Abstract
There exists a substantial body of work describing cardiac support devices to mechanically support the left ventricle (LV); however, these devices lack biological effects. To remedy this, we implemented a cell sheet engineering approach utilizing chondrocytes, which in their natural environment produce a relatively elastic extracellular matrix (ECM) for a cushioning effect. Therefore, we hypothesized that a chondrocyte cell sheet applied to infarcted and borderzone myocardium will biologically enhance the ventricular ECM and increase elasticity to augment cardiac function in a model of ischemic cardiomyopathy (ICM). Primary articular cartilage chondrocytes of Wistar rats were isolated and cultured on temperature-responsive culture dishes to generate cell sheets. A rodent ICM model was created by ligating the left anterior descending coronary artery. Rats were divided into two groups: cell sheet transplantation (1.0 × 107 cells/dish) and no treatment. The cell sheet was placed onto the surface of the heart covering the infarct and borderzone areas. At 4 weeks following treatment, the decreased fibrotic extension and increased elastic microfiber networks in the infarct and borderzone areas correlated with this technology's potential to stimulate ECM formation. The enhanced ventricular elasticity was further confirmed by the axial stretch test, which revealed that the cell sheet tended to attenuate tensile modulus, a parameter of stiffness. This translated to increased wall thickness in the infarct area, decreased LV volume, wall stress, mass, and improvement of LV function. Thus, the chondrocyte cell sheet strengthens the ventricular biomechanical properties by inducing the formation of elastic microfiber networks in ICM, resulting in attenuated myocardial stiffness and improved myocardial function.
Introduction
Left ventricular (LV) remodeling is characterized by progressive dilatation and dysfunction of the LV, leading to severe heart failure (HF). Abnormalities in the composition of the extracellular matrix (ECM) contribute to LV remodeling and HF.1,2 Current treatment for HF entails medical optimization along with limited reconstructive techniques. The cardiac support device is a mesh net designed to prevent LV remodeling. There exists a substantial body of work describing cardiac support devices to mechanically support the LV; however, these devices lack biological matrix remodeling effects.3,4
In contrast, chondrocytes have shown excellent potential for repairing and regenerating elastic ECM components. Recent reports show beneficial effects of chondrocyte transplantation therapy in several animal experimental models and patients with degenerative diseases, such as osteoarthritis.5,6
Therapeutic treatments employing cells or cell-based tissues have been developed to regenerate the damaged myocardium; however, their efficacy has generally been insufficient to repair severe myocardial damage. Thus, a second generation of myocardial regenerative therapeutics, tissue-engineered cardiomyoplasty, is currently being developed.7 Evolving from this basic approach of direct cell injection, our group has employed cell sheet technology. The cell sheet is generated on and removed from special dishes that are grafted with a temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm), which changes from hydrophobic to hydrophilic when the temperature is lowered. The greatest advantage of this technique is that the cell sheet consists of densely adherent cells without an artificial scaffold, does not require enzymatic digestion, is easily manipulated, and has a high ability to integrate with native tissues by retaining the cell–cell junctions as well as the ECM deposited on the basal surface of cell sheet.8–10 Moreover, in a cell sheet, cell–cell adhesions increase the traction stresses within the cell sheet, which may contribute to preventing LV remodeling, reducing myocardial stiffness, and improving the diastolic function.9,11 Furthermore, we focused on the concept that a cell sheet engineering approach utilizing chondrocytes may produce a relatively elastic ECM for a cushioning effect. Thus, we hypothesized that a chondrocyte cell sheet applied to infarcted and borderzone myocardium will biologically and mechanically enhance the myocardial ECM and increase elasticity to mitigate LV remodeling and enhance cardiac function in a model of ischemic cardiomyopathy (ICM).
Materials and Methods
Isolation of chondrocyte cells
Articular cartilage was harvested aseptically from the shoulder joints of neonatal wild-type Wistar rats (Charles River). Chondrocytes were isolated after the digestion of cartilage using collagenase type I (Worthington Biomedical Corp.) at 37°C and 5% CO2 for 24 h and cultured in Dulbecco's Modified Eagle's Medium with 10% fetal bovine serum (FBS; Sigma-Aldrich), gentamicin, and amphotericin B to confluency at 37°C and 5% CO2 for 7 days.5,6
Phenotypes of chondrocyte cells assessed by flow cytometry
To elucidate the phenotypes of cultured chondrocytes, flow cytometry was employed using the specific markers for chondrocytes. Single-cell suspensions of 106/mL were fixed with Fixation/Permeabilization Diluent (00-5223-56; eBioscience) for 30 min on ice. Following washing with 10% FBS in phosphate-buffered saline (PBS), they were incubated with an optimal concentration of an antibody against collagen II (AB3092, 1:100 dilution; Abcam) diluted in 10% FBS in PBS for 2 h on ice. After washing twice with 10% FBS in PBS, cells were incubated with donkey anti-mouse IgG H&L (Alexa Fluor 488®) (AB150105, 1:100 dilution; Abcam) for 2 h on ice. The percentage of cells expressing each cell surface antigen was analyzed with a Becton Dickinson FACSCalibur flow cytometer. Data analysis was performed using FlowJo V10 (Tree Star, Inc.).12 Control samples consisted of cells with FITC-conjugated Rat IgG2b κ isotype control (#556923, 1:100 dilution; BD Pharmingen) diluted in 10% FBS in PBS.
Preparation of chondrocyte cell sheet
The chondrocytes were plated at 1.0 × 107/cm2 in a 35-mm UpCell® dish, which is grafted with temperature-responsive polymers (CellSeed), and then cultured. After 24 h of culture at 37°C and 5% CO2, the dishes were then transferred to another incubator, set at 20°C, for 1 h to release the cultured cells as an intact cell sheet. Under this protocol, confluent chondrocyte cell sheets were spontaneously detached from the plate. The culture area was 8.8 cm2 for the in vivo grafting cell sheet.
Visualization of chondrocyte cell sheet
To confirm densely adherent cells without an artificial scaffold in a cell sheet and ECM deposited on the basal surface of a cell sheet, samples were visualized with scanning electron microscopy (SEM). Samples for SEM were fixed in 2.5% glutaraldehyde and 2.0% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.4, overnight at 4°C after lifting up a cell sheet from the UpCell dish. The samples were postfixed in 2.0% osmium tetroxide for 1 h, washed again in buffer, and dehydrated in a graded ethanol series. Samples were treated with several changes of hexamethyldisilazane and then allowed to air dry before mounting and sputter coating with gold/palladium. SEM examinations were performed in a Philips XL20 SEM.
Rat ICM model and cell sheet transplantation
Male Wistar rats (8-weeks old, 270–300 g; Charles River) were anesthetized with intraperitoneal ketamine (75 mg/kg) and xylazine (7.5 mg/kg), intubated in an endotracheal manner with a 19-gauge catheter, and mechanically ventilated (Hallowell EMC). Anesthesia was maintained by inhalation of 2.0% isoflurane (Clipper Distributing Company, LLC). The proximal left anterior descending coronary artery (LAD) was permanently occluded using a left thoracotomy approach. This produced a consistent and reproducible myocardial infarction (MI) encompassing 35–40% of the left ventricle.12–16 Five minutes after LAD ligation, the rats were allocated into two groups by simple randomization; those that underwent cell sheet transplantation (cell sheet group, n = 10) and those that underwent no intervention (no-treatment group, n = 10). For comparative purposes, rats receiving a sham operation were also studied as a positive control (sham group, n = 5). The rats were allowed to recover under care. In the cell sheet group, the cell sheet, which consisted of 1.0 × 107 chondrocyte cells, was placed on the epicardium covering the ischemic anterior LV. Polyvinylidene fluoride (PVDF) membranes were used as supporting membranes to deliver cell sheets.17 After the delivery, the PVDF membrane was peeled slowly from the edge of the cell sheet with tweezers. The cell sheet was attached and fixed to the epicardial surface without any stitches. Animals were then kept in temperature-controlled individual cages for 4 weeks. The rats were euthanized at 4 weeks after surgery by intravenous injection of 200 mg/kg of pentobarbital and 2 mEq/kg of potassium chloride, under terminal anesthesia, and the heart was excised.
Echocardiographic assessment
Echocardiography was performed under general anesthesia using 1.0% inhaled isoflurane 4 weeks after the treatment procedure (SONOS 7500; Philips Medical Systems) with a 12-MHz transducer at an image depth of 2 cm (cell sheet, n = 5; no treatment, n = 5). Left ventricular end-diastolic diameter and left ventricular end-systolic diameter, and LV end-diastolic anterior and posterior wall thicknesses at the level of the papillary muscles were measured for at least three consecutive cardiac cycles, following the American Society for Echocardiology leading-edge method. LV ejection fraction (LVEF) was calculated as a parameter of systolic function. LV mass was calculated as a parameter of myocardial hypertrophy.12,18,19 Wall stress index was defined as a parameter of LV wall stress.20 All analyses were performed by a single investigator in a group-blinded fashion.
Invasive hemodynamic assessment
Four weeks after the treatment procedure, animals (cell sheet, n = 5; no treatment, n = 5; sham, n = 5) underwent invasive hemodynamic measurements with a pressure–volume (P-V) conductance catheter (SPR-869; Millar Instruments, Inc.). The catheter was calibrated through five-point cuvette linear interpolation with parallel conductance subtraction by the hypertonic saline method.12–14 Rats were anesthetized using 1.0% inhaled isoflurane, and the catheter was introduced into the LV with a closed-chest approach through the right carotid artery. Measurements were obtained before and during inferior vena cava occlusion to produce static and dynamic P-V loops under varying load conditions. Data were recorded and analyzed with LabChart version 6 software (AD Instruments) and ARIA Pressure Volume Analysis software (Millar Instruments, Inc.). After the hemodynamic assessment, the heart was removed for further histological and western blotting analyses.
Mechanical testing of ventricular muscle strips
Myocardial mechanical properties were evaluated in a temperature-regulated bath of PBS maintained at 37°C, as described previously.21 Briefly, the myocardial strip was prepared by excising the infarcted area of LV longitudinally from apex to base (cell sheet, n = 5; no treatment, n = 5). Uniaxial tensile testing was conducted with an Instron 5543 microtester (Instron) with a 10 N six-axis load cell. The specimens were preloaded at 0.05 N, followed by 20 cycles of preconditioning at a rate of 0.1 mm/s and a load ranging from 0.05 to 0.1 N. After a 60-s hold at 0.05 N, three stress-relaxation phases at incremental displacements of 0.5 mm were performed, followed by a ramp to failure at 0.1 mm/s. A custom image acquisition software system (Digi-Velpo, version 1.4.1, run in LabView2010; National Instruments) was used to acquire optical data on tissue displacement. Optical strain data were integrated with the Instron load data using Opticus (custom-made software run in MATLAB), which generated stress–strain curves for each ventricular strip.
Histological and immunohistochemical analyses
Chondrocyte cells were cultured at 37°C for 24 h, washed with Dulbecco's PBS, and blocked with 10% FBS (Sigma-Aldrich) for 30 min. Cells were washed and incubated with an antibody against collagen II (AB3092, 1:100 dilution; Abcam) for 2 h followed by Alexa Fluor 488 donkey anti-mouse (AB150109, 1:100 dilution; Abcam) for 1 h. Slides were washed and mounted using 6-diamidino-2-phenylindole (DAPI; Invitrogen). The chondrocyte cells' phenotype was defined as collagen II+ and DAPI+.
Four weeks after the treatment, the hearts were dissected and embedded in an optimum cutting temperature compound for 10-μm-thick cryosections. The cryosections were used for routine hematoxylin–eosin (HE) staining to assess the myocardial structure. Massons-trichrome staining was performed to assess cardiac fibrotic extension. The fibrotic extension was calculated as the percentage of circumferential fibrotic length of the whole circumferential left ventricular length. Elastica van Gieson was performed to assess elastic microfiber networks. The images were examined by microscopy (Keyence). The elastic fiber content was evaluated from the elastic fiber ratio in the infarct myocardium at a magnification of 200×. The heart cryosections were stained with an antibody against collagen II (AB3092, 1:100 dilution; Abcam) or collagen I (AB34710, 1:100 dilution; Abcam) to detect collagen accumulation. The secondary antibodies were Alexa Fluor 488 donkey anti-mouse (AB150109, 1:100 dilution; Abcam). The cryosections were also stained with an antibody against von Willebrand factor (vWF) (AB8822, 1:100 dilution; Abcam) to assess capillary density, which was calculated as the number of positively stained capillary vessels in five randomly selected fields in the peri-infarct borderzone area, per heart. Cell nuclei were counterstained with DAPI (Vector Laboratories). The images were examined by fluorescence microscopy (Leica). ImageJ software was used for quantitative morphometric analysis.
Western blotting analysis
Tissue homogenates from LV samples in the cell sheet transplanted site (n = 3 in each group, at 4 weeks) were prepared using Halt™ Protease Inhibitor Single-Use Cocktail (ThermoScientific) diluted in T-PER Tissue Protein Extraction Reagent (ThermoScientific) and normalized for total protein content using Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories), as described previously.16,18 The equivalent total protein was loaded onto sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels. Antibodies obtained were anti-elastin (AB 21608, 1:200 dilution; Abcam), anti-collagen I (AB34710, 1:1000 dilution; Abcam), anti-collagen II (AB3092, 1:100 dilution; Abcam), anti-matrix metalloproteinase-1 (MMP-1) (AB118529, 1:500 dilution; Abcam), anti-MMP-2 (AB51125, 1:1000 dilution; Abcam), anti-tissue inhibitor of metalloproteinase-1 (TIMP-1) (AF580, 1:500 dilution; R&D), and anti-GAPDH (AB9484; Abcam). The labeled membrane was stripped and then reprobed. Blots were scanned, and semiquantitative analysis was performed using ImageJ software. The relative proportion of elastin, collagen I, collagen II, MMP-1, MMP-2, and TIMP-1 was compared to the no-treatment group.
Statistical analysis
Continuous variables are expressed as the mean ± standard error. Comparisons between two groups were made using the Wilcoxon–Mann–Whitney U test, because of the small sample sizes. For comparison among three groups, we used the Kruskal–Wallis test, followed by the post hoc pairwise Wilcoxon–Mann–Whitney U test. The multiplicity in pairwise comparisons was corrected by the Bonferroni procedure. A p-value less than 0.05 was considered to be statistically significant. All statistical calculations were performed using the JMP 9.0 (SAS Institute, Inc.).
Animal care and biosafety
Wistar rats were obtained from Charles River. Food and water were provided ad libitum. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania (protocol 803394) and Stanford University (protocol 28921).
Results
Characterization of isolated chondrocyte cells and cell sheet
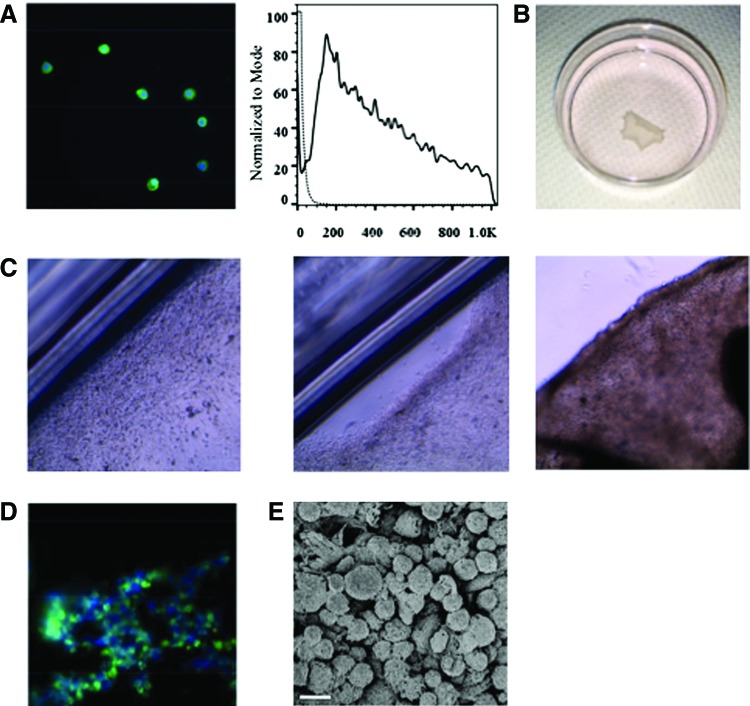
Flow cytometric analysis demonstrated that the percentage of collagen II+ cells in chondrocyte cells was 69.3% ± 11.5%. Histologically, isolated chondrocyte cells showed the expression of collagen II (Fig. 1A). Confluent chondrocyte cells were spontaneously detached as an intact cell sheet from an UpCell dish (Fig. 1B, C). The chondrocyte cell sheet maintained a collagen II-positive layer in vitro (Fig. 1D). SEM revealed the presence of densely adherent cells without an artificial scaffold in a cell sheet and ECM deposited on the surface of a cell sheet (Fig. 1E).
FIG. 1.
Characterization of chondrocyte cells and chondrocyte cell sheet in vitro. (A) Chondrocyte cells showing expression of collagen II. Green indicates collagen II; blue, nuclei. Representative flow cytometry analysis of collagen II+ cells. Black line indicates collagen II+ cells. (B) Confluent chondrocyte cells were spontaneously detached from an UpCell® dish, which is grafted with temperature-responsive polymers (CellSeed). (C) Confluent chondrocyte cells were cultured in a 35-mm UpCell dish at 37°C and 5% CO2 (left panel, magnification 10×). The dishes were then transferred to another incubator, set at 20°C, to release the cultured cells as an intact cell sheet (middle and right panel, magnification 10×). (D) Chondrocyte cell sheet maintained collagen II+ layer in vitro. Green indicates collagen II; blue, nuclei. (E) Scanning electron microscopy revealed the presence of densely adherent cells and the extracellular matrix–cell adhesion without artificial scaffold in a cell sheet. Scale bar = 10 μm. Color images available online at www.liebertpub.com/tea
Cardiac functional recovery and left ventricular reverse remodeling after chondrocyte cell sheet transplantation
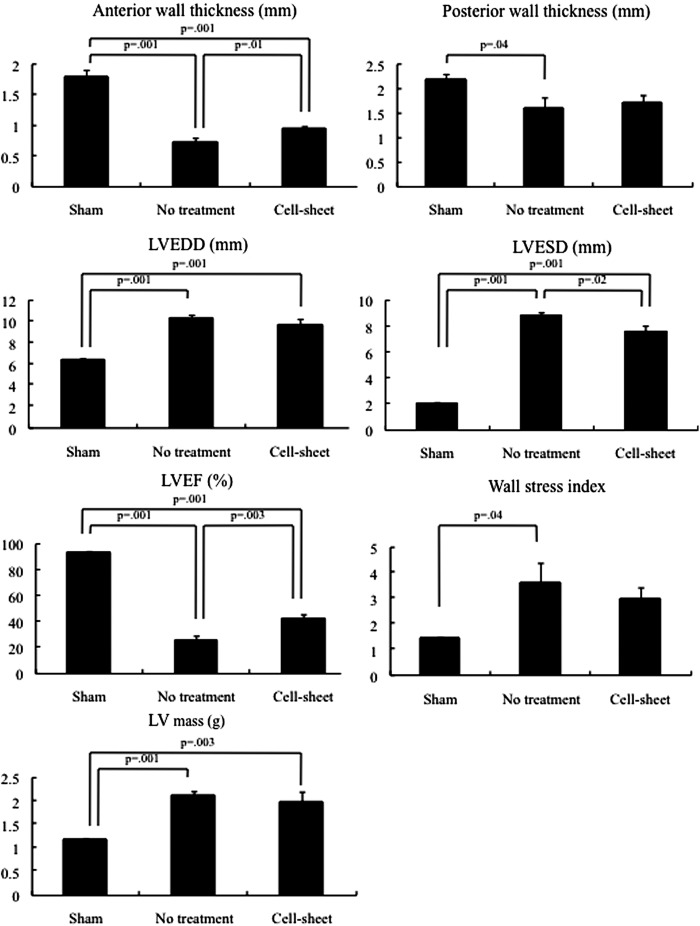
The effects of chondrocyte cell sheet transplantation on cardiac function were assessed in a rat ICM model. Four weeks after permanent occlusion of the LAD, the LV developed echocardiographic features typical of chronic ischemic HF, including decreased LVEF and anterior wall thickness, and increased EDD, ESD, wall stress, and mass, suggesting progressive LV remodeling. At 4 weeks, the chondrocyte cell sheet group had a significantly greater LVEF, improved ESD, and increased wall thickness in the treated area, compared to the no-treatment group (Fig. 2). Wall stress index and LV mass tended to be lower in the cell sheet group than the no-treatment group, although there was no statistical significance (Fig. 2).
FIG. 2.
Echocardiographic measurements of anterior and posterior myocardial thicknesses, left ventricular end-diastolic diameter (LVEDD) and LV end-systolic diameter (ESD), LV ejection fraction (LVEF), LV wall stress index, and LV mass (cell sheet, n = 5; no treatment, n = 5; sham, n = 5). Examinations were performed at the 4-week follow-up after the operation. Four weeks after permanent occlusion of the left anterior descending coronary artery, the no-treatment group showed decreased wall thickness and LVEF, and increased EDD, ESD, wall stress, and mass, compared with the baseline. (anterior wall thickness, p = 0.002; posterior wall thickness, p = 0.007; LVEDD, p = 0.005; left ventricular end-systolic diameter [LVESD], p = 0.004; LVEF, p = 0.002; LV wall stress index, p = 0.008; LV mass, p = 0.007; Wilcoxon-Mann-Whitney U test). Anterior wall thickness was significantly greater at 4 weeks following the chondrocyte cell sheet transplantation compared with the no-treatment group (p = 0.01), while there was no significant difference in posterior wall thickness between these two groups (p = 0.10). LVEDD and LVESD was lower at 4 weeks in the cell sheet group (LVEDD, p = 0.20; LVESD, p = 0.02). LVEF was significantly higher at 4 weeks in the cell sheet group, compared with the no-treatment group (p = 0.003). Wall stress index and LV mass tended to be attenuated in the cell sheet group compared with the no-treatment group (LV wall stress index, p = 0.42; LV mass, p = 0.47).
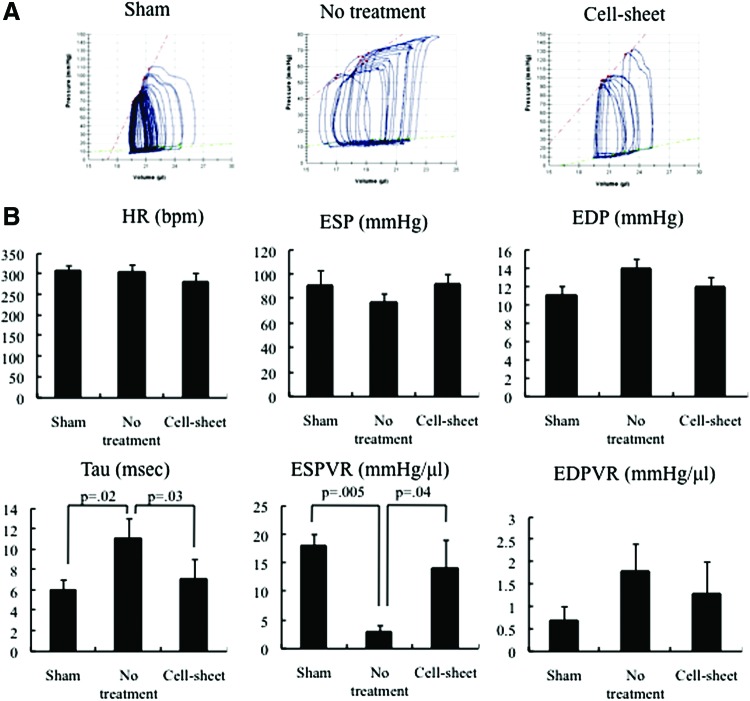
Assessment by PV catheter further confirmed the cell sheet-induced functional enhancement demonstrated by the echocardiographic data. Four weeks after transplantation, tau and the end-systolic pressure–volume relationship were significantly enhanced in the cell sheet group compared with the no-treatment group. There was no significant difference in heart rate, end-systolic pressure, end-diastolic pressure, or end-diastolic pressure–volume relationship (Fig. 3).
FIG. 3.
Hemodynamic measurements determined using cardiac catheterization after cell sheet transplantation (cell sheet, n = 5), no treatment (no treatment, n = 5), and sham (sham, n = 5). Examinations were performed at the 4-week follow-up after the operation. (A) Representative pressure–volume loops during inferior vena cava occlusion from the cell sheet, no treatment, and sham groups. (B) There was no significant difference in the heart rate (HR), end-systolic pressure (ESP), end-diastolic pressure (EDP), or end-diastolic pressure–volume relationship (EDPVR) (HR, p = 0.28; ESP, p = 0.54; EDP, p = 0.23; EDPVR, p = 0.33; Kruskal–Wallis test). Tau and end-systolic pressure–volume relationship (ESPVR) significantly improved in the cell sheet group, compared with the no-treatment group (tau, p = 0.04; ESPVR, p = 0.01; Kruskal–Wallis test). Color images available online at www.liebertpub.com/tea
Attenuated tensile modulus after chondrocyte cell sheet transplantation
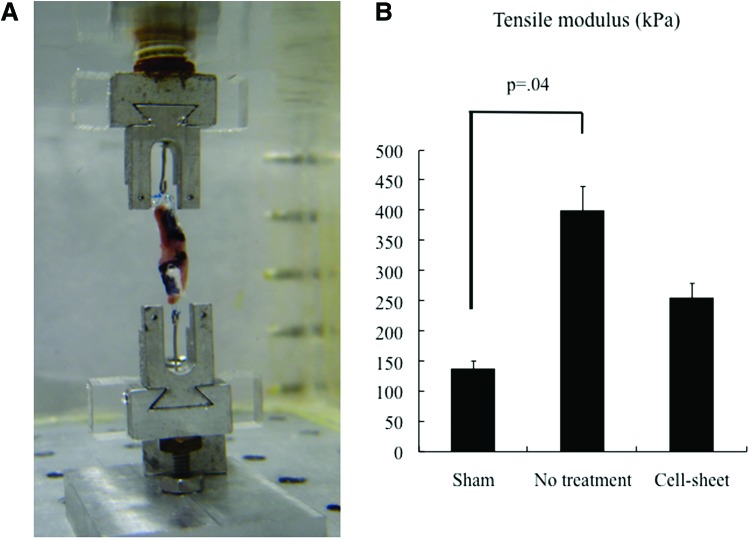
At 4 weeks after permanent occlusion of the LAD, the tensile modulus, which was calculated from the slope of the stress–strain curves, was significantly larger than that of the sham group (p = 0.04; Kruskal–Wallis test), suggesting a decreased myocardial compliance. The tensile modulus was attenuated in the cell sheet group, compared to the no-treatment group, but the difference was not significant (Fig. 4).
FIG. 4.
(A) Image of ventricular strip inside the temperature-regulated bath, connected to the Instron microtester (Instron). (B) The tensile modulus, which was calculated from the slope of the stress–strain curves, was lower in the cell sheet group, compared to the no-treatment group (cell sheet, n = 5; no treatment, n = 5; sham, n = 5, p = 0.003; Kruskal–Wallis test). Color images available online at www.liebertpub.com/tea
Left ventricular remodeling after chondrocyte cell sheet transplantation
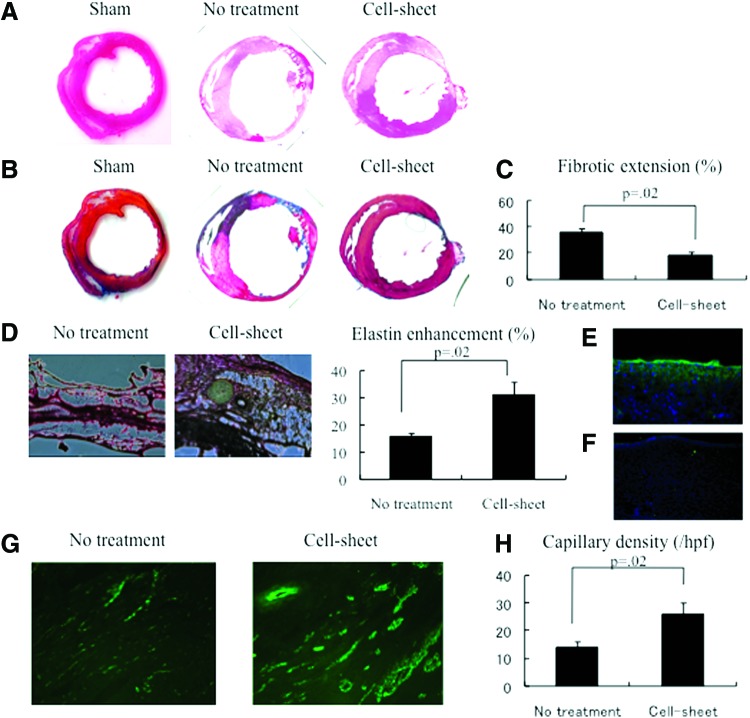
The LV myocardial structure was superiorly maintained after cell sheet transplantation compared to no treatment, as assessed by HE staining (Fig. 5A). In the no-treatment group, Masson's trichrome staining demonstrated an increased collagen deposition and fibrotic changes in the infarct area compared to the cell sheet transplantation group (Fig. 5B, C). Following the cell sheet therapy, abundant elastic microfiber networks were observed in the cell sheet-transplanted myocardium, as shown by Elastica van Gieson staining (Fig. 5D). The elastic fiber content was significantly enhanced in the cell sheet group (30.6% ± 5.0%) compared with the no-treatment group (15.5% ± 1.4%) (p = 0.02; Wilcoxon–Mann–Whitney U test).
FIG. 5.
(A) Representative hematoxylin–eosin staining of the heart. The myocardial structure was superiorly maintained after cell sheet transplantation compared to the no-treatment group. (B) Representative Masson's trichrome staining of the heart. (C) Quantification of cardiac fibrotic extension. Fibrosis was significantly suppressed in the cell sheet group, compared to the no-treatment group (cell sheet, n = 5; no treatment, n = 5; p = 0.02). (D) Representative Elastica van Gieson staining in the peri-infarct borderzone myocardium, showing abundant elastic microfiber networks. Quantification of elastin enhancement. The elastic fiber content was significantly enhanced in the cell sheet group, compared with the no-treatment group (p = 0.02; Wilcoxon–Mann–Whitney U test). (E) Immunostaining microscopy showed anti-collagen II-positive cells both on the epicardium and within the myocardium. Green indicates collagen II; blue, nuclei. (F) Immunostaining microscopy showed no significant amount of anti-collagen I-positive cells both on the epicardium and within the myocardium. Green indicates collagen I; blue, nuclei. (G) Representative von Willebrand factor staining of the borderzone myocardium. (H) Quantification of capillary density. Capillary density was enhanced in the cell sheet group, compared with the no-treatment group (cell sheet, n = 5; no treatment, n = 5; p = 0.02). Color images available online at www.liebertpub.com/tea
ECM remodeling after chondrocyte cell sheet transplantation
Four weeks after the treatment, a collagen II+ membrane-like construct was observed on the epicardium, coupled with a robust number of collagen II+ cells located within the myocardium following chondrocyte cell sheet therapy (Fig. 5E). Notably, a collagen I+ construct was not observed within the myocardium following chondrocyte cell sheet therapy (Fig. 5F).
Enhanced capillary density after chondrocyte cell sheet transplantation
A greater number of vWF-positive blood vessels were detected in the peri-infarct borderzone myocardium following cell sheet therapy compared to no treatment (Fig. 5G). This demonstrated a superior enhancement of capillary density in the cell sheet group (Fig. 5H).
Upregulation of ECM components after chondrocyte cell sheet transplantation
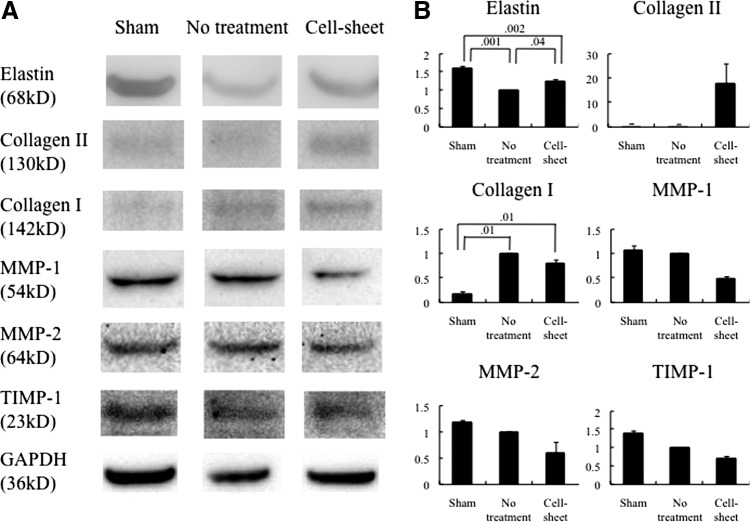
Major ECM components relevant to LV remodeling were analyzed by western blotting. Elastin was significantly increased after chondrocyte cell sheet transplantation (Fig. 6). Collagen I decreased in the heart following transplantation of the chondrocyte cell sheet, compared with the no-treatment group (Fig. 6).
FIG. 6.
(A) Representative immunoblots for elastin, collagen I, collagen II, matrix metalloproteinase-1 (MMP-1), MMP-2, tissue inhibitor of metalloproteinase-1 (TIMP-1), and GAPDH between the cell sheet, no treatment, and sham groups (cell sheet, n = 3; no treatment, n = 3; sham, n = 3). (B) At 4 weeks, elastin and collagen II increased (elastin, p = 0.02; collagen II, p = 0.05; Kruskal–Wallis test), while collagen I, MMP-1, MMP-2, and TIMP-1 decreased (collagen I, p = 0.02; MMP-1, p = 0.06; MMP-2, p = 0.10; TIMP-1, p = 0.06) after chondrocyte cell sheet transplantation. GAPDH staining was performed to demonstrate equivalent protein loading between the three groups.
Among the MMP/TIMP family of molecules, MMP-1, MMP-2, and TIMP-1 decreased after the chondrocyte cell sheet transplantation (Fig. 6).
Discussion
This study revealed a multifaceted mechanism by which the targeted transplantation of a chondrocyte cell sheet enhances the myocardial function in a rodent ICM model. Isolated chondrocytes and cell sheets exhibited collagen II expression and phenotypic markers consistent with chondrocytes in vitro. A significant ECM modification was observed in vivo where chondrocyte cell sheet transplantation induced the upregulation of elastin and collagen II, in addition to the downregulation of collagen I, MMP-1, MMP-2, and TIMP-1. The decreased fibrotic extension and increased elastic microfiber networks in the infarct and borderzone areas correlate with this technology's potential to stimulate elastic ECM formation. The enhanced ventricular elasticity was further confirmed by the axial stretch test, which revealed that the cell sheet tended to attenuate tensile modulus, a parameter of stiffness. The observed increased capillary density in the borderzone elucidated the significant in vivo angiogenic potential of this technology. These biomechanical effects translated to increased wall thickness in the infarct area, reduced ventricular volume, attenuated wall stress, and mass, thereby improving systolic and diastolic functions as indicated by echocardiography and pressure–volume analyses.
The mechanism by which the chondrocyte cell sheet transplantation attenuated LV remodeling has not been fully elucidated and is likely complex. LV remodeling is a pathologic consequence of MI and has a significant impact on the development of HF.1,2 The initial response after MI is that the surviving myocardium undergoes cellular and molecular changes, such as hypertrophy, apoptosis of cardiomyocytes, collagen accumulation, and pathological remodeling of ECM, leading to cardiac enlargement and dysfunction.1,9 LV reverse remodeling is a critical aspect of myocardial repair. There are accumulating reports showing that cell therapy improves the cardiac function by modulating LV remodeling.22 The present study focused on various mechanical aspects of the LV reverse remodeling process in a detailed fashion, such as the myocardial wall thickness, LV volume, wall stress, mass, and functional performance, as measured by echocardiography. Together with the concept of Grossman,23 it is suggested that the placement of a chondrocyte cell sheet over ischemic myocardium enhances anterior wall thickness, reduces ventricular volume, wall stress, and myocardial mass, resulting in an improved cardiac function.
In addition, among the many complicated biological processes associated with LV reverse remodeling, the transplanted chondrocyte cell sheet can cause changes in the molecular signals that promote hypertrophy and ECM turnover affecting LV geometry. More specifically, our semiquantitative molecular analysis here demonstrated that elastin and collagen II were upregulated, while collagen I, MMP-1, MMP-2, and TIMP-1 were downregulated, 4 weeks following the chondrocyte cell sheet transplantation. The MMP/TIMP family is known to play an important role in regulation of the ECM composition in post-MI remodeling.1–3,22 One explanation for the above-mentioned findings is that chondrocyte cell sheet transplantation favorably modified collagen formation, modulated an important ECM proteolytic pathway, and induced LV reverse remodeling by balancing MMPs and TIMPs.1,2,9,24 However, further investigation is warranted regarding, as to which marker is best suited to reflect changes in the setting of MI, because of the complex dynamics of the myocardial ECM responses.25
Moreover, elastin is one of the major ECM components. In the process of recovering from MI, the necrotic myocardium is replaced by collagen fibers, especially collagen type I and type III, and elasticity is lost in the scarred area. However, our data demonstrated increases in elastin and the development of elastic microfiber networks in the recipient myocardium after chondrocyte cell sheet transplantation. The mechanism of promoting elastin into the damaged myocardium following chondrocyte cell sheet transplantation is potentially multifaceted based on current discussions in the literature. Isolated chondrocytes utilized in this study can potentially include several kinds of stem cells (e.g., fibroblasts, myoblasts, myofibroblasts), which can secrete tropoelastin. In addition, stem cell recruitment and cytokine release might have been evoked.26 These are potential mechanisms for the enhanced elastin presence demonstrated in this study. Previous work has revealed that the elastic fiber network induces the essential properties of elasticity and resilient recoil, along with maintaining the integrity of tissue architecture against repeated expansion.27 Taken together, the underlying mechanism of reverse LV remodeling may involve the augmented elasticity of the infarcted area, permitting recoil in response to the stresses of contraction, leading to reduced scar thinning and LV dilatation. Ultimately, this manifests as an improved LV diastolic function.
Another key component of the study is the hypoxic milieu present for the transplanted chondrocyte cell sheet post-MI. It has been reported that the VEGF expression is activated under hypoxic conditions in chondrocytes partially mediated through hypoxia-inducible factor 1 alpha (HIF-1α).28 This can promote angiogenesis, attenuate ischemic injury, and upon reperfusion, enhance the recovery of myocardial performance.29 Hypoxia applied during the collagen synthesis phase has shown to result in decreased collagen content, although the major cell type that migrates to the infarct area post-MI is the fibroblast, which mainly produces a granulation tissue with predominantly collagen type I. Moreover, for stem cells, hypoxia has been shown to be effective and sufficient in inducing a chondrocytic phenotype during certain times, which might be related to upregulation of collagen II.30 Thus, we speculate that the recovery of diastolic function, ECM remodeling, and angiogenesis are major components of the regenerative mechanism in chondrocyte cell sheet transplantation.
This treatment strategy for acute MI is not yet directly applicable to the clinical setting. However, the finding that this therapy yielded marked cardioprotective effects through ECM reconstruction should be beneficial for treating other types of cardiac pathologies, such as the chronic phase of MI or idiopathic dilated cardiomyopathy. Another potential limitation of this study is that other stem cells, such as induced pluripotent stem cells, mesenchymal stem cells, and endothelial stem cells, can be used as an alternative cell source.9 Further study will be necessary to compare the use of chondrocyte cells with other cells for the treatment of HF. In addition, further studies that include an extended timeframe are needed to examine a longer term restoration of heart function post-MI. Although chondrocyte cell sheet transplantation seemed to induce favorable ECM remodeling without significant bony structure at 4 weeks, the transplanted neocartilage, after completing the maturation process, might cause a restrictive type of HF. Our tensile testing method was performed by uniaxial testing and could estimate myocardial properties along a longitudinally sectioned ventricle only. A possible limitation of this uniaxial testing is that it could not show the material properties as either isotropic or anisotropic. This will be overcome by introducing a biaxial testing method.13,31 An additional limitation is that this test was not conducted in different directions. Multidirectional myocardial properties could be obtained with the information of myocardial alignment.
In conclusion, the chondrocyte cell sheet strengthens the ventricular biomechanical properties by inducing the formation of elastic microfiber networks and modulating the ECM components in ischemic cardiomyopathic rats, resulting in attenuated myocardial stiffness and improved systolic and diastolic function. These findings suggest that this new technology represents a novel strategy for myocardial repair in advanced cardiomyopathy.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) Grant 1R01HL089315-01 (Y.J.W.): American Heart Association Great Rivers Affiliate Postdoctoral Fellowship cosponsored by the Claude R. Joyner Fund for Young Medical Researchers (#12POST12060567) (Y.S.): Uehara Memorial Foundation for Research Fellow, Japan (Y.S.): Shinya Fund for International Exchange, Japan (Y.S.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Pfeffer M., and Braunwald E. Ventricular remodeling after myocardial infaction. Experimental observations and clinical implications. Circulation 81, 1161, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Spinale F.G. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res 90, 520, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Blom A.S., Mukherjee R., Pilla J.J., Lowry A.S., Yarbrough W.M., Mingoia J.T., Hendrick J.W., Stroud R.E., McLean J.E., Affuso J., Gorman R.C., Gorman J.H., III, Acker M.A., and Spinale F.G. Cardiac support device modifies left ventricular geometry and myocardial structure after myocardial infarction. Circulation 112, 1274, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Philla J.J., Blom A.S., Brockman D.J., Bowen F., Yuan Q., Giammarco J., Ferrari V.A., Gorman J.H., III, Gorman R.C., and Acker M.A. Ventricular constraint using the acorn cardiac support device reduces myocardial akinetic area in an ovine model of acute infarction. Circulation 106, I207, 2002 [PubMed] [Google Scholar]
- 5.Yano F., Hojo H., Ohba S., Saito T., Honnami M., Mochizuki M., Takato T., Kawaguchi H., and Chung U. Cell-sheet technology combined with a thienoindazole derivative small compound TD-198946 for cartilage regeneration. Biomaterials 34, 5581, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Kokubo M., Sato M., Yamato M., Mitani G., Kutsuna T., Ebihara G., Okano T., and Mochida J. Characterization of chondrocyte sheets prepared using a co-culture method with temperature-responsive culture inserts. J Tissue Eng Regen Med 2013; DOI: 10.1002/term.1764 [DOI] [PubMed] [Google Scholar]
- 7.Sawa Y., and Miyagawa S. Cell sheet technology for heart failure. Curr Pharm Biotechnol 14, 61, 2013 [PubMed] [Google Scholar]
- 8.Okano T., Yamada N., Sakai H., and Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-iso-propylacrylamide). J Biomed Mater Res 27, 1243, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Miyagawa S., Roth M., Saito A., Sawa Y., and Kostin S. Tissue-engineered cardiac constructs for cardiac repair. Ann Thorac Surg 91, 320, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Miyagawa S., Sawa Y., Sakakida S., Taketani S., Kondoh H., Memon I.A., Imanishi Y., Shimizu T., Okano T., and Matsuda H. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardium. Transplantation 80, 1586, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Weber G.F., Bjerke M.A., and DeSimone D.W. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell 22, 104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shudo Y., Cohen J.E., MacArthur J.W., Atluri P., Hsiao P.F., Yang E.C., Fairman A.S., Trubelja A., Patel J., Miyagawa S., Sawa Y., and Woo Y.J. Spatially-oriented, temporally-sequential SMC-EPC bi-level cell-sheet neovascularizes ischemic myocardium. Circulation 128(26 Suppl 1): S59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atluri P., Trubelja A., Fairman A.S., Hsiao P.F., MacArthur J.W., Cohen J.E., Shudo Y., Frederick J., and Woo Y.J. Normalization of post-infarct biomechanics utilizing a novel tissue engineered angiogenic construct. Circulation 128(26 Suppl 1): S95, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacArthur J.W., Purcell B., Shudo Y., Cohen J.E., Fairman A.S., Trubelja A., Patel J., Hsiao P.F., Yang E.C., Lloyd K., Hiesinger W., Atluri P., Burdick J., and Woo Y.J. Sustained release of engineered stromal cell-derived factor 1-alpha from injectable hydrogels effectively recruits endothelial progenitor cells and preserves ventricular function following myocardial infarction. Circulation 128(26 Suppl 1): S79, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiesinger W., Perez-Aguilar J.M., Atluri P., Marotta N.A., Frederick J.R., Fitzpatrick J.R., III, McCormick R.C., Muenzer J.R., Yang E.C., Levit R.D., Yuan L.J., MacArthur J.W., Saven J.G., and Woo Y.J. Computational protein design to reengineer stromal cell-derived factor-1α generates an effective and translatable angiogenic polypeptide analog. Circulation 124, S18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frederick J.R., Fitzpatrick J.R., III, McCormick R.C., Harris D.A., Kim A.Y., Muenzer J.R., Marotta N., Smith M.J., Cohen J.E., Hiesinger W., Atluri P., and Woo Y.J. Stromal cell-derived factor-1 alpha activation of tissue-engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis. Circulation 122, S107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamato M., Utsumi M., Kushida A., Konno C., Kikuchi A., and Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng 7, 473, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Shudo Y., Miyagawa S., Ohkura H., Fukushima S., Saito A., Kawaguchi N., Matsuura N., Shimizu T., Okano T., Matsuyama A., and Sawa Y. Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng Part A 20, 728, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shudo Y., Miyagawa S., Fukushima S., Saito A., Shimizu T., Okano T., and Sawa Y. Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J Thorac Cardiovasc Surg 142, 1188, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Mourouzis I., Giagourta I., Galanopoulos G., Mantzouratou P., Kostakou E., Kokkinos A.D., Tentolouris N., and Pantos C. Thyroid hormone improves the mechanical performance of the post-infarcted diabetic myocardium: a response associated with up-regulation of Akt/mTOR and AMPK activation. Metabolism 62, 1387, 2013 [DOI] [PubMed] [Google Scholar]
- 21.MacArthur J.W., Jr, Trubelja A., Shudo Y., Hsiao P., Fairman A., Yang E., Hiesinger W., Sarver J.J., Atluri P., and Woo Y.J. Mathematically engineered stromal cell-derived factor-1a stem cell cytokine analog enhances mechanical properties of infarcted myocardium. J Thorac Cardiovasc Surg 145, 278, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ducharme A., Frantz S., Aikawa M., Rabkin E., Lindsey M., Rohde L.E., Schoen F.J., Kelly R.A., Werb Z., Libby P., and Lee R.T. Targeted delotion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106, 55, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process. Am J Med 69, 576, 1980 [DOI] [PubMed] [Google Scholar]
- 24.Shudo Y., Taniguchi K., Takeda K., Sakaguchi T., Funatsu T., Kondoh H., and Sawa Y. Serial multidetector computed tomography assessment of left ventricular reverse remodeling, mass, and regional wall stress after restrictive mitral annuloplasty in dilated cardiomyopathy. J Thorac Cardiovasc Surg 143(4 Suppl): S43, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Li A.H., Liu P.P., Villarreal F.J., and Garcia R.A. Dynamic changes in myocardial matrix and relevance to disease: translational perspectives. Circ Res 114, 916, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Uchinaka A., Kawaguchi N., Hamada Y., Miyagawa S., Saito A., Mori S., Sawa Y., and Matsuura N. Transplantation of elastin-secreting myoblast sheets improves cardiac function in infarcted rat heart. Mol Cell Biochem 368, 203, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T., Lozano P.R., Ikeda Y., Iwanaga Y., Hinek A., Minamisawa S., Cheng C.F., Kobuke K., Dalton N., Takeda Y., Tashiro K., Ross J., Jr., Honjo T., and Chien K.R. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415, 171, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Zhang C., Li Y., Cornelia R., Swisher S., and Kim H. Regulation of VEGF expression by HIF-1a in the femoral head cartilage following ischemia osteonecrosis. Sci Rep 2, 650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shudo Y., Miyagawa S., Nakatani S., Fukushima S., Sakaguchi T., Saito A., Asanuma T., Kawaguchi N., Matsuura N., Shimizu T., Okano T., and Sawa Y. Myocardial layer-specific effect of myoblast cell-sheet implantation evaluated by tissue strain imaging. Circ J 77, 1063, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Makris E.A., Hu J.C., and Athanasiou K.A. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical proper. Osteoarthritis Cartilage 21, 634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trubelja A., MacArthur J.W., Sarver J.J., Cohen J.E., Hung G., Shudo Y., Fairman A.S., Patel J., Edwards B.B., Damrauer S.M., Hiesinger W., Atluri P., and Woo Y.J. Bioengineered stromal cell-derived factor-1a analogue delivered as an angiogenic therapy significantly restores viscoelastic material properties of infarcted cardiac muscle. J Biomech Eng 136, 2014; DOI: 10.1115/1.4027731 [DOI] [PMC free article] [PubMed] [Google Scholar]