2 |
| Sampling and recruitment described | 2 | 2 |
| Inclusion/exclusion criteria described | 2 | 2 |
| Adequate participation | 2 | 2 |
| Baseline study sample described | 2 | 2 |
| Prognostic factor measurement | 8 (5–10) | 12 |
| Prognostic factors are clearly described or defined | 1.5 | 2 |
| Continuous variables reported, or appropriate cut-off points used | 1.5 | 2 |
| Prognostic factors are valid and reliable | 2 | 2 |
| Study sample has complete data for prognostic factors | 2 | 2 |
| Same method and setting of measurement for all study participants | 2 | 2 |
| Appropriate methods used for missing prognostic data | 0 | 2 |
| Outcome measurement | 6 (5,6) | 6 |
| Definition of the outcome described | 2 | 2 |
| Outcome measure and method are valid and reliable | 2 | 2 |
| Same method and setting of measurement for all study participants | 2 | 2 |
| Confounding factor measurement | 8 (1–12) | 14 |
| Important confounders are measured | 1 | 2 |
| Clear definitions of confounders described | 2 | 2 |
| Measurement of all confounders is valid and reliable | 1 | 2 |
| Same method and setting of measurement for all study participants | 1.5 | 2 |
| Appropriate methods used for missing confounder data | 0 | 2 |
| Important confounders accounted for in the study design | 1.5 | 2 |
| Important confounders accounted for in the analysis | 1 | 2 |
| Analysis | 6 (3–8) | 8 |
| Sufficient presentation of data to assess the adequacy of the analysis | 1.5 | 2 |
| Strategy for model building appropriate and based on a conceptual model | 1.5 | 2 |
| Adequate selection of model for the design of the study | 2 | 2 |
| No selective reporting of results | 2 | 2 |
| Median quality score of studies | 38 (16–45) | 50 |
Discussion
Clinical application
Risk stratification tools systematically classify patients to a level of risk and have an established role in modern surgical practice, heralded by the widespread use of the American Society of Anesthesiologists (ASA) grading system.27,28 Scores have been developed to predict the general risk of peri-operative morbidity and mortality after pancreatic surgery. These include the Nebraska Nomogram,29 the John Hopkins mortality model30 and the HPB risk calculator,3 and a degree of risk stratification occurs routinely in many institutions by the use of exercise testing such as cardiopulmonary exercise testing (CPET).31,32 Although these scores may act as a general guide to the clinician they are not applicable to individual complications.
In the era of patient centred care, strategies to quantify the risk of a POPF after a PD are highly desirable. The systems by Yamamoto, Wellner and Roberts comprise of easily measurable radiological and demographic variables and may be constructed pre-operatively.17,18,20 Pre-operative risk stratification has certain advantages including the opportunity to individualize patient consent. Whether this information would affect patient or clinician decisions to proceed then or not with surgery is not known. Although it is thought that a surgeon's ‘gut feeling’ performs equally accurately to existing models of morbidity, predictive models can add objectivity to these decisions.33 These scores highlight that the risk of POPF is very strongly modified by patient variables. There are also clear academic applications of pre-operative risk scores. Most studies reviewing the impact of various interventions have failed to demonstrate a significant reduction in POPF with the proposed treatments. If patients with a predicted low risk of POPF were to be excluded from clinical trials, the numbers of participants needed within the study could be lowered while increasing the likelihood of observing a treatment effect. In addition, understanding a patient's risk of POPF may inform resource planning for the health care provider. The management of a patient with a grade C fistula will cost around six times more than a patient with no complications after PD ($119053 and $18075, respectively).1
The technique of pancreatic reconstruction is not included in any score reflecting current evidence that fails to demonstrate consistently the optimum reconstructive technique of a pancreatoenterostomy.34 Furthermore, evidence of the impact of other interventions, such as internal or external pancreatic stents and octreotide, upon POPF is conflicting (suggesting that patient-related factors are more critical in the causation of POPF.35–41 With the absence of effective strategies to decrease POPF, it can be argued that these prognostic scores have no clinical usefulness. However, although recent evidence suggests that drains should not be omitted post-operatively even in low-risk patients, these patients may be suitable for early drain removal.42,43 This has been shown to reduce intra-abdominal infections in patients without symptoms of POPF,44,45 allowing for expedited mobilization to potentially reduce the length of stay thus forming part of an enhanced recovery programme.45,46 Callery's group advocate placement of at least two post-operative drains in patients who accrue a high intra-operative risk score and may omit drains altogether in patients with a low fistula risk score.22 They also consider the use of feeding jejunostomy tubes in elderly patients with a moderate-to-high post-operative risk score.22 The score by Graham et al., who take a conservative approach to the management of POPF by instigating at least 4 weeks of drainage and parenteral nutrition, may be valuable in managing patient expectation with regard to length of stay and the start of adjuvant therapy.26
Risk stratification of a POPF may have a role in facilitating training opportunities. A PD is considered one of the most technically demanding surgical procedures and is a challenging operation for both trainers to teach and trainees to learn.47,48 Recognizing patients at a lower risk of POPF may permit the trainee to perform the pancreatoenteric anastomosis, and conversely an experienced pancreatic surgeon may choose to construct this anastomosis in a high-risk patient. In addition, the adoption of a reliable tool to calculate individual patient risk would establish surgical performance more accurately by allowing a correction to be made for centres and surgeons that treat more high-risk patient groups.
Extrapolating intra-operative variables to form a pre-operative score
A soft pancreas is a widely accepted risk factor for POPF6,9,49–51 and if it were not an intra-operative measurement would likely feature more prominently in predictive scoring systems. The relationship between pancreatic texture and other covariables is used in the pre-operative score proposed by Wellner et al. which utilized factors related to gland texture as surrogate risk factors for POPF.17
Pancreatic steatosis has been independently implicated in the development of POPF25,50 as well as being associated with a high BMI and increasing age.25,51,52 Despite featuring in the score by Ganjoux et al., the clinical application of including the histologically measured pancreatic fat infiltration in a predictive scoring system is limited. The radiological quantification by pancreatic steatosis via computerized tomography (CT),53 magnetic resonance imaging (MRI)54,55 and ultrasound (US)56 has shown promise although none of the scores utilize these assessments.
Pancreatic duct width is strongly (inversely) related to POPF risk6,11,17,57–59 and was included in over half the scoring systems reviewed. Pre-operative measurement using CT was utilized in three scores11,20 and intra-operative assessment in 1.22 Using an intra-operative measurement of pancreatic duct width has not been shown to increase the accuracy of POPF prediction as demonstrated when substituted for the pre-operative radiological measurement in the scoring system by Roberts et al.21 Yamamoto et al. found the MPD index to be the strongest predictor of the occurrence of POPF and Callery et al. report each 1 mm decrease of the pancreatic duct width to result in a 76% increase in the likelihood of developing POPF.11,22 Roberts et al. suggests that PD width should be considered as a continuous variable as considering duct width as narrow or wide (usually based on a cut off of 3 mm) underplays the significant association between duct width and POPF. They also report a strong correlation between the pre-operative radiological interpretation of pancreatic duct width by surgeons and hepatobiliary radiologists suggesting this measurement could be easily and accurately performed in the outpatient setting.20
Radiological prediction
With the average PD patient undergoing at least one pre-operative radiological investigation in conjunction with a multi-disciplinary team review of these images, the use of imaging as a component of pre-operative fistula prediction appears to be ideal. The utility of CT to predict the occurrence of POPF has been recently reported57,60,61 and imaging forms a key component of the score proposed by Yamamoto et al. where four of five variables are radiological findings.18
Further scores to predict POPF
Additional scores have been proposed to predict POPF in patients undergoing a general, pancreatic resection. These authors include a distal pancreatectomy, middle pancreatectomy and partial resections in the modelling cohorts. Belyaev et al. proposed a score based on histomorphological features of the pancreatic remnant62 and Fujiwara et al. proposed a post-operative inflammatory score based on serum albumin and c-reactive protein on day one after a resection.63 In addition, several authors have examined the use of pre-established prognostic indicators in predicting POPF including CPET testing64 and the surgical Apgar score,31 demonstrating a low and high result, respectively, to be associated with POPF.
Quality assessment and study limitations
The quality of included studies was variable, and two studies failed to describe the baseline characteristics of the modelling population adequately. All studies when appropriate attempted to limit bias by blinding radiology and histology assessors to patient outcome; however, other sources of potential bias, such as the subjective nature of the assessment of texture of the pancreatic gland intra-operatively were not controlled. There was also a disparity in the use of continuous variables with sometimes no justification into the cut-off values used in the conversion of continuous to categorical values. Two scoring systems lacked internal validation so the reproducibility of these scores, even in a similar patient cohort (as applied in the remaining four studies), is not known. Frequently the use of potential confounders such as octreotide and stents was not adequately described, accounting for the low overall scores in this bias assessment domain.
The use of non-independent variables in the scores raises the issue of multicollinearity. Multicollinearity can increase the variance of the coefficient estimates and make the estimates very sensitive to minor changes in the model.65 Both BMI and pancreatic fat that are known to be closely correlated are included in the score by Ganjoux et al.25,50,66 The score proposed by Callery et al. again includes non-independent variables such as pancreatic duct width and gland firmness.21,22
Despite the majority of studies analysing similar variables in the initial analysis, there were inconsistencies in those identified as independent predictors of POPF and, therefore, included in the final score. Although there is no doubt that POPF is a multifactorial condition, the disparities between the proposed scoring systems suggest that particular variables may be more significant in certain patient cohorts than others. Sample size may account for these differences as although several studies included a large number of patients, the actual number of these patients who developed POPF, particularly when split into subgroups based on fistula grade, was often small. Also, institutional differences in patient selection, surgical technique and post-operative management may account for these differences between patient cohorts.
Conclusion
These six scores demonstrate a variation in approach to predict POPF. The prospective validation of these scores in a large external multicentre cohort is now required to allow direct comparison of score validity. Although individualized patient complication prediction after a PD is in the development stage, an individualized assessment of the risk of POPF is now possible and may be used as part of informed consent.
Funding sources
None.
Conflict of interest
None to declare.
References
- Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM. Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245:443–451. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D, Kelly K, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–2135. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- Kneuertz P, Pitt H, Bilimoria K, Smiley J, Cohen M, Ko C, et al. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg. 2012;16:1727–1735. doi: 10.1007/s11605-012-1938-y. [DOI] [PubMed] [Google Scholar]
- Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Pratt W, Callery M, Vollmer C. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–428. doi: 10.1007/s00268-007-9388-5. [DOI] [PubMed] [Google Scholar]
- Poon RTP, Lo SH, Fong D, Fan ST, Wong J. Prevention of pancreatic anastomotic leakage after pancreaticoduodenectomy. Am J Surg. 2002;183:42–52. doi: 10.1016/s0002-9610(01)00829-7. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Cameron J, Yeo C, Riall T, Lillemoe K. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–959. doi: 10.1016/j.gassur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Sato N, Yamaguchi K, Chijiiwa K, Tanaka M. Risk analysis of pancreatic fistula after pancreatic head resection. Arch Surg. 1998;133:1094–1098. doi: 10.1001/archsurg.133.10.1094. [DOI] [PubMed] [Google Scholar]
- Kawai M, Kondo S, Yamaue H, Wada K, Sano K, Motoi F, et al. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18:601–608. doi: 10.1007/s00534-011-0373-x. [DOI] [PubMed] [Google Scholar]
- Akamatsu N, Sugawara Y, Komagome M, Shin N, Cho N, Ishida T, et al. Risk factors for postoperative pancreatic fistula after pancreaticoduodenectomy: the significance of the ratio of the main pancreatic duct to the pancreas body as a predictor of leakage. J Hepatobiliary Pancreat Sci. 2010;17:322–328. doi: 10.1007/s00534-009-0248-6. [DOI] [PubMed] [Google Scholar]
- Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Onishi S, Hanazaki K. Risk factors, predictors and prevention of pancreatic fistula formation after pancreatoduodenectomy. J Hepatobiliary Pancreat Surg. 2007;14:557–563. doi: 10.1007/s00534-007-1242-5. [DOI] [PubMed] [Google Scholar]
- Parikh P, Shiloach M, Cohen ME, Bilimoria KY, Ko CY, Hall BL, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB. 2010;12:488–497. doi: 10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG The Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- Wellner UF, Kayser G, Lapshyn H, Sick O, Makowiec F, Hoppner J, et al. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB. 2010;12:696–702. doi: 10.1111/j.1477-2574.2010.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Sakamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2011;35:2747–2755. doi: 10.1007/s00268-011-1253-x. [DOI] [PubMed] [Google Scholar]
- Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature: III. How to use an article about a diagnostic test B. What are the results and will they help me in caring for my patients? JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- Roberts KJ, Hodson J, Mehrzad H, Marudanayagam R, Sutcliffe RP, Muiesan P, et al. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB. 2014;16:620–628. doi: 10.1111/hpb.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KJ, Sutcliffe RP, Marudanayagam R, Hodson J, Isaac J, Muiesan P, et al. Scoring System to Predict Pancreatic Fistula After Pancreaticoduodenectomy: A UK Multicenter Study. Ann Surg. 2015;261:1191–7. doi: 10.1097/SLA.0000000000000997. [DOI] [PubMed] [Google Scholar]
- Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM., Jr A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Miller B, Christein J, Behrman S, Drebin J, Pratt W, Callery M, et al. A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg. 2014;18:172–180. doi: 10.1007/s11605-013-2337-8. [DOI] [PubMed] [Google Scholar]
- Kunstman JW, Kuo E, Fonseca AL, Salem RR. Evaluation of a recently described risk classification scheme for pancreatic fistulae development after pancreaticoduodenectomy without routine post-operative drainage. HPB. 2014;16:987–993. doi: 10.1111/hpb.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaujoux S, Cortes A, Couvelard A, Noullet S, Clavel L, Rebours V, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15–23. doi: 10.1016/j.surg.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Graham JA, Kayser R, Smirniotopoulos J, Nusbaum JD, Johnson LB. Probability prediction of a postoperative pancreatic fistula after a pancreaticoduodenectomy allows for more transparency with patients and can facilitate management of expectations. J Surg Oncol. 2013;108:137–138. doi: 10.1002/jso.23362. [DOI] [PubMed] [Google Scholar]
- Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–284. [Google Scholar]
- Lewis RS, Jr, Vollmer CM., Jr Risk scores and prognostic models in surgery: pancreas resection as a paradigm. Curr Probl Surg. 2012;49:731–795. doi: 10.1067/j.cpsurg.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Are C, Afuh C, Ravipati L, Sasson A, Ullrich F, Smith L. Preoperative nomogram to predict risk of perioperative mortality following pancreatic resections for malignancy. J Gastrointest Surg. 2009;13:2152–2162. doi: 10.1007/s11605-009-1051-z. [DOI] [PubMed] [Google Scholar]
- Venkat R, Puhan MA, Schulick RD, Cameron JL, Eckhauser FE, Choti MA, et al. Predicting the risk of perioperative mortality in patients undergoing pancreaticoduodenectomy: a novel scoring system. Arch Surg. 2011;146:1277–1284. doi: 10.1001/archsurg.2011.294. [DOI] [PubMed] [Google Scholar]
- Assifi MM, Lindenmeyer J, Leiby B, Grunwald Z, Rosato E, Kennedy E, et al. Surgical Apgar score predicts perioperative morbidity in patients undergoing pancreaticoduodenectomy at a high-volume center. J Gastrointest Surg. 2012;16:275–281. doi: 10.1007/s11605-011-1733-1. [DOI] [PubMed] [Google Scholar]
- Junejo MA, Mason JM, Sheen AJ, Bryan A, Moore J, Foster P, et al. Cardiopulmonary exercise testing for preoperative risk assessment before pancreaticoduodenectomy for cancer. Ann Surg Oncol. 2014;21:1929–1936. doi: 10.1245/s10434-014-3493-0. [DOI] [PubMed] [Google Scholar]
- Hartley MN, Sagar PM. The surgeon's ‘gut feeling’ as a predictor of post-operative outcome. Ann R Coll Surg Engl. 1994;76:227–228. [PubMed] [Google Scholar]
- Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Huttner FJ, Antes G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2014;11:Cd006053. doi: 10.1002/14651858.CD006053.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor S, Alexakis N, Garden OJ, Leandros E, Bramis J, Wigmore SJ. Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg. 2005;92:1059–1067. doi: 10.1002/bjs.5107. [DOI] [PubMed] [Google Scholar]
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev. 2012;6:Cd008370. doi: 10.1002/14651858.CD008370.pub2. [DOI] [PubMed] [Google Scholar]
- Koti RS, Gurusamy KS, Fusai G, Davidson BR. Meta-analysis of randomized controlled trials on the effectiveness of somatostatin analogues for pancreatic surgery: a Cochrane review. HPB. 2010;12:155–165. doi: 10.1111/j.1477-2574.2010.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay A, Mackenzie S, Sutherland FR, Bathe OF, Doig C, Dort J, et al. Meta-analysis of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy. Br J Surg. 2006;93:929–936. doi: 10.1002/bjs.5407. [DOI] [PubMed] [Google Scholar]
- Wente MN, Shrikhande SV, Müller MW, Diener MK, Seiler CM, Friess H, et al. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg. 2007;193:171–183. doi: 10.1016/j.amjsurg.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Zhang Q, Han S, Yu Z, Zheng M, Zhou M, et al. Efficacy of somatostatin and its analogues in prevention of postoperative complications after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. Pancreas. 2008;36:18–25. doi: 10.1097/mpa.0b013e3181343f5d. [DOI] [PubMed] [Google Scholar]
- McMillan MT, Christein JD, Callery MP, Behrman SW, Drebin JA, Kent TS, et al. Prophylactic octreotide for pancreatoduodenectomy: more harm than good? HPB. 2014;16:954–962. doi: 10.1111/hpb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener MK, Tadjalli-Mehr K, Wente MN, Kieser M, Buchler MW, Seiler CM. Risk-benefit assessment of closed intra-abdominal drains after pancreatic surgery: a systematic review and meta-analysis assessing the current state of evidence. Langenbecks Arch Surg. 2011;396:41–52. doi: 10.1007/s00423-010-0716-0. [DOI] [PubMed] [Google Scholar]
- McMillan MT, Fisher WE, Van Buren G, 2nd, McElhany A, Bloomston M, Hughes SJ, et al. The value of drains as a fistula mitigation strategy for pancreatoduodenectomy: something for everyone? Results of a randomized prospective multi-institutional study. J Gastrointest Surg. 2015;19:21–30. doi: 10.1007/s11605-014-2640-z. ; discussion -1. [DOI] [PubMed] [Google Scholar]
- Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1–7. doi: 10.1097/01.sla.0000218077.14035.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren G, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, et al. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014;259:605–612. doi: 10.1097/SLA.0000000000000460. [DOI] [PubMed] [Google Scholar]
- Sutcliffe RP, Battula N, Haque A, Ali A, Srinivasan P, Atkinson SW, et al. Utility of drain fluid amylase measurement on the first postoperative day after pancreaticoduodenectomy. World J Surg. 2012;36:879–883. doi: 10.1007/s00268-012-1460-0. [DOI] [PubMed] [Google Scholar]
- Marangoni G, Morris-Stiff G, Deshmukh S, Hakeem A, Smith A. A modern approach to teaching pancreatic surgery. J Gastrointest Surg. 2012;16:1597–1604. doi: 10.1007/s11605-012-1934-2. [DOI] [PubMed] [Google Scholar]
- Wamser P, Stift A, Passler C, Goetzinger P, Sautner T, Jakesz R, et al. How to pass on expertise: pancreatoduodenectomy at a teaching hospital. World J Surg. 2002;26:1458–1462. doi: 10.1007/s00268-002-5958-8. [DOI] [PubMed] [Google Scholar]
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–939. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058–1064. doi: 10.1097/SLA.0b013e31814a6906. [DOI] [PubMed] [Google Scholar]
- Rosso E, Casnedi S, Pessaux P, Oussoultzoglou E, Panaro F, Mahfud M, et al. The role of “fatty pancreas” and of BMI in the occurrence of pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2009;13:1845–1851. doi: 10.1007/s11605-009-0974-8. [DOI] [PubMed] [Google Scholar]
- Olsen T. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol Microbiol Scand A. 1978;86 A:367–373. doi: 10.1111/j.1699-0463.1978.tb02058.x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim H, Cho JY, Lim S, Cha K, Lee KH, et al. Quantitative assessment of pancreatic fat by using unenhanced CT: pathologic correlation and clinical implications. Radiology. 2014;271:104–112. doi: 10.1148/radiol.13122883. [DOI] [PubMed] [Google Scholar]
- Schwenzer NF, Machann J, Martirosian P, Stefan N, Schraml C, Fritsche A, et al. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest Radiol. 2008;43:330–337. doi: 10.1097/RLI.0b013e31816a88c6. [DOI] [PubMed] [Google Scholar]
- Lingvay I, Esser V, Legendre J, Price A, Wertz K, Adams-Huet B, et al. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009;94:4070–4076. doi: 10.1210/jc.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ræder H, Haldorsen IS, Ersland L, Grüner R, Taxt T, Søvik O, et al. Pancreatic lipomatosis is a structural marker in nondiabetic children with mutations in carboxyl-ester lipase. Diabetes. 2007;56:444–449. doi: 10.2337/db06-0859. [DOI] [PubMed] [Google Scholar]
- Roberts KJ, Storey R, Hodson J, Smith AM, Morris-Stiff G. Pre-operative prediction of pancreatic fistula: is it possible? Pancreatology. 2013;13:423–428. doi: 10.1016/j.pan.2013.04.322. [DOI] [PubMed] [Google Scholar]
- Yang YM, Tian XD, Zhuang Y, Wang WM, Wan YL, Huang YT. Risk factors of pancreatic leakage after pancreaticoduodenectomy. World J Gastroenterol. 2005;11:2456–2461. doi: 10.3748/wjg.v11.i16.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duconseil P, Turrini O, Ewald J, Berdah S, Moutardier V, Delpero J-R. Biliary complications after pancreaticoduodenectomy: skinny bile ducts are surgeons’ enemies. World J Surg. 2014;38:2946–51. doi: 10.1007/s00268-014-2698-5. [DOI] [PubMed] [Google Scholar]
- Tranchart H, Gaujoux S, Rebours V, Vullierme MP, Dokmak S, Levy P, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg. 2012;256:139–145. doi: 10.1097/SLA.0b013e318256c32c. [DOI] [PubMed] [Google Scholar]
- Kirihara Y, Takahashi N, Hashimoto Y, Sclabas GM, Khan S, Moriya T, et al. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: the use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann Surg. 2013;257:512–519. doi: 10.1097/SLA.0b013e31827827d0. [DOI] [PubMed] [Google Scholar]
- Belyaev O, Munding J, Herzog T, Suelberg D, Tannapfel A, Schmidt WE, et al. Histomorphological features of the pancreatic remnant as independent risk factors for postoperative pancreatic fistula: a matched-pairs analysis. Pancreatology. 2011;11:516–524. doi: 10.1159/000332587. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Misawa T, Shiba H, Shirai Y, Iwase R, Haruki K, et al. A novel postoperative inflammatory score predicts postoperative pancreatic fistula after pancreatic resection. Anticancer Res. 2013;33:5005–5010. [PubMed] [Google Scholar]
- Ausania F, Snowden CP, Prentis JM, Holmes LR, Jaques BC, White SA, et al. Effects of low cardiopulmonary reserve on pancreatic leak following pancreaticoduodenectomy. Br J Surg. 2012;99:1290–1294. doi: 10.1002/bjs.8859. [DOI] [PubMed] [Google Scholar]
- Farrar DE, Glauber RR. Multicollinearity in regression analysis: the problem revisited. Rev Econ Stat. 1967;49:92–107. [Google Scholar]
- Olsen TS. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol Microbiol Scand A. 1978;86a:367–373. doi: 10.1111/j.1699-0463.1978.tb02058.x. [DOI] [PubMed] [Google Scholar]