Abstract
Malaria is the most important parasitic disease worldwide, accounting for 1 million deaths each year. Severe malaria is a systemic illness characterized by dysfunction of brain tissue and of one or more peripheral organs as lungs and kidney. The most severe and most studied form of malaria is associated with cerebral complications due to capillary congestion and the adhesion of infected erythrocytes, platelets, and leukocytes to brain vasculature. Thus, leukocyte rolling and adhesion in the brain vascular bed during severe malaria is singular and distinct from other models of inflammation. The leukocyte/endothelium interaction and neutrophil accumulation are also observed in the lungs. However, lung interactions differ from brain interactions, likely due to differences in the blood-brain barrier and blood-air barrier tight junction composition of the brain and lung endothelium. Here, we review the importance of endothelial dysfunction and the mechanism of leukocyte/endothelium interaction during severe malaria. Furthermore, we hypothesize a possible use of adjunctive therapies to antimalarial drugs that target the interaction between the leukocytes and the endothelium.
1. Introduction
Malaria is the most important parasitic disease worldwide. It is present in more than 100 countries, putting 1.2 billion people at risk and accounting for more than 800 thousand deaths each year [1, 2]. Cerebral malaria (CM) is the most severe form of malaria and is usually found in children under five years old [3]. Clinically, CM is defined by the identification of P. falciparum in peripheral blood, convulsions, and coma, after ruling out any other cause of coma such as meningitis [4, 5]. Pathological findings such as capillary congestion, production of proinflammatory cytokines, and adhesion of infected red blood cells (iRBC) to brain vasculature are responsible for cerebral complications associated with CM [6]. In some patients, a systemic illness called severe malaria (SM) is observed which is characterized by one or more peripheral organ dysfunctions as acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) [7] and acute kidney injury [8, 9] and can be combined with cerebral malaria signals [10]. Some authors suggest that SM is due to pathological events such as parasitized erythrocytes, leukocyte adhesion to the organ microvasculature, systemic production of cytokines, and cytotoxic lymphocyte activation [11, 12]. Despite systemic activation, the leukocyte/endothelial cell interaction differs depending on the studied organ. Here, we discuss endothelial dysfunction during severe malaria and the mechanisms by which leukocytes adhere to the endothelium in distinct organs during this pathology.
2. Leukocyte-Endothelium Interaction during Cerebral Malaria
A main characteristic of brain physiology is the immune privilege conferred by the BBB to brain tissue [13]. However, the BBB composition, especially in the postcapillary venule, allows leukocyte diapedesis during nonmalarial brain injury [14, 15].
During human cerebral malaria, the importance of infected red blood cells adhesion to brain microvasculature is well established [5]. Necropsy of fatal cases of severe malaria shows the adhesion of iRBC in the venules and capillaries, causing congestion [6, 16, 17]. The mechanism of iRBC adhesion to brain microvasculature is well described and depends on expression of membrane proteins such as P. falciparum erythrocyte membrane proteins (PfEMP1) [18]. However, the leukocyte-endothelium interaction during human cerebral malaria is not completely clarified [12, 16, 19, 20]. Indeed, it is well established that both endothelium [21] and leukocyte [22, 23] are activated in patients diagnosed with CM; however, how they orchestrate the brain injury to develop CM is still not well understood.
Endothelium activation markers have been used in clinical studies to predict malaria severity [24, 25]. During CM, the endothelium can be activated by different mechanisms as the binding of soluble proteins present in host serum [24], direct contact with iRBC [6], and activation induced by parasite-derived molecules as hemozoin [26] and GPI [27]. Necropsy performed in fatal cases of CM showed increased expression of adhesion molecules on brain microvasculature [28] supporting the idea that the endothelium is able to promote leukocyte adhesion. Some studies show the presence of leukocyte in brain vasculature lumen [16] or in perivascular space [29], although there are no lines of evidence of the importance of leukocyte adhesion to brain vasculature in development of human CM. However, it cannot be ruled out considering the lack of knowledge in this issue [16, 20].
The interaction between leukocytes and endothelial cells during human CM could not depend on cell-cell contact. Instead, leukocytes and lymphocytes produce inflammatory mediators as TNF-α which activate endothelial cells [28, 30]. Endothelial activation induced by TNF-α accounts for many factors involved in development of CM [31] as increased iRBC adhesion [30], expression of leukocyte chemotactic factors [32] and, costimulated by iRBC, increases ICAM-1 expression that improve iRBC adhesion [30].
On the other hand, the adhesion of leukocytes to brain vasculature is often observed during experimental cerebral malaria [33, 34]. A recent report revealed that the majority of leucocytes accumulated in the brain during experimental severe malaria are monocytes. These cells are responsible for the recruitment of CD4+ and CD8+ T cells to the CNS vasculature [35]. However, in the absence of monocytes, T cells are still recruited to the brain to initiate experimental cerebral malaria [35]. Observation of the microvessels within the brains of live animals demonstrated the marginalization of leukocytes and platelets aggregates in postcapillary brain venules but not in capillaries of P. berghei-infected mice, showing that leukocytes do not accumulate in brains tissue but induce endothelium dysfunction, leading to vascular leakage, neurological signs, and coma [35, 36]. The role of adhesion molecules, especially ICAM-1, in the leukocyte/endothelium interaction to promote cerebral dysfunction during experimental severe malaria is controversial. The impairment of the ICAM-1/β2-integrin complex abolishes the development of cerebral dysfunction associated with P. berghei infection [36–38]. However, Ramos and colleagues deleted ICAM-1 in different cells and showed that only ICAM-1 expressed in leukocytes accounts for experimental severe malaria [39]. The authors speculated that because endothelial cells do not express ICAM-1 counter receptor, leukocytes, platelets, and iRBC aggregates occlude brain microvessels and promote cerebral malaria [39].
A new approach of leukocyte and endothelium interaction in brain during CM has been proposed through interaction between MHC class I molecules and CD8+ T lymphocytes. Recent studies regarding experimental CM show that the membranes of endothelial cells and iRBC fuse by trogocytosis, resulting in the expression of Plasmodium antigens [40]. Endothelial cells preferentially phagocytize merozoites and, via proteasome digestion, present plasmodial antigens by MHC class I molecules to CD8+ T lymphocytes, thus contributing to the adaptive immune response to P. berghei infection [41]. It is noteworthy that the same results were observed within P. falciparum phagocytosis by human endothelial cells [41]. However, P. falciparum phagocytosis by endothelial cells in vivo and its clinical relevance remain to be elucidated.
Overall, microvascular congestion observed in both human and experimental CM leads to severe cerebral endothelial damage, resulting in the breakdown of the BBB mainly at the level of postcapillary venules [16, 29, 31, 42]. The postcapillary venule BBB (Figure 1) is functionally distinct from other BBB areas and is in direct contact with the perivascular space [42]. In light of the new findings concerning brain anatomy in which the authors described the presence of lymphatic vessels in direct contact with the perivascular space in the central nervous system, in the next few years, the dynamics of the interaction between leukocytes and the endothelium during cerebral malaria will likely be unveiled [43].
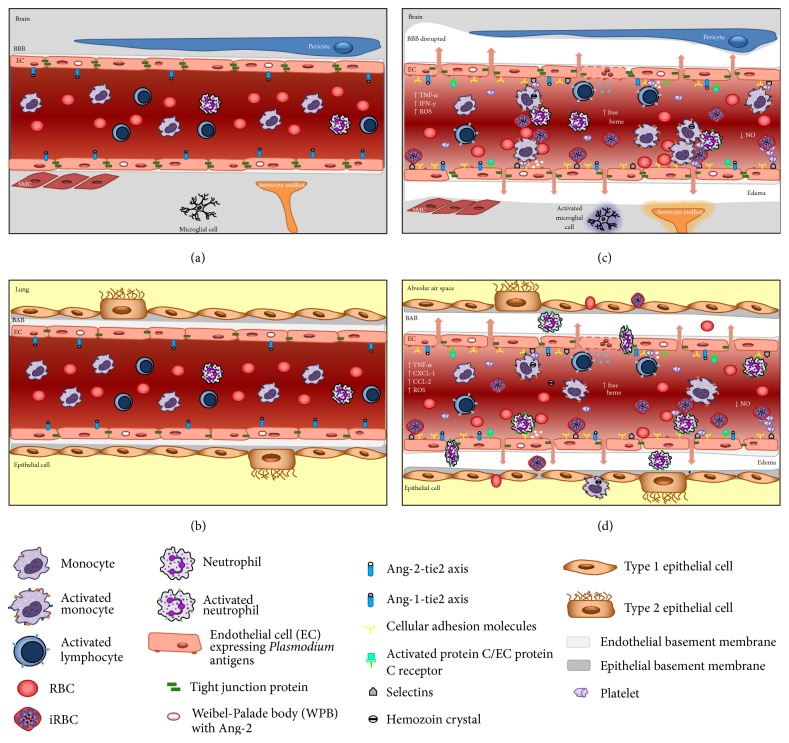
Figure 1.
Blood barrier differences between brain and lung during malaria. (a) Cerebral microvasculature and (b) lung microvasculature without leucocytes attached in postcapillary venules and EC expressing Ang-1, under physiological conditions. (c) During severe malaria, we observe production of proinflammatory cytokines, increase of cellular adhesion molecules expression, release of Ang-2, decrease of NO, and adhesion of iRBC and leukocytes (mainly mononuclear cells) to brain vasculature leading to capillary congestion, BBB dysfunction, and edema. Such events activate the subjacent tissue (microglial cells and astrocytes). (d) Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) associated with malaria. The augment of inflammatory cytokines and chemokines, release of Ang-2, and decrease of NO are responsible for activation of EC that increases the expression of cellular adhesion molecules allowing the margination and infiltration of iRBC, leucocytes, and platelets into blood vessels, interstitial tissue, and consequently alveolar air space. BBB: blood-brain barrier; BAB: blood-air barrier; EC: endothelial cell; ROS: reactive oxygen species; SMC: smooth muscle cell.
3. Leukocyte-Endothelium Interaction in the Lung during Malaria
The brain is not the only organ affected during severe malaria. Twenty percent of patients diagnosed with severe malaria develop acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [7, 9]. ALI/ARDS is a syndrome derived from pathological conditions such as sepsis and traumatic brain injury. ALI/ARDS diagnosis includes the identification of respiratory failure, diffuse alveolar damage, and inflammatory infiltration in lung tissue [44]. Necropsy in fatal cases of severe malaria revealed that patients present classical symptoms of ALI, including pulmonary edema, pulmonary capillary congestion, thickened alveolar septa, marked inflammatory response in lung tissue, and macrophages in the lumen of the pulmonary capillaries [11]. Murine experimental models of severe malaria also present pulmonary pathology such as edema, cell infiltration, tissue damage, and lung mechanical impairment [45–48]. Furthermore, the lung appears to be a large reservoir of metabolically active parasites, as described in an elegant study by Lovegrove et al. who evaluated the transcriptional responses to Plasmodium in different organs [49].
The lung vasculature in malaria infection is essential to initiate the Plasmodium cycle within the host. When merozoites leave the liver, they are located inside host-derived buds named merosomes, whose membranes are disrupted within the pulmonary capillary beds to allow merozoites to reach the erythrocytes [50, 51]. The close contact between infected erythrocytes and pulmonary endothelial cells triggers a remarkable inflammatory response 24 h after infection, characterized by intense inflammatory cell infiltration as well as the production of proinflammatory cytokines and chemokines in lung tissue that persists for at least five days after infection [45–47]. The quantity of parasites in lung tissue defines the extent of chemokine production in lung tissue [52]. Chemokines such as CCL2, CXCL1, and CCL5 are produced in lungs during experimental malaria and are correlated with macrophage and neutrophil accumulation in pulmonary tissue [45, 53, 54]. Intravital studies in lungs of Plasmodium-infected mice reveled edema formation and the migration of monocytes and neutrophils to lung tissue [37]. However, due to the technical limitations in studying leukocyte mobility within the lung [55], until now, there have been no available data on the dynamics of leukocytes and lung endothelium during malaria-triggered ALI [37]. Indeed, lung endothelial cells are activated during malaria infection and express adhesion molecules. P-selectin, in addition to L- and E-selectin, is part of a family of calcium-dependent (C-type) lectins whose activation induces the expression of β2-integrins and consequent leukocyte arrest in the vasculature [56, 57]. P-selectin is expressed in both lung and brain endothelium during experimental malaria. This molecule mediates leukocyte rolling in brain microvessels of P. berghei-infected mice; however, it is not essential for development of experimental cerebral malaria signals [58]. On the other hand, the monocyte/macrophage accumulation in lungs of P. berghei-infected mice depends on the expression of ICAM-1 [52, 59], while ICAM-1 expression in the brains of infected mice does not account for leukocyte adhesion [60]. It is interesting to note that while inflammatory cell infiltration in cerebral tissue was not observed in the brain, neutrophil and macrophage infiltration is frequently observed in pulmonary interstitial lung tissue during malaria [45]. Indeed, differences in the blood-brain barrier and blood-air barrier tight junction constitution of the brain and lung are responsible for this phenomenon (Figure 1).
The morphological and biochemical differences between lung and brain endothelial cells account for the distinct inflammatory responses in both organs. Despite both endothelial cell types containing nonfenestrated endothelium, brain endothelial cells present fewer caveolae and are richer in tight junctions than lung endothelium [61, 62]. The lung endothelial bed is rich in adherens junctions and P-selectins and allows leukocyte transmigration by paracellular and transcellular pathways [61, 62]. Endothelial cells from lung tissue can be activated by VEGF [4], TNF-α [63], LPS [64], and P. falciparum infected erythrocytes, resulting in the reorganization of their junctional proteins [63]. In addition to inflammatory mediators and pathogen-associated molecular pattern (PAMP), the leukocyte contact also contributes to endothelial cell reorganization, triggering a dephosphorylation cascade followed by the endocytosis of VE-cadherins, which support leukocyte transmigration through lung endothelial cells [65]. Of note, in most organs, leukocyte transmigration happens almost exclusively in postcapillary venules. However, in the lung leukocyte transmigration occurs in capillaries of the blood-air barrier which are surrounded by epithelium forming alveoli [61].
In addition to the direct interaction between leukocytes and the endothelium described earlier, leukocytes can bind platelets and then adhere to the endothelium. Piguet and colleagues showed that platelet and mononuclear cell trapping occurs in the lungs of P. berghei-infected mice [66]. In addition, the authors observed that the impairment of platelet activation decreased leukocyte adhesion to the lung vasculature of P. berghei-infected mice [66]. The stimulation of the receptor P2Y1 but not P2Y12 on platelets induces the downstream activation of the RhoA pathway, resulting in platelet/leukocyte aggregation and migration to the lung [67]. In addition, platelets also contribute to the leukocyte/endothelium interaction by releasing microparticles. Neutrophils stimulated with platelet-derived microparticles increased the expression of αM integrin and adhered to pulmonary endothelial cells via ICAM-1 [68].
The study of leukocyte/endothelium interactions within the lung during malaria is limited but extremely important. Mice depleted of neutrophils showed reduced malaria associated ALI and delayed mortality [38], suggesting that further studies are necessary to show the mechanism of the leukocyte/endothelial interaction in the lung during severe malaria.
4. Leukocyte/Endothelium Interaction during Malaria as a Target for Treatment
In accordance with the findings presented above both in human and animals, the leukocyte/endothelium interaction plays a role in the development of pathogenesis of severe malaria particularly in malaria-induced ALI [9, 12, 45, 47]. In fact, lung dysfunction triggered in both human and experimental malaria shares similarities with lung mechanics impairment, pulmonary edema, production of inflammatory cytokines, and inflammatory cells infiltration in lung tissue [9, 45]. Furthermore, the inflammatory response persists even after the host is cured of infection [10, 69, 70] (unpublished data), which suggests that modulation of inflammatory response in addition to antimalarial therapy would be helpful to patient outcome [71]. The leukocyte-endothelium interaction is not the most important factor regarding development of human cerebral malaria pathogenesis; however, it should not be neglected as actor in severe malaria-induced organ dysfunction.
Recently, Frosch and John suggested that an adjunctive therapy that impaired the inflammatory response induced during malaria should be combined with antimalarial drugs [72]. Several approaches have already aimed at the modulation of the malaria-induced inflammatory response. Figure 2 illustrates several potential targets described in the literature. Patients diagnosed with severe malaria have been treated with modulators of TNF-α production [73], CD36 expression [74, 75], NO precursors [70, 76], or adhesion of iRBC to vasculature [77] and presented decreased inflammation scores when compared to a placebo treated group. Despite evidence suggesting that the modulation of leukocyte and endothelial activation supports the outcome of severe malaria, it is not clear whether an adjunctive therapy targeting the leukocyte/endothelium interaction would predict patient outcome. It is worth noting that the most important class of antimalarial drug to treat severe malaria is artemisinin and its derivatives [78], which also have immunomodulatory activities in pathologies such as microbial infections, tumor growth, and inflammatory diseases [79–82]. Our group demonstrated that, in addition to its antimalarial properties, artesunate exerted a protective effect against severe malaria via its immunomodulatory properties by inhibiting endothelial cell activation, NF-κB nuclear translocation, and the subsequent expression of ICAM-1 [83].
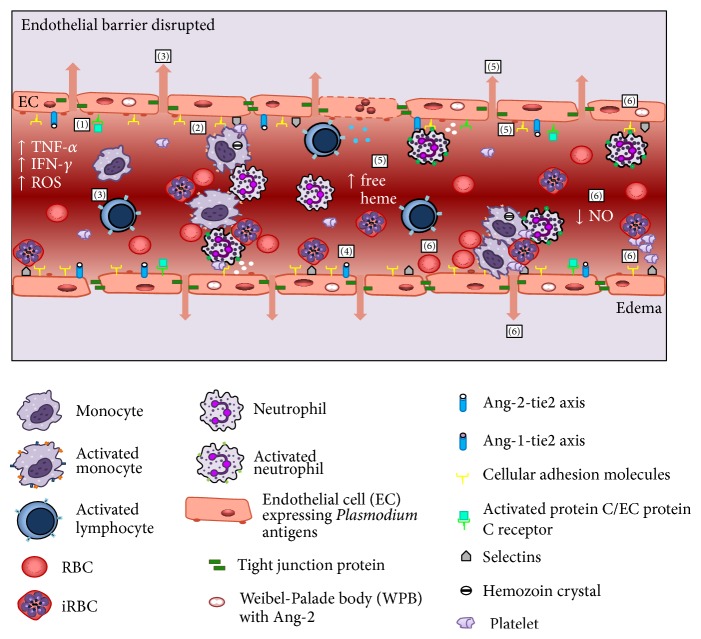
Figure 2.
Targets of adjuvant therapies during malaria. Scheme showing several approaches that have been investigated aiming at modulation of malaria-induced inflammatory response. EC: endothelial cell; ROS: reactive oxygen species; SMC: smooth muscle cell. (1) Activated protein C binds to protein C receptor in activated EC cells decreasing the expression of adhesion molecules. (2) Statins decrease the production of chemokine and diminished the adhesion of leukocytes to brain microvasculature. (3) Sphingosine-1-phosphate (S1P) decreases the numbers of lymphocytes in brain vasculature and stabilizes the tight junction protein ZO-1 in brains. (4) Neuregulin-1 and bone marrow mesenchymal stromal cells induce Ang-1, which promotes stabilization of EC tight junctions, EC desensitization to TNF-α, and downregulation of ICAM-1 and VCAM-1. (5) Lipoxin A 4 decreases production of proinflammatory cytokines, impairs EC activation, and inhibits the expression of cellular adhesion molecules involved in leukocyte adhesion by stimulating the activity of HO-1, which catabolizes free heme. (6) L-Arginine or inhaled NO (iNO) reduces pulmonary edema and, in addition, decreases cytoadherence of iRBC, hemorrhagic foci, and leukocyte and platelets adherence to brain vasculature by inhibiting of WPB exocytosis that impairs the release of Ang-2 and inhibiting TNF-α production and procoagulant activity of endothelial cells.
Srinivas and colleagues studied the effect of treatment with activated protein C on a patient with severe malaria coinfected with leptospirosis and observed a rapid outcome [84]. The binding of activated protein C to endothelial cell protein C receptor in activated endothelial cells avoided NF-κB p65 phosphorylation and induced AKT signaling, which decreased the expression of adhesion molecules on the endothelial cell surface [18, 85]. Thus, activated protein C in malaria would increase endothelial barrier integrity, induce antiapoptotic pathways, and decrease adhesion molecule expression [86]. Other modulators of endothelial functions have been used to evaluate malaria outcome in humans and experimental models. Studies in which P. berghei-infected mice were treated with statins, a class of drugs that inhibit the rate-limiting step in cholesterol synthesis and that show pleiotropic effects, demonstrated that statins decreased the production of chemokines [87] and decreased the adhesion of leukocytes to the brain microvasculature [88] probably by inhibiting the binding site of LFA-1 on leukocytes [89]. Accordingly, in vitro treatment of human endothelial cells with statins followed by stimulation with P. falciparum-infected erythrocytes decreased the expression of adhesion molecules, suggesting that statins could exert an antiadhesive role in the treatment of severe malaria [90]. Statins have not been tested in clinical trials for malaria adjunctive treatment. However, statins diminished the risk of sepsis-related mortality in patients, probably by decreasing the inflammatory response triggered during sepsis [91].
The endothelial barrier stabilizer sphingosine-1-phosphate (S1P) also rescued mice from severe malaria by decreasing the numbers of CD8+, CD4+, and CD45+ cells in the brain vasculature of P. berghei-infected mice, likely decreasing ICAM-1 expression and stabilizing the tight junction protein ZO-1 in brains [36, 92]. Transfection of bone marrow mesenchymal stromal cells and administration of S1P and other endothelial barrier stabilizers such as neuregulin-1 induce the endogenous Ang-1 anti-inflammatory pathway, which promotes decreased vascular permeability by stabilizing endothelial cell tight junctions, endothelial cell desensitization to TNF-α, and downregulating ICAM-1 and VCAM-1. These Ang-1 actions result in decreased leukocyte/endothelial interaction and, consequently, host outcome [4, 93–97].
Another family of lipids has been studied for its anti-inflammatory activity during severe malaria. Lipoxins (LX) are products of arachidonic acid metabolism and are produced through sequential lipoxygenase activity following cell-cell interactions in the inflammatory milieu (reviewed by [98]). The interaction of LXA4 and its receptor ALX has anti-inflammatory and proresolving activity in inflammatory models such as allergic airway inflammation [99] and autoimmune diseases [100] by reducing leukocyte adhesion to endothelial cells [101]. The administration of LXA4 improved survival in P. berghei-infected mice by decreasing the production of proinflammatory cytokines but not the accumulation of CD8+/IFN-γ + cells in brain tissue [102]. In addition to LXA4 impairment of leukocyte activation, the mechanism of action of LXA4 on endothelium during severe malaria was recently disclosed by intravital studies of the microvasculature of P. berghei-infected mice. The authors showed that treatment with LXA4 did not modulate leukocyte adhesion to the brain vasculature or decrease the expression of β2-integrin in leukocytes (unpublished data). On the other hand, treatment with LXA4 impaired endothelial activation during severe malaria and restored the blood flow in brains of P. berghei-infected mice [33]. The authors also showed that LXA4 exerted its effects by stimulating the activity of heme oxygenase 1 (HO-1), an isoenzyme that catabolizes free heme released under pathological conditions, especially in pathologies such as malaria which are associated with intravascular hemolysis [33]. HO-1 upregulation helps maintain BBB integrity under pathological conditions [103]. During the inflammatory response, HO-1 inhibits the expression of several adhesion molecules involved in leukocyte adhesion to endothelial cells [104, 105]. During experimental severe malaria, HO-1 is differentially regulated in certain tissues at different stages of Plasmodium life cycle [106, 107]. Furthermore, HO-1 production in brain tissue is associated with mouse survival, decreased cerebral edema, and decreased leukocyte adhesion to brain vasculature [106].
In hemolytic disorders such as malaria, low bioavailability of NO is observed, as free hemoglobin is a potent scavenger of this gaseous molecule [108]. Therefore, the administration of L-arginine or inhaled NO (iNO) has also been tested as adjunctive therapy in the treatment of severe malaria [4, 76]. Yeo and collaborators showed that impaired endothelial NO production occurred in severe malaria in both children and adults, supporting the idea that further trials of drugs that led to increased endothelial NO bioavailability could attenuate severe malaria symptoms [109]. Studies in which severe malaria patients were treated with inhaled nitric oxide demonstrated that NO reduced pulmonary edema in patients with malaria-derived ALI and decreased pulmonary capillary pressure through selective vasodilatory effects on postcapillary venules [110]. Thus, in severe malaria, nitric oxide is hypothesized to promote vascular quiescence, decrease cytoadherence of parasitized erythrocytes to the microvascular endothelium as a critical mediator of VEGF and Ang-1, and dampen inflammatory responses and thrombosis [4]. Nitric oxide (NO) is a short-lived free radical formed from L-arginine conversion that is involved in many important biological functions including neurotransmission, immune system, cytokine modulation platelet inhibition, vascular homeostasis, and regulation of hematopoiesis [111]. Its production occurs through three different NO synthase (NOS) enzyme isoforms: neuronal NOS (nNOS or NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3) [111]. The constitutive isoforms (neuronal and endothelial) are calcium/calmodulin dependent and permanently active, generating low concentrations of NO. The inducible isoform (iNOS) is only expressed when its transcription is activated by a variety of cytokines, growth factors, and inflammatory stimuli on target cells, leading to the release of high levels of NO [112]. In experimental severe malaria, treatment with exogenous NO (NO donor dipropylenetriamine NONOate, DPTA-NO) showed improved pial blood flow, diminished hemorrhagic foci, and reduced leukocyte and platelet adherence to the brain vasculature [113, 114]. The authors hypothesize that NO attenuates malaria symptoms by (a) inhibition of Weibel-Palade body exocytosis and the consequent release of Ang-2 and increase in Ang-1 expression; (b) decreasing the endothelial expression of ICAM-1 and VCAM-1; (c) inhibiting TNF-α production; (d) inhibiting the procoagulant activity of endothelial cells; and (e) decreasing intravascular platelet aggregation [108, 110, 111].
Indeed, pathophysiological phenomena experienced during experimental severe malaria are not fully translated to human severe malaria [115–118]. Therefore, further studies should be performed before initiating clinical studies of immunomodulatory drugs as adjunctive therapy for severe malaria.
Acknowledgments
This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Disclosure
Tatiana Pádua is a student of the Graduate Program in Cellular and Molecular Biology from Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro, Brazil.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.WHO. Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization; 2015. WHO guidelines approved by the guidelines review committee. [Google Scholar]
- 2.Murray C., Chambers R. Keeping score: fostering accountability for children's lives. The Lancet. 2015;386(9988):3–5. doi: 10.1016/s0140-6736(15)61171-0. [DOI] [PubMed] [Google Scholar]
- 3.Seydel K. B., Kampondeni S. D., Valim C., et al. Brain swelling and death in children with cerebral malaria. The New England Journal of Medicine. 2015;372(12):1126–1137. doi: 10.1056/nejmoa1400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H., Higgins S., Liles W. C., Kain K. C. Endothelial activation and dysregulation in malaria: a potential target for novel therapeutics. Current Opinion in Hematology. 2011;18(3):177–185. doi: 10.1097/moh.0b013e328345a4cf. [DOI] [PubMed] [Google Scholar]
- 5.Wassmer S. C., Taylor T. E., Rathod P. K., et al. Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. The American Journal of Tropical Medicine and Hygiene. 2015;93(3, supplement):42–56. doi: 10.4269/ajtmh.14-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor T. E., Molyneux M. E. The pathogenesis of pediatric cerebral malaria: eye exams, autopsies, and neuroimaging. Annals of the New York Academy of Sciences. 2015;1342(1):44–52. doi: 10.1111/nyas.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohan A., Sharma S. K., Bollineni S. Acute lung injury and acute respiratory distress syndrome in malaria. Journal of Vector Borne Diseases. 2008;45(3):179–193. [PubMed] [Google Scholar]
- 8.Abdul Manan J., Ali H., Lal M. Acute renal failure associated with malaria. Journal of Ayub Medical College, Abbottabad. 2006;18(4):47–52. [PubMed] [Google Scholar]
- 9.Taylor W. R. J., Hanson J., Turner G. D. H., White N. J., Dondorp A. M. Respiratory manifestations of malaria. Chest. 2012;142(2):492–505. doi: 10.1378/chest.11-2655. [DOI] [PubMed] [Google Scholar]
- 10.Maguire G. P., Handojo T., Pain M. C. F., et al. Lung injury in uncomplicated and severe falciparum malaria: a longitudinal study in Papua, Indonesia. Journal of Infectious Diseases. 2005;192(11):1966–1974. doi: 10.1086/497697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milner D., Jr., Factor R., Whitten R., et al. Pulmonary pathology in pediatric cerebral malaria. Human Pathology. 2013;44(12):2719–2726. doi: 10.1016/j.humpath.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milner D. A., Jr., Whitten R. O., Kamiza S., et al. The systemic pathology of cerebral malaria in African children. Frontiers in Cellular and Infection Microbiology. 2014;4, article 104 doi: 10.3389/fcimb.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris M. G., Hulseberg P., Ling C., et al. Immune privilege of the CNS is not the consequence of limited antigen sampling. Scientific Reports. 2014;4, article 4422 doi: 10.1038/srep04422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfieri A., Srivastava S., Siow R. C. M., et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radical Biology and Medicine. 2013;65:1012–1022. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- 15.Silva N. M., Manzan R. M., Carneiro W. P., et al. Toxoplasma gondii: the severity of toxoplasmic encephalitis in C57BL/6 mice is associated with increased ALCAM and VCAM-1 expression in the central nervous system and higher blood-brain barrier permeability. Experimental Parasitology. 2010;126(2):167–177. doi: 10.1016/j.exppara.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Dorovini-Zis K., Schmidt K., Huynh H., et al. The neuropathology of fatal cerebral malaria in Malawian children. The American Journal of Pathology. 2011;178(5):2146–2158. doi: 10.1016/j.ajpath.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. The American Journal of Pathology. 1985;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- 18.Turner L., Lavstsen T., Berger S. S., et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013;498(7455):502–505. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patnaik J. K., Das B. S., Mishra S. K., Mohanty S., Satpathy S. K., Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. American Journal of Tropical Medicine and Hygiene. 1994;51(5):642–647. [PubMed] [Google Scholar]
- 20.Punsawad C., Maneerat Y., Chaisri U., Nantavisai K., Viriyavejakul P. Nuclear factor kappa B modulates apoptosis in the brain endothelial cells and intravascular leukocytes of fatal cerebral malaria. Malaria Journal. 2013;12, article 260:12. doi: 10.1186/1475-2875-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García F., Cebrián M., Dgedge M., et al. Endothelial cell activation in muscle biopsy samples is related to clinical severity in human cerebral malaria. The Journal of Infectious Diseases. 1999;179(2):475–483. doi: 10.1086/314598. [DOI] [PubMed] [Google Scholar]
- 22.Krupka M., Seydel K., Feintuch C. M., et al. Mild Plasmodium falciparum malaria following an episode of severe malaria is associated with induction of the interferon pathway in Malawian children. Infection and Immunity. 2012;80(3):1150–1155. doi: 10.1128/iai.06008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worku S., Troye-Blomberg M., Christensson B., Björkman A., Fehniger T. Activation of T cells in the blood of patients with acute malaria: proliferative activity as indicated by Ki-67 expression. Scandinavian Journal of Immunology. 2001;53(3):296–301. doi: 10.1046/j.1365-3083.2001.00861.x. [DOI] [PubMed] [Google Scholar]
- 24.Conroy A. L., Glover S. J., Hawkes M., et al. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study∗ . Critical Care Medicine. 2012;40(3):952–959. doi: 10.1097/ccm.0b013e3182373157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain V., Lucchi N. W., Wilson N. O., et al. Plasma levels of angiopoietin-1 and -2 predict cerebral malaria outcome in Central India. Malaria Journal. 2011;10, article 383 doi: 10.1186/1475-2875-10-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prato M., D'Alessandro S., Van den Steen P. E., et al. Natural haemozoin modulates matrix metalloproteinases and induces morphological changes in human microvascular endothelium. Cellular Microbiology. 2011;13(8):1275–1285. doi: 10.1111/j.1462-5822.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 27.Tachado S. D., Gerold P., McConville M. J., et al. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. Journal of Immunology. 1996;156(5):1897–1907. [PubMed] [Google Scholar]
- 28.Armah H., Wiredu E. K., Dodoo A. K., Adjei A. A., Tettey Y., Gyasi R. Cytokines and adhesion molecules expression in the brain in human cerebral malaria. International Journal of Environmental Research and Public Health. 2005;2(1):123–131. doi: 10.3390/ijerph2005010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown H., Hien T. T., Day N., et al. Evidence of blood-brain barrier dysfunction in human cerebral malaria. Neuropathology and Applied Neurobiology. 1999;25(4):331–340. doi: 10.1046/j.1365-2990.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y., Szestak T., Stins M., Craig A. G. Amplification of P. falciparum cytoadherence through induction of a pro-adhesive state in host endothelium. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0024784.e24784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storm J., Craig A. G. Pathogenesis of cerebral malariaâ—inflammation and cytoadherence. Frontiers in Cellular and Infection Microbiology. 2014;4, article 100 doi: 10.3389/fcimb.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravorty S. J., Carret C., Nash G. B., Ivens A., Szestak T., Craig A. G. Altered phenotype and gene transcription in endothelial cells, induced by Plasmodium falciparum-infected red blood cells: pathogenic or protective? International Journal for Parasitology. 2007;37(8-9):975–987. doi: 10.1016/j.ijpara.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Souza M. C., Pádua T. A., Torres N. D., et al. Lipoxin A4 attenuates endothelial dysfunction during experimental cerebral malaria. International Immunopharmacology. 2015;24(2):400–407. doi: 10.1016/j.intimp.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 34.Nacer A., Movila A., Sohet F., et al. Experimental cerebral malaria pathogenesis—hemodynamics at the blood brain barrier. PLoS Pathogens. 2014;10(12) doi: 10.1371/journal.ppat.1004528.e1004528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pai S., Qin J., Cavanagh L., et al. Real-time imaging reveals the dynamics of leukocyte behaviour during experimental cerebral malaria pathogenesis. PLoS Pathogens. 2014;10(7) doi: 10.1371/journal.ppat.1004236.e1004236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nacer A., Movila A., Baer K., Mikolajczak S. A., Kappe S. H. I., Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathogens. 2012;8(10) doi: 10.1371/journal.ppat.1002982.e1002982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frevert U., Nacer A., Cabrera M., Movila A., Leberl M. Imaging Plasmodium immunobiology in the liver, brain, and lung. Parasitology International. 2014;63(1):171–186. doi: 10.1016/j.parint.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senaldi G., Vesin C., Chang R., Grau G. E., Piguet P. F. Role of polymorphonuclear neutrophil leukocytes and their integrin CD11a (LFA-1) in the pathogenesis of severe murine malaria. Infection and Immunity. 1994;62(4):1144–1149. doi: 10.1128/iai.62.4.1144-1149.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos T. N., Bullard D. C., Darley M. M., McDonald K., Crawford D. F., Barnum S. R. Experimental cerebral malaria develops independently of endothelial expression of intercellular adhesion molecule-1 (ICAM-1) The Journal of Biological Chemistry. 2013;288(16):10962–10966. doi: 10.1074/jbc.c113.457028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jambou R., Combes V., Jambou M.-J., Weksler B. B., Couraud P.-O., Grau G. E. Plasmodium falciparum adhesion on human brain microvascular endothelial cells involves transmigration-like cup formation and induces opening of intercellular junctions. PLoS Pathogens. 2010;6(7) doi: 10.1371/journal.ppat.1001021.e1001021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howland S. W., Poh C. M., Rénia L., Langhorne J. Activated brain endothelial cells cross-present malaria antigen. PLOS Pathogens. 2015;11(6) doi: 10.1371/journal.ppat.1004963.e1004963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owens T., Bechmann I., Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. Journal of Neuropathology & Experimental Neurology. 2008;67(12):1113–1121. doi: 10.1097/nen.0b013e31818f9ca8. [DOI] [PubMed] [Google Scholar]
- 43.Louveau A., Smirnov I., Keyes T. J., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweatt A. J., Levitt J. E. Evolving epidemiology and definitions of the acute respiratory distress syndrome and early acute lung injury. Clinics in Chest Medicine. 2014;35(4):609–624. doi: 10.1016/j.ccm.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Souza M. C., Silva J. D., Pádua T. A., Capelozzi V. L., Rocco P. R. M., Henriques M. D. G. Early and late acute lung injury and their association with distal organ damage in murine malaria. Respiratory Physiology & Neurobiology. 2013;186(1):65–72. doi: 10.1016/j.resp.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Aitken E. H., Negri E. M., Barboza R., et al. Ultrastructure of the lung in a murine model of malaria-associated acute lung injury/acute respiratory distress syndrome. Malaria Journal. 2014;13, article 230 doi: 10.1186/1475-2875-13-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epiphanio S., Campos M. G., Pamplona A., et al. VEGF promotes malaria-associated acute lung injury in mice. PLoS Pathogens. 2010;6(5) doi: 10.1371/journal.ppat.1000916.e1000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortolan L. S., Sercundes M. K., Barboza R., et al. Predictive criteria to study the pathogenesis of malaria-associated ALI/ARDS in mice. Mediators of Inflammation. 2014;2014:12. doi: 10.1155/2014/872464.872464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lovegrove F. E., Peña-Castillo L., Mohammad N., Liles W. C., Hughes T. R., Kain K. C. Simultaneous host and parasite expression profiling identifies tissue-specific transcriptional programs associated with susceptibility or resistance to experimental cerebral malaria. BMC Genomics. 2006;7, article 295 doi: 10.1186/1471-2164-7-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baer K., Klotz C., Kappe S. H. I., Schnieder T., Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathogens. 2007;3, article e171 doi: 10.1371/journal.ppat.0030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thiberge S., Blazquez S., Baldacci P., et al. In vivo imaging of malaria parasites in the murine liver. Nature Protocols. 2007;2(7):1811–1818. doi: 10.1038/nprot.2007.257. [DOI] [PubMed] [Google Scholar]
- 52.Deroost K., Tyberghein A., Lays N., et al. Hemozoin induces lung inflammation and correlates with malaria-associated acute respiratory distress syndrome. American Journal of Respiratory Cell and Molecular Biology. 2013;48(5):589–600. doi: 10.1165/rcmb.2012-0450oc. [DOI] [PubMed] [Google Scholar]
- 53.Van den Steen P. E., Geurts N., Deroost K., et al. Immunopathology and dexamethasone therapy in a new model for malaria-associated acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2010;181(9):957–968. doi: 10.1164/rccm.200905-0786oc. [DOI] [PubMed] [Google Scholar]
- 54.Belnoue E., Potter S. M., Rosa D. S., et al. Control of pathogenic CD8+ T cell migration to the brain by IFN-gamma during experimental cerebral malaria. Parasite Immunology. 2008;30(10):544–553. doi: 10.1111/j.1365-3024.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 55.Looney M. R., Thornton E. E., Sen D., Lamm W. J., Glenny R. W., Krummel M. F. Stabilized imaging of immune surveillance in the mouse lung. Nature Methods. 2011;8(1):91–96. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnamurthy V. R., Sardar M. Y., Ying Y., et al. Glycopeptide analogues of PSGL-1 inhibit P-selectin in vitro and in vivo . Nature Communications. 2015;6, article 6387 doi: 10.1038/ncomms7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parish C. R. The role of heparan sulphate in inflammation. Nature Reviews Immunology. 2006;6(9):633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 58.Combes V., Rosenkranz A. R., Redard M., et al. athogenic role of P-selectin in experimental cerebral malaria: importance of the endothelial compartment. American Journal of Pathology. 2004;164(3):781–786. doi: 10.1016/s0002-9440(10)63166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Favre N., da Laperousaz C., Ryffel B., et al. Role of ICAM-1 (CD54) in the development of murine cerebral malaria. Microbes and Infection. 1999;1(12):961–968. doi: 10.1016/s1286-4579(99)80513-9. [DOI] [PubMed] [Google Scholar]
- 60.Li J., Chang W.-L., Sun G., et al. Intercellular adhesion molecule 1 is important for the development of severe experimental malaria but is not required for leukocyte adhesion in the brain. The Journal of Investigative Medicine. 2003;51(3):128–140. doi: 10.1136/jim-51-03-15. [DOI] [PubMed] [Google Scholar]
- 61.Aird W. C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circulation Research. 2007;100(2):158–173. doi: 10.1161/01.res.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 62.Craig L. E., Spelman J. P., Strandberg J. D., Zink M. C. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvascular Research. 1998;55(1):65–76. doi: 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- 63.Gillrie M. R., Krishnegowda G., Lee K., et al. Src-family kinase-dependent disruption of endothelial barrier function by Plasmodium falciparum merozoite proteins. Blood. 2007;110(9):3426–3435. doi: 10.1182/blood-2007-04-084582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu P., Usatyuk P. V., Lele A., et al. c-Abl mediated tyrosine phosphorylation of paxillin regulates LPS-induced endothelial dysfunction and lung injury. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2015;308(10):L1025–L1038. doi: 10.1152/ajplung.00306.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wessel F., Winderlich M., Holm M., et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nature Immunology. 2014;15(3):223–230. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 66.Piguet P. F., Da Laperrousaz C., Vesin C., Tacchini-Cottier F., Senaldi G., Grau G. E. Delayed mortality and attenuated thrombocytopenia associated with severe malaria in urokinase- and urokinase receptor-deficient mice. Infection and Immunity. 2000;68(7):3822–3829. doi: 10.1128/iai.68.7.3822-3829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amison R. T., Momi S., Morris A., et al. RhoA signaling through platelet P2Y(1) receptor controls leukocyte recruitment in allergic mice. Journal of Allergy and Clinical Immunology. 2014;135(2):528–538. doi: 10.1016/j.jaci.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 68.Xie R. F., Hu P., Wang Z. C., et al. Platelet-derived microparticles induce polymorphonuclear leukocyte-mediated damage of human pulmonary microvascular endothelial cells. Transfusion. 2015;55(5):1051–1057. doi: 10.1111/trf.12952. [DOI] [PubMed] [Google Scholar]
- 69.Alves C., Chen J.-T., Patel N., et al. Extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome in severe malaria. Malaria Journal. 2013;12, article 306 doi: 10.1186/1475-2875-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeo T. W., Lampah D. A., Gitawati R., et al. Impaired nitric oxide bioavailability and L-arginine-reversible endothelial dysfunction in adults with Falciparum malaria . The Journal of Experimental Medicine. 2007;204(11):2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abreu T. P., Silva L. S., Takiya C. M., et al. Mice rescued from severe malaria are protected against renal injury during a second kidney insult. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0093634.e93634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frosch A. E. P., John C. C. Immunomodulation in Plasmodium falciparum malaria: experiments in nature and their conflicting implications for potential therapeutic agents. Expert Review of Anti-Infective Therapy. 2012;10(11):1343–1356. doi: 10.1586/eri.12.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watt G., Jongsakul K., Ruangvirayuth R. A pilot study of N-acetylcysteine as adjunctive therapy for severe malaria. QJM. 2002;95(5):285–290. doi: 10.1093/qjmed/95.5.285. [DOI] [PubMed] [Google Scholar]
- 74.Boggild A. K., Krudsood S., Patel S. N., et al. Use of peroxisome proliferator-activated receptor γ agonists as adjunctive treatment for Plasmodium falciparum malaria: a randomized, double-blind, placebo-controlled trial. Clinical Infectious Diseases. 2009;49(6):841–849. doi: 10.1086/605431. [DOI] [PubMed] [Google Scholar]
- 75.Serghides L., McDonald C. R., Lu Z., et al. PPARγ agonists improve survival and neurocognitive outcomes in experimental cerebral malaria and induce neuroprotective pathways in human malaria. PLoS Pathogens. 2014;10(3) doi: 10.1371/journal.ppat.1003980.e1003980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yeo T. W., Lampah D. A., Rooslamiati I., et al. A randomized pilot study of L-arginine infusion in severe Falciparum malaria: preliminary safety, efficacy and pharmacokinetics. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0069587.e69587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dondorp A. M., Silamut K., Charunwatthana P., et al. Levamisole inhibits sequestration of infected red blood cells in patients with falciparum malaria. Journal of Infectious Diseases. 2007;196(3):460–466. doi: 10.1086/519287. [DOI] [PubMed] [Google Scholar]
- 78.Dondorp A., Nosten F., Stepniewska K., Day N., White N. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. The Lancet. 2005;366(9487):717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 79.Sarciron M. E., Saccharin C., Petavy A. F., Peyron F. Effects of artesunate, dihydroartemisinin, and an artesunate-dihydroartemisinin combination against Toxoplasma gondii . The American Journal of Tropical Medicine and Hygiene. 2000;62(1):73–76. doi: 10.4269/ajtmh.2000.62.73. [DOI] [PubMed] [Google Scholar]
- 80.Efferth T., Dunstan H., Sauerbrey A., Miyachi H., Chitambar C. R. The anti-malarial artesunate is also active against cancer. International Journal of Oncology. 2001;18(4):767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 81.Kaptein S. J. F., Efferth T., Leis M., et al. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo . Antiviral Research. 2006;69(2):60–69. doi: 10.1016/j.antiviral.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 82.Wang J., Zhou H., Zheng J., et al. The antimalarial artemisinin synergizes with antibiotics to protect against lethal live Eschenchia coli challenge by decreasing proinflammatory cytokine release. Antimicrobial Agents and Chemotherapy. 2006;50(7):2420–2427. doi: 10.1128/aac.01066-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Souza M. C., Paixao F. H., Ferraris F. K., Ribeiro I., Henriques M. Artesunate exerts a direct effect on endothelial cell activation and NF-kappaB translocation in a mechanism independent of plasmodium killing. Malaria Research and Treatment. 2012;2012:12. doi: 10.1155/2012/679090.679090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srinivas R., Agarwal R., Gupta D. Severe sepsis due to severe falciparum malaria and leptospirosis co-infection treated with activated protein C. Malaria Journal. 2007;6, article 42 doi: 10.1186/1475-2875-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guitton C., Cottereau A., Gérard N., et al. Protective cross talk between activated protein C and TNF signaling in vascular endothelial cells: implication of EPCR, noncanonical NF-κB, and ERK1/2 MAP kinases. American Journal of Physiology—Cell Physiology. 2011;300(4):C833–C842. doi: 10.1152/ajpcell.00003.2010. [DOI] [PubMed] [Google Scholar]
- 86.Riewald M., Schuepbach R. A. Protective signaling pathways of activated protein C in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(1):1–3. doi: 10.1161/ATVBAHA.107.157321. [DOI] [PubMed] [Google Scholar]
- 87.Dormoi J., Briolant S., Pascual A., Desgrouas C., Travaillé C., Pradines B. Improvement of the efficacy of dihydroartemisinin with atorvastatin in an experimental cerebral malaria murine model. Malaria Journal. 2013;12, article 302 doi: 10.1186/1475-2875-12-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reis P. A., Estato V., da Silva T. I., et al. Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathogens. 2012;8(12) doi: 10.1371/journal.ppat.1003099.e1003099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weitz-Schmidt G., Welzenbach K., Brinkmann V., et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Medicine. 2001;7(6):687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 90.Taoufiq Z., Pino P., N'Dilimabaka N., et al. Atorvastatin prevents Plasmodium falciparum cytoadherence and endothelial damage. Malaria Journal. 2011;10, article 52 doi: 10.1186/1475-2875-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ou S.-Y., Chu H., Chao P.-W., et al. Effect of the use of low and high potency statins and sepsis outcomes. Intensive Care Medicine. 2014;40(10):1509–1517. doi: 10.1007/s00134-014-3418-1. [DOI] [PubMed] [Google Scholar]
- 92.Finney C. A., Hawkes C. A., Kain D. C., et al. S1P is associated with protection in human and experimental cerebral malaria. Molecular Medicine. 2011;17(7-8):717–725. doi: 10.2119/molmed.2010.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Souza M. C., Silva J. D., Padua T. A., et al. Mesenchymal stromal cell therapy attenuated lung and kidney injury but not brain damage in experimental cerebral malaria. Stem Cell Research & Therapy. 2015;6:102–117. doi: 10.1186/s13287-015-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nacer A., Claes A., Roberts A., et al. Discovery of a novel and conserved Plasmodium falciparum exported protein that is important for adhesion of PfEMP1 at the surface of infected erythrocytes. Cellular Microbiology. 2015;17(8):1205–1216. doi: 10.1111/cmi.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Solomon W., Wilson N. O., Anderson L., et al. Neuregulin-1 attenuates mortality associated with experimental cerebral malaria. Journal of Neuroinflammation. 2014;11, article 9 doi: 10.1186/1742-2094-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schweizer R., Kamat P., Schweizer D., et al. Bone marrow-derived mesenchymal stromal cells improve vascular regeneration and reduce leukocyte-endothelium activation in critical ischemic murine skin in a dose-dependent manner. Cytotherapy. 2014;16(10):1345–1360. doi: 10.1016/j.jcyt.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 97.Maron-Gutierrez T., Laffey J. G., Pelosi P., Rocco P. R. M. Cell-based therapies for the acute respiratory distress syndrome. Current Opinion in Critical Care. 2014;20(1):122–131. doi: 10.1097/mcc.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 98.Ryan A., Godson C. Lipoxins: regulators of resolution. Current Opinion in Pharmacology. 2010;10(2):166–172. doi: 10.1016/j.coph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 99.Haworth O., Cernadas M., Yang R., Serhan C. N., Levy B. D. Resolvin E1 regulates interleukin 23, interferon-γ and lipoxin A4 to promote the resolution of allergic airway inflammation. Nature Immunology. 2008;9(8):873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan M. M.-Y., Moore A. R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. The Journal of Immunology. 2010;184(11):6418–6426. doi: 10.4049/jimmunol.0903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fiorucci S., Distrutti E., Mencarelli A., et al. Evidence that 5-lipoxygenase and acetylated cyclooxygenase 2-derived eicosanoids regulate leukocyte-endothelial adherence in response to aspirin. British Journal of Pharmacology. 2003;139(7):1351–1359. doi: 10.1038/sj.bjp.0705356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shryock N., McBerry C., Gonzalez R. M. S., Janes S., Costa F. T. M., Aliberti J. Lipoxin A4 and 15-epi-lipoxin A4 protect against experimental cerebral malaria by inhibiting IL-12/IFN-γ in the brain. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061882.e61882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y.-F., Gu Y.-T., Qin G.-H., Zhong L., Meng Y.-N. Curcumin ameliorates the permeability of the blood-brain barrier during hypoxia by upregulating heme oxygenase-1 expression in brain microvascular endothelial cells. Journal of Molecular Neuroscience. 2013;51(2):344–351. doi: 10.1007/s12031-013-9989-4. [DOI] [PubMed] [Google Scholar]
- 104.Lu C.-Y., Yang Y.-C., Li C.-C., Liu K.-L., Lii C.-K., Chen H.-W. Andrographolide inhibits TNFα-induced ICAM-1 expression via suppression of NADPH oxidase activation and induction of HO-1 and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1 pathways in human endothelial cells. Biochemical Pharmacology. 2014;91(1):40–50. doi: 10.1016/j.bcp.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 105.Belhaj A., Dewachter L., Kerbaul F., et al. Heme oxygenase-1 and inflammation in experimental right ventricular failure on prolonged overcirculation-induced pulmonary hypertension. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0069470.e69470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pamplona A., Ferreira A., Balla J., et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nature Medicine. 2007;13(6):703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 107.Seixas E., Gozzelino R., Chora Â., et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Moura Carvalho L. J., da Silva Moreira A., Daniel-Ribeiro C. T., Martins Y. C. Vascular dysfunction as a target for adjuvant therapy in cerebral malaria. Memorias do Instituto Oswaldo Cruz. 2014;109(5):577–588. doi: 10.1590/0074-0276140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yeo T. W., Lampah D. A., Kenangalem E., et al. Decreased endothelial nitric oxide bioavailability, impaired microvascular function, and increased tissue oxygen consumption in children with falciparum malaria. The Journal of Infectious Diseases. 2014;210(10):1627–1632. doi: 10.1093/infdis/jiu308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hawkes M., Opoka R. O., Namasopo S., et al. Inhaled nitric oxide for the adjunctive therapy of severe malaria: protocol for a randomized controlled trial. Trials. 2011;12, article 176 doi: 10.1186/1745-6215-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bergmark B., Bergmark R., Beaudrap P. D., et al. Inhaled nitric oxide and cerebral malaria: basis of a strategy for buying time for pharmacotherapy. Pediatric Infectious Disease Journal. 2012;31(12):e250–e254. doi: 10.1097/inf.0b013e318266c113. [DOI] [PubMed] [Google Scholar]
- 112.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends in Immunology. 2015;36(3):161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 113.Cabrales P., Zanini G. M., Meays D., Frangos J. A., Carvalho L. J. M. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. Journal of Infectious Diseases. 2011;203(10):1454–1463. doi: 10.1093/infdis/jir058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zanini G. M., Cabrales P., Barkho W., Frangos J. A., Carvalho L. J. M. Exogenous nitric oxide decreases brain vascular inflammation, leakage and venular resistance during Plasmodium berghei ANKA infection in mice. Journal of Neuroinflammation. 2011;8, article 66 doi: 10.1186/1742-2094-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lou J., Lucas R., Grau G. E. Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clinical Microbiology Reviews. 2001;14(4):810–820. doi: 10.1128/cmr.14.4.810-820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Souza J. B., Hafalla J. C. R., Riley E. M., Couper K. N. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2010;137(5):755–772. doi: 10.1017/s0031182009991715. [DOI] [PubMed] [Google Scholar]
- 117.Craig A. G., Grau G. E., Janse C., et al. The role of animal models for research on severe malaria. PLoS Pathogens. 2012;8(2) doi: 10.1371/journal.ppat.1002401.e1002401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carvalho L. J. M. Murine cerebral malaria: how far from human cerebral malaria? Trends in Parasitology. 2010;26(6):271–272. doi: 10.1016/j.pt.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]