Abstract
Background: Scaffolds are a key component of tissue-engineered heart valves (TEHVs). Several approaches had been adopted in the design of scaffolds using both natural and synthetic resources. We have investigated the suitability of parylene C (PC), a vapor deposited polymeric material, for the use as a scaffold in TEHV.
Aims: To evaluate the adsorption of extracellular matrix components onto plasma-activated PC and study the biocompatibility of PC by measuring cellular adhesion, viability, apoptosis, and phenotypic expression of valve endothelial and interstitial cells. Finally, the mechanical properties of PC were compared with those of native aortic valve cusp tissue.
Methods: PC slides were plasma activated and then coated with gelatin, type I collagen, or fibronectin. Porcine pulmonary valve endothelial and interstitial cells were then grown on plasma oxidized PC with different types of coatings and their adhesion was observed after 20 h of incubation. Cell viability was tested using the MTS assay, and apoptosis was estimated using TUNEL staining. The mechanical properties of PC and valve tissue were measured using a Bose Mechanical Tester. Finally, cell-seeded PC films were exposed to pulsatile pressure and aortic shear stress, respectively, to test their durability in a dynamic environment.
Results: Our findings show that collagen and fibronectin could bind to plasma oxidized PC. Both valve endothelial and interstitial cells adhered to protein-coated ECM. PC had a profile of mechanical stiffness and ultimate tensile strength that were comparable with or in excess of those seen in porcine aortic valve cusps. Cells were still attached to PC films after 3 days of exposure to up to 50 mmHg pulsatile pressure or aortic levels of shear stress.
Conclusion: PC is a promising candidate for use as a scaffold in tissue engineering heart valves. Additional studies are required to determine both the durability and long-term performance of cell-seeded PC when in a similar hemodynamic environment to that of the aortic valve.
Introduction
Diseases of the heart are the number one cause of death worldwide, resulting in 9.4 million deaths annually according to the World Health Organization. A considerable proportion of these deaths are caused by valvular heart disease (VHD), where the valve fails to function properly, leading to heart failure.1 The only treatment of end-stage VHD is the surgical replacement of the diseased valve with a prosthetic substitute.2 Although the available prostheses increase the life quality and expectancy, they are still associated with limitations that affect their performance, such as thrombogenicity and structural failure.1 Therefore, efforts are focused on providing a biocompatible viable valve replacement that overcomes the limitations of the available prostheses, in addition to providing an intrinsic repair mechanism, with the ability to remodel and adapt to the hemodynamic changes.
Tissue engineering can offer a solution to this problem. Several approaches have been made to design scaffolds suitable for tissue-engineered heart valves (TEHVs), both from natural and synthetic resources, but most of the investigated materials have limitations, especially in regard to their mechanical properties. Examples of natural scaffolds are those made of collagen and fibrin, which provide good interaction with the cells, but show weak mechanical properties.3,4 Synthetic materials from the aliphatic polyester family had been studied, such as polyglycolic acid (PGA) and polylactic acid (PLA). These polymers are highly stiff and nonpliable, making the fabrication of scaffolds a difficult process.5 To overcome the limitations of both types of scaffolds, several studies attempted to combine thin synthetic films with extracellular matrix (ECM) components, thus integrating the structural and mechanical properties of synthetic scaffolds with the natural biocompatible ingredients of ECM.6 In the current study, we adapt this approach, testing parylene C (PC) thin polymeric films supported with collagen or fibronectin.
Poly(chloro-para-xylynene) or PC is a member of the family of parylene polymers that are produced through the chemical vapor deposition (CVD) process and are widely used in biomedical applications.7 PC is characterized by its biocompatibility and mechanical robustness and therefore it has been used in coating surgical tools and implantable biomedical devices.8,9 Since PC can be deposited as a continuous nonporous layer, it has been used as a coating to protect sensitive components of biomedical implants, such as blood pressure sensors and cardiac assist devices.10 In addition, PC stencils have been used for cell and protein patterning and for coculture generation.11 Recently, the use of PC as a scaffolding material in tissue engineering has been studied.12–15 To evaluate PC suitability for TEHV, a thorough understanding of its mechanical and physical properties is essential. PC is easy to fabricate, forming a thin uniform pinhole-free coating.12 The process of depositing PC starts by the decomposition of vaporized dichlorodi-p-xylylene, a low-molecular-weight dimer, to produce chloro-p-xylylene, which is then polymerized to the high-molecular-weight PC.7,12 In terms of mechanical properties, PC possesses high strength and stiffness.11 In addition, it is a nondegradable, chemically inert nontoxic polymer that is highly stable in the biological system, possessing a high level of biocompatibility, according to the US Pharmacopeia.12 Nonetheless, one factor that could limit the use of PC in biomedical applications is its hydrophobicity. This can be resolved by plasma oxidizing PC films resulting in the destruction of chemical bonds, making them hydrophilic.12,16
The biocompatibility and ease of fabrication of PC make it a promising candidate as a scaffolding material for TEHV. Thus, we hypothesize that PC thin films will provide the desired interaction with valve cells and will be mechanically suitable for a scaffold of TEHVs. The aim of the current study is to assess PC film compatibility for use in heart valve tissue engineering. In this very early stage of designing a PC scaffold, we have evaluated PC adsorption to collagen and fibronectin, and its biocompatibility with valve endothelial and interstitial cells by assessing cellular adhesion, viability, apoptosis, and phenotypic expression. In addition, we have compared PC mechanical properties to that of the aortic valve by measuring Young's modulus, yield stress, and ultimate tensile strength (UTS).
Materials and Methods
Deposition and plasma oxidation of PC
PC was deposited on microscope glass slides by CVD. Briefly, standard glass slides (76×26 mm) were cleaned in acetone and isopropanol and rinsed with deionized water. Slides were then dehydrated for 60 s at 90°C. Thin films of PC (1.5–6 μm) were deposited through CVD using a (PDS2010) coater (SCS Coating) by vaporizing at 150°C followed by PC dimer pyrolization at 690°C. The film thickness was determined through calibration samples where PC was removed and measured by a Dektak 6M Stylus profiler (Veeco Instruments). PC-coated slides were then oxygen plasma treated using the FEMTO plasma system (Diener Electronics) at 0.1 mbar and 50 W for 10 min. Two PC-coated slides were kept untreated (hydrophobic) to compare hydrophilicity and cellular adhesion. To test the wettability of PC, 10 μL of trypan blue was pipetted on the surface of each sample, and images were captured using high-resolution mobile camera. The contact angle was measured using ImageJ (NIH).
Surface protein staining
To determine PC adsorption to ECM proteins, a plasma oxidized PC slide was divided using a flexiPERM reusable 12-well cell culture chamber (Greiner Bio-One), and wells were coated with either 2.1 mg/m type I rat tail collagen (First Link LTD) for 30 min or 10 μg/mL human fibronectin (PromoCell) for 1 h. Both coatings were done at room temperature, and uncoated wells were included as negative controls. The sample was compared to a microscope slide with the same treatments. After incubation, qualitative staining was performed using picrosirius red staining of collagen, and immunofluorescent staining of fibronectin-coated wells using antifibronectin MAbs (Novotec).
Isolation and cell culture of pulmonary valve endothelial and interstitial cells
Whole hearts from pigs were obtained from an abattoir (Turner's Abattoir). Pulmonary valve endothelial cells (VECs) and pulmonary valve interstitial cells (VICs) were isolated from freshly dissected pig pulmonary valves by collagenase digestion, as previously described.17 Valve cusps were dissected from the root, washed several times with Dulbecco's phosphate-buffered saline (DPBS) (Sigma Aldrich), and then incubated in collagenase type II (Sigma Aldrich) for 10 min at 37°C with shaking. Collagenase was deactivated by fetal bovine serum (FBS) (Sigma Aldrich), the digest was centrifuged, and the endothelial cell pellet was resuspended in the endothelial cell growth medium (EGM) (PromoCell) and transferred to six-well plates coated with 1% gelatin (Sigma Aldrich). To isolate VICs, valves were further incubated in collagenase for 45 min at 37°C. Collagenase was then deactivated by FBS, and cells were pelleted followed by resuspension in Dulbecco's Modified Eagle's Medium (DMEM) (Sigma Aldrich). VICs were then seeded into six-well plates. Cells were grown to confluence and then transferred to T25 flasks. Pulmonary valve endothelial cells (PVECs) were grown in the ECM 2 supplemented with endothelial cell growth medium (EGM-2) (PromoCell), 1% penicillin–streptomycin, 1% glutamine, and 20% fetal calf serum (FCS), whereas pulmonary valve interstitial cells (PVICs) were maintained in DMEM supplemented with 10% FCS, 1% penicillin–streptomycin, and 1% glutamine (all obtained from Sigma Aldrich). All cell cultures were maintained in a 37°C incubator with 5% carbon dioxide, and media were renewed every 3 days.
Assessment of PC cell compatibility
To validate the effect of PC on VEC morphology and adhesion, three groups of plasma oxidized PC-coated slides were used with the following treatments: (1) 1% gelatin coating for 20 min, (2) 2.1 mg/mL type I rat tail collagen coating for 30 min, and (3) 10 μg/mL fibronectin coating for 1 h. Fibronectin and collagen were chosen since they are both present in the valve ECM, while gelatin is a standard protein used to coat glass slides to allow cells to attach and grow on the slides. Another group of PC-coated slides without plasma oxidation was included as a negative control. All of these treatments were compared to the control group grown on sterile microscope slides with 1% gelatin coating. Similar groups of coated slides were used to evaluate the effect of PC on the morphology of VICs, except that no gelatin coating was necessary for interstitial cell attachment to glass or PC. Additional experiments were conducted to evaluate the effect of PC on the proliferation of VECs and VICs. The proliferation of cells on slides coated with plasma oxidized PC without additional coating was compared to plasma oxidized slides coated with PC and a range of concentrations of collagen or fibronectin (10–1000 μg/mL). Slides were attached to 12-well cell culture chambers, and each well was seeded with 10–15×103 cells from passages 4–6 and incubated overnight. Cell number and apoptosis (at a single concentration of each ECM protein) were then studied on each of the slides as described below.
Cell adhesion
After 20 h of incubation, cells were visualized using the Olympus light microscope, and three to four different fields were captured to compare cellular adhesion and morphology.
MTS cell proliferation assay
To determine the number of viable cells, the CellTiter 96® AQueous NonRadioactive Cell Proliferation assay (Promega) was performed following the manufacturer's instructions. The kit is based on the reduction of MTS (a tetrazolium compound) by metabolically active cells to form a water soluble colored formazan compound that is measured at 490 nm. The amount of measured absorbance is directly proportional to the number of viable cells. A standard curve for each type of coating was performed, using five standards for both PVIC and PVEC lines (10, 7, 5, 2, 1×103 cells). Samples and standards were washed with DPBS to remove dead cells, and 100 μL of fresh media was added to each well. A mixture of PMS/MTS solution was prepared by adding 100 μL of PMS solution to 2 mL MTS solution. Each well was then loaded with 20 μL of PMS/MTS mixture and was incubated for 1 h at 37°C. Absorbance was measured at 490 nm using an ELISA plate reader, standard curves were plotted using absorbance and cell numbers, and a trend line was drawn using excel. The trend line's equation was used to determine numbers and percentages of viable cells.
Terminal deoxynucleotidyl transferase dUTP nick end labeling
To estimate the percentage of apoptotic cells, the TUNEL assay was performed using an in situ Cell Death Detection Kit (Roche). Apoptosis detection was assessed by terminal deoxynucleotidyl transferase (TdT) labeling of DNA breaks with modified nucleotides. Cells were fixed in freshly prepared 4% paraformaldehyde in phosphate-buffered saline (v/v) for 1 h at room temperature and were then incubated for 2 min in a permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate). The TUNEL reaction mixture was prepared by mixing 10 μL of TdT with 90 μL of labeling solution. Cells were then washed, and 30 μL of TUNEL reaction mixture was added to each well and incubated for 1 h at 37°C. Cells were then stained with DAPI (4′,6-diamidino-2-phenylindole) nuclear stain, and slides were mounted with mounting media. Cells were counted in five to eight fields, and the percentage of apoptosis was determined. A negative control was included for each treatment, where the TdT enzyme was not added. In addition, positive controls were induced by treatment with 100 μmol H2O2 (1 h in PVECs and 30 min in PVICs), and the suitable treatment duration was determined through a time course.
Mechanical testing of PC and comparison with aortic valves
Preparation of aortic valves
Porcine aortic valve leaflets (right coronary, left coronary, and noncoronary) were isolated from the root and transferred to DPBS. Leaflets were cut in two directions, circumferentially or radially, with a width of 5 mm, and were mechanically tested using the Bose instrument.
Uniaxial testing
Uniaxial testing of heart valves and PC membranes was carried out using the Bose instrument (Bose Electroforce), and data were acquired by WinTest software. The width of the samples was measured using a caliper, and the initial distance between the clamps was set up to 8 mm. The thickness of PC was measured using a stylus profiler as described in the Materials and Methods section, while leaflet thicknesses were determined microscopically. Samples were loaded into the clamps, and tensile testing was applied. Since PC films are very thin, films were attached to the clamps using tape at each end to avoid slipping of samples during testing.
Tissue samples were exposed to cyclic uniaxial extension for 20 cycles with a frequency of 0.1 Hz and a displacement of 4 mm. PC samples were tested using the stretch to failure method, where samples were stretched at 0.1 Hz with a speed of 0.05 mm/min to a maximum displacement of 5.8 mm. The machine applies gradual tensile load on the sample and records force and displacement throughout the experiment.
Young's modulus and UTS were calculated for each sample. This was done by drawing a stress–strain curve, where the slope of the linear region of the curve is Young's modulus, and the maximum load the sample reaches before failure is the UTS. To draw a stress–strain curve, stress (ơ) was calculated by dividing the force (F) by the cross-sectional area (A), where the cross-sectional area is equal to width (W) multiplied by thickness (h) (Equation 1.1). Strain (Ɛ) was calculated by dividing the change in length (Δl) by initial length (l˚) (Equation 1.2). Data analysis and curve generation were done using Excel.
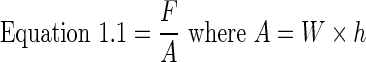 |
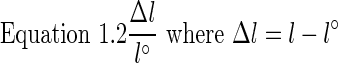 |
Dynamic evaluation of PC films with a pulsatile bioreactor
Briefly, the bioreactor comprised two parts: the central part being a pressurized windkessel chamber (by means of an adjustable volume of air above the tissue culture medium), surrounded by a second cylinder forming an external reservoir vented to the atmosphere with a 0.2-μm syringe filter to maintain sterility and allow gas exchange. The outer reservoir and the inner chamber were connected to the inlet and outlet of a ventricular assist device (VAD), respectively. The two chambers were also connected by silicon tubing around which an adjustable gate clamp could be placed. PC film mounted cassettes were mounted over holes in the wall between the inner and outer chamber. Instantaneous pressure profiles within the windkessel and the reservoir were simultaneously recorded with transducers (6600 series amplifier; Edwards Lifesciences) that had been precalibrated with a water column. The transducer output was connected to an analog-to-digital converter (Model Micro 1401; Cambridge Electronic Design) with proprietary data capture and analysis software (Spike 2, version 7). This allowed the instantaneous pressure gradient across the valve to be determined. The VAD was attached to a stepper motor (Placepower UK Ltd).18 Once assembled, the bioreactor was moved into an incubator at 37°C and 5% CO2 attached to the VAD. Pressure gradient between the windkessel and the reservoir was set at 0–50 mmHg with the stepper motor set at 60 beats/min. These conditions were kept for 3 days. PC films were then removed from the cassettes and stained as previously described with primary antibodies against vimentin (1:100) (Dako).
Exposure of PC films to aortic flow patterns
PVECs were seeded overnight on the different coated PC films; films were then peeled off, cut, and mounted in a purpose-built flow chamber.19 The chamber is composed of a spinning cone made with a 179° angle and a cylindrical plate containing nine equally spaced wells where PC films can be mounted. PC films were exposed to a flow pattern simulating that of the aortic side of the aortic valve for 3 days. This period was chosen since we were confident from previous studies that the cells would remain viable in the bioreactor over this time frame. During exposure to flow, samples were kept at 37°C, 5% CO2 with DMEM supplemented with 0.4% FCS, 1% penicillin–streptomycin, and 1% glutamine (all obtained from Sigma Aldrich). After 3 days, films were washed with DPBS and staining was performed as previously described: cells were stained with TRITC-conjugated phalloidin to look at cell shape and nuclei were stained with DAPI.
Statistical analysis
Statistical analysis was carried out using GraphPad prism statistical software. Data are expressed as mean±standard deviation. Samples were tested using ANOVA, and upon significance, multiple comparisons were done using the t-test, followed by Bonferroni correction. A p-value<0.05 was considered significant.
Results
PC hydrophobicity and contact angle
To check the effect of plasma oxidation in inducing membrane hydrophobicity, the contact angles of plasma-treated and nontreated PC membranes were compared. The mean contact angle of the trypan blue drop was significantly decreased from 55.5°±3° to 18°±1.3° after plasma treatment of PC with 50% O2 for 10 min (n=2, p=0.0001) (Fig. 1).
FIG. 1.
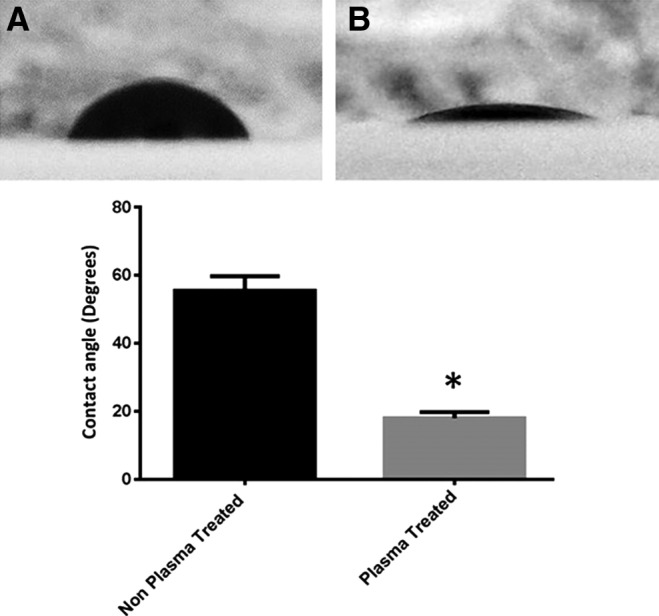
Wettability of parylene C (PC). The contact angle of 10 μL trypan blue drop with PC was measured before plasma treatment (A) and after plasma treatment (B) (n=2, p<0.0001).
Biocompatibility of PC
Adsorption of ECM proteins onto PC
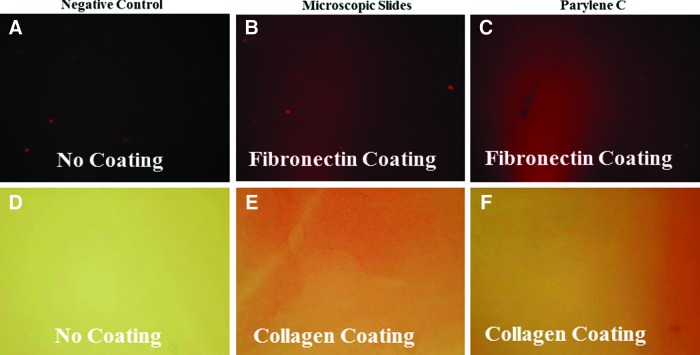
Plasma oxidized PC slides were coated with collagen or fibronectin and then stained to check for their adsorption of each protein. Fibronectin adsorption was detected by immunofluorescence, and fibronectin-coated PC showed positivity in a similar pattern to the staining of fibronectin-coated microscopic slide (n=2) (Fig. 2A–C). Similarly, collagen-coated slides were stained with picrosirius red, where collagen-coated PC slides showed positivity (n=2) (Fig. 2D–F).
FIG. 2.
PC adsorption of fibronectin and collagen. (A–C) Staining of fibronectin using antifibronectin fluorescent antibody (red stain) and (D–F) detection of collagen adsorption using Picrosirius (red stain). A plain microscopic slide was used as negative control. Microscopic slides coated with either collagen or fibronectin were used as positive controls and were compared to PC-coated slides. Color images available online at www.liebertpub.com/tea
PC compatibility with cell adhesion and viability
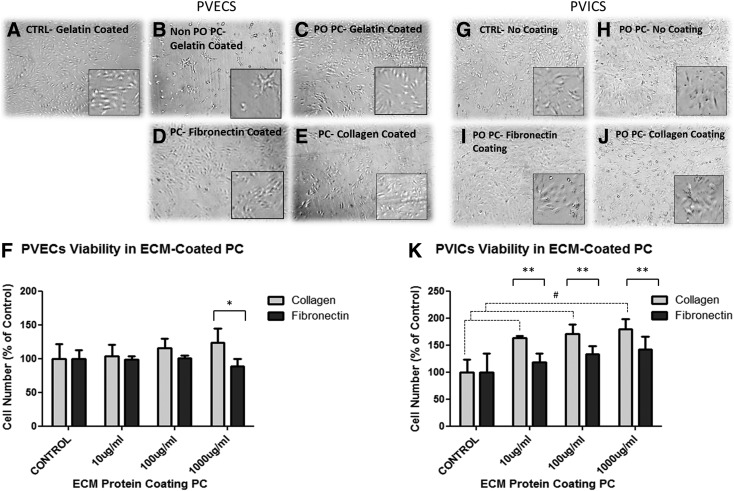
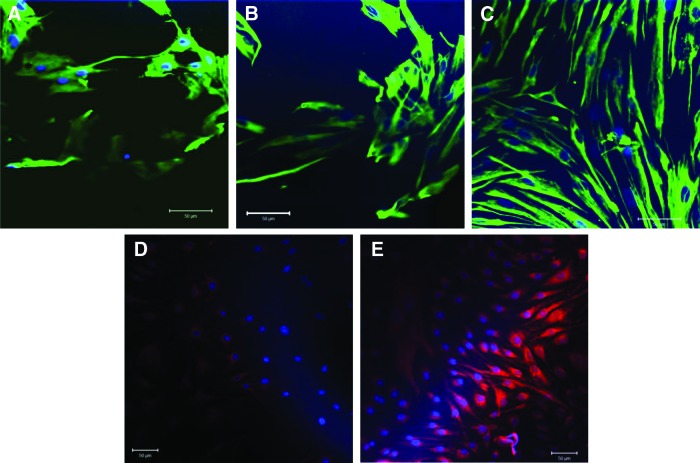
PVECs show poor adhesion to plain glass slides; thus, to allow attachment, it was necessary to coat the surface of the control slides with gelatin. Cells attached only weakly to nonplasma oxidized gelatin-coated PC slides. In contrast, cell morphology and attachment were enhanced on plasma-treated gelatin-coated PC slides, with a similar pattern to control grown on gelatin-coated glass slides (Fig. 3A–C). The number of PVECs grown on plasma oxidized PC membranes remained stable, while those grown on PC without plasma treatment were significantly reduced (Fig. 3F). Subsequently, all the following experiments were carried out using plasma-treated PC.
FIG. 3.
Cell adhesion and viability of pulmonary valve endothelial cells (PVECs) and pulmonary valve interstitial cells (PVICs) grown on PC. The upper left hand panel shows morphology of PVECs grown on slides with (A) gelatin, (B) PC without plasma oxidation, (C) PC with plasma oxidation, (D) PC with plasma oxidation and coated with fibronectin, and (E) PC with plasma oxidation coated with collagen. (F) Shows the number of adherent cells on slides treated with 10–1000 μg/mL of either collagen or fibronectin. Coating PC membranes with collagen significantly enhanced PVEC number when compared to cells grown on slides coated with fibronectin (*p<0.001, N=4). The upper right hand panel shows the morphology of PVICs on slides with (G) no coating, (H) PC without plasma oxidation, (I) PC with plasma oxidation and coated with fibronectin, and (J) PC with plasma oxidation coated with collagen. (K) Shows the number of adherent cells on slides treated with 10–1000 μg/mL of either collagen or fibronectin. Coating PC with either collagen enhances PVIC number compared to control and slides coated with fibronectin (#p<0.05 [control compared to collagen], **p<0.001 [collagen compared to fibronectin], respectively, N=4).
The adhesion of PVECs to plasma oxidized PC was tested with different types of coatings and their morphology was compared to gelatin-coated PC slides and plain gelatin-coated glass slides. PVECs grown on collagen and fibronectin showed comparable morphology to cells on glass and plasma oxidized PC-only slides (Fig. 3C–E). The PVEC number remained stable on collagen- and fibronectin-coated slides at all concentrations studied compared to uncoated slides; however, the cell number was significantly higher on slides coated with 1000 μg/mL of collagen compared to the same slides coated with the same concentration of fibronectin (p<0.01, n=4) (Fig. 3F).
In contrast, PVICs are able to attach to plain uncoated glass slides. The adhesion and morphology of PVICs grown on plasma oxidized PC showed similar patterns to those grown on uncoated glass slides, and plasma-treated PC slides coated with collagen and fibronectin, with a typical spindly morphology (Fig. 3G–J). The PVIC number was enhanced when plasma oxidized PC slides were coated with 10, 100, and 1000 μg/mL of collagen compared to uncoated slides (n=4, p<0.005 and p<0.001, respectively), but not those coated with the same concentrations of fibronectin. The cell number was significantly greater at all coating concentrations on slides coated with collagen compared to those coated with fibronectin (p<0.05, n=4) (Fig. 3K).
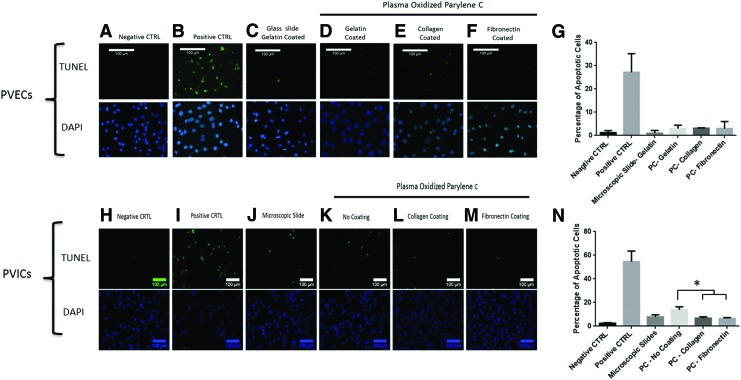
The incidence of apoptotic cells confirmed the MTS assay findings, where programmed cell death percentage of PVECs was less than 3.4% for all cells grown on all coatings (N=2) (Fig. 4A–G). In PVICs, cells grown on uncoated PC showed slightly higher levels of apoptosis, where 13.9%±2.5% of cells were apoptotic (p=0.01), while coating PC with collagen and fibronectin resulted in a lower incidence of apoptosis of 6.82%±0.56% (p=0.002) and 6.58%±0.39% (p=0.002), respectively (N=2) (Fig. 4H–N).
FIG. 4.
Effect of different coatings on the incidence of apoptosis. TUNEL and DAPI staining of PVECs (A–G) and PIVCs (H–N) on different coatings. The negative control is where TdT enzyme was omitted, and the positive control is represented with cells treated with H2O2. PVECs and PVICs were grown in standard culture conditions on slides coated with gelatin alone (or plan microscopic slides for PVICs) and slides coated with PC and gelatin (or PC without coating for PVICs), collagen or fibronectin. Cells cultured on PC with different coatings showed very low levels of apoptosis, with no significant difference when compared to cells grown on microscopic slides. *p<0.05. Color images available online at www.liebertpub.com/tea
Mechanical properties of PC
Comparison to aortic valve biomechanics
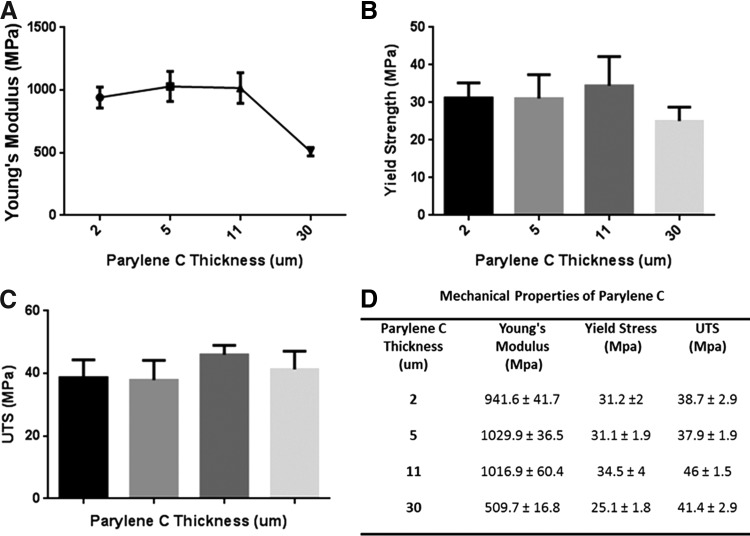
Uniaxial testing of different thicknesses of PC revealed that there is no significant change in Young's modulus for membranes of thickness less than 11 μm (n=4, p=0.77). However, a significant drop in the Young's modulus was observed for the 30-μm-thick membranes (n=4, p<0.0001) in comparison with each of the three other thicknesses of PC studied. In contrast, yield strength and UTS did not show any significant difference in PC films of different thicknesses (n=4, p=0.19 and p=0.13, respectively) (Fig. 5).
FIG. 5.
Mechanical properties of different thicknesses of PC. PC was tested for Young's modulus (A), yield strength (B), and ultimate tensile strength (UTS) (C). Young's modulus was the same for thicknesses less than 11 μm, but tended to decrease in 30 μm thickness (p<0.0001, N=4). UTS and yield strength are not affected by thickness. (D) Table summarizes PC mechanical properties of different thicknesses.
PC mechanical properties in comparison to aortic valve
Mechanical testing of the aortic valve revealed a significant difference in the Young's modulus between the circumferential (2.8±2.09) and the radial direction (0.6±0.19) (data not shown). These findings agree with what was previously reported by Li et al., where they showed that Young's modulus on the circumferential direction is six times higher than that in the radial direction, due to the circumferential orientation of the collagen fibers.20
To compare the aortic valve stiffness with that of PC, we pooled the data of PC with thicknesses between 2 and 11 μm (since Young's modulus was consistent in these films) and compared the Young's modulus of the aortic valve at the radial and circumferential direction. The results suggest a strong significance in the Young's modulus between all PC thicknesses with the aortic valve in both directions (p<0.0001) (Table 1).
Table 1.
Stiffness of PC in Comparison to the Aortic Valve
| PC | Aortic valve | |||
|---|---|---|---|---|
| Sample | PC (<11 μm) | PC (30 μm) | Radial direction | Circumferential direction |
| Young's modulus (Mpa) | 1008.6±114.1 | 509.7±16.8 | 0.64±0.2 | 2.82±2.09 |
PC, parylene C.
Exposure of PC films to pulsatile pressure
PC films seeded with PVICs were exposed to pulsatile pressure with a pressure profile of 0–50 mmHg. After 3 days, cells on uncoated PC films and fibronectin-coated PC were sparsely distributed, loosely attached, and they did not express a fibroblast-like morphology (Fig. 6A, B). In contrast, cells on PC with collagen were more uniformly distributed, with good attachment and a spindle-like elongated morphology (Fig. 6C).
FIG. 6.
Effect of exposure to flow and pressure in PVECs and PVICs, respectively. PVICs exposed to 0–50 mmHg cyclical pressure at a frequency of 1.0 Hz seeded on PC films uncoated (A), coated with fibronectin (B) and collagen (C), and stained for Vimentin and nuclei counterstained with DAPI. PVECs exposed to aortic flow pattern for 3 days on PC films uncoated (D), coated with collagen (E), and stained with TRITC-conjugated phalloidin. Scale bars=50 μm. Color images available online at www.liebertpub.com/tea
Exposure of PC films to aortic flow pattern
PC films seeded with PVECs were exposed to the aortic flow pattern. After 3 days of exposure, PVECs were still attached to PC films, regardless of the coating. Due to the PC films being very thin, once they have been peeled off it is difficult to mount them completely flat on a coverslip. This created different focal planes and the appearance of a shaded area, although the staining was uniform (Fig. 6D, E).
Discussion
Biocompatibility and mechanical strength are important factors in evaluating the suitability of synthetic materials for future use in tissue engineering.21 In the current work, we showed that ECM proteins are able to absorb onto plasma-activated PC and allow PC to be biocompatible with endothelial and interstitial cells. We also showed that different thicknesses of PC exhibit a higher tensile strength and distensibility than aortic valve tissue.
Interactions of scaffolds used in tissue engineering with the biological system are determined by their surface chemistry. It is desirable to modify these surfaces in such a manner that enhances interactions with cells.22 Cell adhesion in vivo occurs through the binding of integrins on the cell surface with specific regions of different ECM proteins. Therefore, precoating the scaffold with ECM components provides a favorable environment for cellular adhesion which, in turn, facilitates their migration into the scaffold and subsequent proliferation. The ability of the scaffold material to adsorb ECM proteins is important in providing such an environment.13 In addition, surface wettability is an important factor in enhancing cell adhesion and protein adsorption.23 In this study, we applied two surface modifications to PC: first, we plasma oxidized PC to induce its hydrophilicity, and then we coated the material with a range of concentrations of ECM proteins to provide a favorable environment for cellular adhesion and growth. Collagen and fibronectin were chosen since type 1 collagen is the most abundant type of collagen in the normal valve and fibronectin is an important glycoprotein for interstitial cell repair.24 In our experience, it was difficult to get the PC films to lie completely flat, which made en face imaging and quantification of the staining for collagen or fibronectin problematic. Our findings suggest that ECM proteins are able to bind to plasma-treated PC and that binding to collagen promotes VIC proliferation and that VECs are more proliferative when in contact with collagen compared to the same concentrations of fibronectin.
Many studies had investigated the biocompatibility of PC with the growth of different cell types, such as fibroblasts (NIH 3T3), hepatocytes,12 human umbilical vain endothelial cells,13 and retinal pigment epithelial cells.14 In addition, a study by Schleicher et al. showed that PC exhibits low thrombogenicity and excellent hemocompatibility after oligonucleotide coating.13 In the current study, we showed that cellular morphology, phenotype, and viability of PVECs and PVICs grown on plasma-treated PC showed similar patterns to cells grown in normal in vitro conditions.
Since interstitial and endothelial cells are the two resident cell types in the normal valve, studying their growth behavior and functionality is a crucial step in evaluating PC suitability as a valve scaffold. Although some studies reported the use of fully differentiated interstitial and endothelial cells in valve implants, this approach is associated with surgical intervention to harvest donor vascular tissue from which cells can be isolated and expanded in culture.25 An effective alternative is to use stem cells that have the ability to differentiate and function as valve interstitial and endothelial cells. A recent study showed that adipose-derived stem cells exhibit the ability to produce and remodel ECM proteins.17 Schmidt et al. showed the ability of endothelial progenitor cells derived from peripheral blood to endothelialize TEHV in vivo.26 The next step should be to test the compatibility of PC with the growth and functionality of stem cells and to study their ability to differentiate and secrete their own ECM over a longer time frame than used in the current study.
The biocompatibility of the scaffold should be combined with mechanical strength equivalent to that of the normal valve. We tested the mechanical properties of PC at four different thicknesses (2, 5, 11, and 30 μm) and found that all four thicknesses possess a high elastic modulus (509.7–1008.5 MPa), which is more than 180-fold higher compared with the normal valve. Our findings also suggest that PC of thicknesses up to 11 μm exhibit the same range of stiffness with no significant difference, while the highest thickness showed a significant decrease in the elastic modulus. In theory, the elastic modulus should not be affected by the thickness, since it is normalized to the cross-sectional area of the sample. Our findings could be related to an increase of the material's surface roughness that is associated with increased thickness. This has not been proved for PC and we could not find any studies investigating the effect of thickness on the elastic modulus except in the nanoscale. Another possibility is that sample processing before mechanical testing affected the obtained results and that this trend is associated with technical errors rather than being an actual decrease. Handling very thin films presented a number of technical difficulties in loading the thin films onto the testing rig. These included unintentional stretching of the sample before loading, positioning in the clamps of the test rig, and the ability of the clamp to hold the membranes tightly. Some of these issues were solved by first fixing the sample to the clamp using tape, while they were closed tightly. However, it was clear from the data obtained that the mechanical strength of PC in all thicknesses studied is highly resistant to fracture and plastic deformation, as indicated by their high yield strength and UTS.
Although our initial data showed that PVICs attached well to both fibronectin- and collagen-coated films, those experiments were performed into a static environment without any dynamic conditioning. To test the PC-seeded films in a more physiological environment, we exposed them to pulsatile pressure and to the aortic flow pattern. While the cells remained attached to the PC under conditions of pressure and flow, regardless of which ECM coating was used, the data obtainable on the phenotype of the cells were limited due to technical issues. Since collagen contains many different cell-binding sites compared to fibronectin, it is possible that with the exposure to pulsatile pressure or flow, the phenotype of cells may differ when compared to either PC only or fibronectin. A second consideration is the stiffness of the underlying substrate. There will be a significant influence of the stiffness of the glass slides used in the first part of this study compared to when the PC is mounted as a free unsupported material in the pulsatile bioreactor. Additional studies are required to ascertain the exact influence of the stiffness of PC on cell function and phenotype under physiological conditions of flow (using flow patterns experienced by either surface of the valve) and pressure.
This study has utilized pulmonary valve VICs and VECs. Phenotypically, these cells are similar to those isolated from the aortic valve27,28; however, some differences have been reported in the remodeling response of cells from each valve.29 The data presented in this study with pulmonary valve cells serve mainly as a proof on concept that valve cells can adhere and proliferate on plasma-activated PC coated with ECM proteins. Further studies should focus on the remodeling potential of the cells as well as on the use of human valve cells, ideally from the aortic valve.
Our preliminary findings suggest that PC holds a promise for successful use as a scaffolding material for TEHV. Its proven biocompatibility with the biological system, in combination with its high mechanical properties, and the ability to enhance the adhesion and phenotypic characteristics of valve cells indicate that it is a good candidate for future use. PC overcomes other synthetic polymers like PGA and PLA with their relatively lower level of biocompatibility, as evidenced by their poor rate of cell growth.4 In addition, these polymers are nonpliable, in contrast to the flexible nature of PC that could be easily modified and fabricated to cope with TEHV requirements.
There are a number of potential limitations to the use of PC, which include a lack of porosity,14 a lack of anisotropy in its mechanical properties, and the fact that the stiffness of the material is an order of magnitude greater compared with the native valve. These limitations would prevent cellular migration into the structure of the scaffold and would provide a miss-match to the normal mechanical properties of native valve tissue. However, there are various strategies that could be employed to circumvent these limitations. In addition, the current studies of the effect of PC on cells under physiological conditions were only performed over a short time frame (3 days). Validation of the apparatus used in these experiments containing cells and PC over a much greater time frame is required before longer term experiments can be reliably performed.
Porosity could be changed by introducing holes to the PC films through standard lithographic techniques and oxygen plasma etching,16 which has been demonstrated to be feasible through cell patterning experiments.30 Porosity of the scaffolding material is important for cell growth and migration, which allows ECM secretion and three-dimensional (3D) regeneration of the tissue.21 Pores are also effective in decreasing necrosis caused by clustering of cells, where cell growth will be confined to the size of the pore.31
In the native valve, the collagen matrix is highly organized in a way that provides the necessary strength circumferentially and the flexibility in the radial direction.32 Previous studies showed that PC could be fabricated in the form of nanowires by vapor deposition from a directional vapor source, which exhibits anisotropic mechanical features, in addition to porosity.33
With regard to the stiffness of PC films, this could be adjusted by mixing PC with other polymeric materials such as polycaprolactone (PCL), which is a biocompatible biodegradable polymer that could be manufactured in a unique porous structure, therefore neutralizing the strength of PC. In addition, it had been previously shown that there is an inverse relationship between Young's modulus and porosity, thus creating pores in PC membranes, as mentioned above, may also potentially decrease the stiffness to a desirable level.34,35 The potential to make composite scaffolds with other materials remains a possibility. This may include the synthesis of sandwich scaffolds containing a layer of PC. This study illustrates that if PC is incorporated into the body of the scaffold, then it can be compatible with ECM proteins and cells that may populate the scaffold.
As a first step, further studies are required to gain additional information about the interaction of PC with cells that may be used in TEHV. The biocompatibility of PC with stem cells of different sources (bone marrow, adipose tissue, and others) should be studied. In addition, evaluating the ability of the cells to survive and function for prolonged periods is crucial and will provide a better understanding of cell behavior and growth pattern on PC. Furthermore, different strategies to decorate the surface of PC with protein sequences that will attract and bind specific cell types can be developed to maximize cell attachment in vitro or in vivo. Models for fabricating PC scaffolds for TEHV could also include the development of 3D PC scaffold through layer-by-layer assembly of PC thin nanowires with cells seeding on each layer.
In conclusion, this study showed that PC is compatible with valve cells and ECM adsorption. The in vivo behavior of this scaffold needs to be evaluated.
Acknowledgments
This study was funded from a grant awarded by the M.H.Y. Institute. I.M. was funded by support from the Qatar Foundation Research and Development Division, Doha-Qatar.
Disclosure Statement
No competing financial interests exist.
References
- 1.Carabello B.A., and Crawford F.A., Jr Valvular heart disease. N Engl J Med 337, 32, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Mahmood K.T., and Asghar A.M. A valvular heart diseases—a review. J Biomed Sci Res 3, 315 2011 [Google Scholar]
- 3.Shaikh F.M., Callanan A., Kavanagh E.G., Burke P.E., Grace P.A., and McGloughlin T.M. Fibrin: a natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs 188, 333, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Mendelson K., and Schoen F.J. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng 34, 1799, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasi L.P., Simon H.A., Sucosky P., and Yoganathan A.P. Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol 36, 225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon D., and Fisher J. Natural and synthetic polymeric scaffolds. In: Narayan R., ed. Biomedical Materials. Springer Science & Business Media LLC, New York, 2009, pp. 415 [Google Scholar]
- 7.Tan C.P., and Craighead H.G. Surface engineering and patterning using parylene for biological applications. Materials 3, 1803, 2010 [Google Scholar]
- 8.Kammer S., Wien S., Koch K.P., Robitzki A., and Stieglitz T. [Coating material of parlene C as encapsulation material for biomedical micro-implants]. Biomed Tech (Berl) 47 Suppl 1 Pt 2, 823, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Wolgemuth L. Crystal-clear coating covers components. Med Des 6, 2006 [Google Scholar]
- 10.Stark N. Literature review: biological safety of parylene C. Med Plastic Biomater 3, 30, 1996 [Google Scholar]
- 11.Wright D., Rajalingam B., Karp J.M., Selvarasah S., Ling Y., Yeh J., Langer R., Dokmeci M.R., and Khademhosseini A. Reusable, reversibly sealable parylene membranes for cell and protein patterning. J Biomed Mater Res A 85, 530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang T.Y., Yadav V.G., De Leo S., Mohedas A., Rajalingam B., Chen C.L., Selvarasah S., Dokmeci M.R., and Khademhosseini A. Cell and protein compatibility of parylene-C surfaces. Langmuir 23, 11718, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Schleicher M., Hansmann J., Elkin B., Kluger P.J., Liebscher S., Huber A.J., Fritze O., Schille C., Muller M., Schenke-Layland K., Seifert M., Walles H., Wendel H.P., and Stock U.A. Oligonucleotide and parylene surface coating of polystyrene and ePTFE for improved endothelial cell attachment and hemocompatibility. Int J Biomater 2012, 397813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu B., Zhu D., Hinton D., Humayun M.S., and Tai Y.C. Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomedical Microdevices 14, 659, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Trantidou T., Rao C., Barrett H., Camelliti P., Pinto K., Yacoub M.H., Athanasiou T., Toumazou C., Terracciano C.M., and Prodromakis T. Selective hydrophilic modification of parylene C films: a new approach to cell micro-patterning for synthetic biology applications. Biofabrication 6, 025004, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Trantidou T., Prodromakis T., and Toumazou C. Oxygen plasma induced hydrophilicity of parylene-C thin films. Appl Surf Sci 261, 43, 2012 [Google Scholar]
- 17.Colazzo F., Sarathchandra P., Smolenski R.T., Chester A.H., Tseng Y.T., Czernuszka J.T., Yacoub M.H., and Taylor P.M. Extracellular matrix production by adipose-derived stem cells: implications for heart valve tissue engineering. Biomaterials 32, 119, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Bowles C.T., New S.E.P., Van Loon R., Dreger S.A., Biglino G., Chan C., Parker K.H., Chester A.H., Yacoub M.H., and Taylor P.M. Hydrodynamic evaluation of a bioreactor for tissue engineering heart valves. Cardiovasc Eng Tech 1, 10, 2010 [Google Scholar]
- 19.Sucosky P., Padala M., Elhammali A., Balachandran K., Jo H., and Yoganathan A.P. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J Biomech Eng 130, 035001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J., Luo X.Y., and Kuang Z.B. A nonlinear anisotropic model for porcine aortic heart valves. J Biomech 34, 1279, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Yang S., Leong K.F., Du Z., and Chua C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng 7, 679, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Vendra V.K., Wu L., and Krishnan S. Polymer thin films for biomedical applications. In: Nanotechnologies for the Life Sciences. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2007 [Google Scholar]
- 23.Lee J.H., and Lee H.B. A wettability gradient as a tool to study protein adsorption and cell adhesion on polymer surfaces. J Biomater Sci Polym Ed 4, 467, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Taylor P.M., Cass A.E.G., and Yacoub M.H. Extracellular matrix scaffolds for tissue engineering heart valves. Prog Pediatr Cardiol 21, 219, 2006 [Google Scholar]
- 25.Weber B., Emmert M.Y., and Hoerstrup S.P. Stem cells for heart valve regeneration. Swiss Med Wkly 142, w13622, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt D., Dijkman P.E., Driessen-Mol A., Stenger R., Mariani C., Puolakka A., Rissanen M., Deichmann T., Odermatt B., Weber B., Emmert M.Y., Zund G., Baaijens F.P., and Hoerstrup S.P. Minimally-invasive implantation of living tissue engineered heart valves: a comprehensive approach from autologous vascular cells to stem cells. J Am Coll Cardiol 56, 510, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Taylor P.M., Allen S.P., and Yacoub M.H. Phenotypic and functional characterization of interstitial cells from human heart valves, pericardium and skin. J Heart Valve Dis 9, 150, 2000 [PubMed] [Google Scholar]
- 28.Brand N.J., Roy A., Hoare G., Chester A., and Yacoub M.H. Cultured interstitial cells from human heart valves express both specific skeletal muscle and non-muscle markers. Int J Biochem Cell Biol 38, 30, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Merryman W.D., Liao J., Parekh A., Candiello J.E., Lin H., and Sacks M.S. Differences in tissue-remodeling potential of aortic and pulmonary heart valve interstitial cells. Tissue Eng 13, 2281, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Eshraghi S., and Das S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater 6, 2467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhandayuthapani B, Yoshida Y., Maekawa T., and Kumar D.S. Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci 2011, 2011 [Google Scholar]
- 32.Bouten C.V., Dankers P.Y., Driessen-Mol A., Pedron S., Brizard A.M., and Baaijens F.P. Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev 63, 221, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Demirel M.C., Boduroglu S., Cetinkaya M., and Lakhtakia A. Spatially organized free-standing poly(p-xylylene) nanowires fabricated by vapor deposition. Langmuir 23, 5861, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Alam P. A mixtures' model for porous particle–polymer composites. Mech Res Commun 37, 389, 2010 [Google Scholar]
- 35.Zeleniakienė D., Kleveckas T., Liukaitis J., and Fataraitė E. The influence of porosity value and mode on soft polymer materials behaviour. Mater Sci 9, 201, 2003 [Google Scholar]