Abstract
OBJECTIVES
While anthracycline-based treatment can cure diffuse large B-cell lymphoma, most patients over age 80 do not receive doxorubicin due to toxicity concerns. This study evaluated this practice, as patients age 80 and older are largely excluded from clinical trials. The primary outcome of interest was overall survival. Secondary outcomes included treatment-related mortality and anthracycline dose intensity.
MATERIALS AND METHODS
We assembled a cohort of 530 newly diagnosed diffuse large B-cell lymphoma patients age 80 or older diagnosed within United States Veterans Health Administration. Treatment and survival information were obtained to determine associations between anthracycline use, dose intensity, treatment-related mortality and overall survival.
RESULTS
Of the 530 patients, 285 received systemic treatment and 193 received an anthracycline. After controlling for potential confounders, rituximab decreased mortality (hazard ratio, 0.62; 95% confidence interval [CI]: 0.44 – 0.88), while doxorubicin was not significantly associated with mortality (hazard ratio, 0.87; 95% CI: 0.64 – 1.17). Completion of treatment with anthracycline dose intensity ≥85% of expected was only 14%. Patients treated with anthracycline dose intensity <85% had better one year survival compared to those treated at ≥85% (70% vs. 59%, p = 0.029).
CONCLUSION
These results suggest that full dose anthracycline therapy may be less important in the treatment of diffuse large B-cell lymphoma patients over age 80. The low frequency of completion of full dose intensity treatment suggests standard doses are an unrealistic standard of care for patients this age. Alternate treatment strategies and risk stratification should be considered for these patients.
Keywords: LYMPHOMA, VETERANS, ELDERLY, DOXORUBICIN
INTRODUCTION
Between the years 2000 and 2030, the proportion of the population over 65 years old will more than double in the United States and the rest of the world.(1, 2) As an age-dependent malignancy, the incidence of non-Hodgkin lymphoma (NHL) in the United States among individuals age 65 and older will also increase from 39 000 in 2010 to an estimated 65 000 in 2030.(1, 3) Three large phase III, randomized, prospective clinical trials shaped the current standard of care in diffuse large B-cell lymphoma (DLBCL), the most common NHL diagnosis. These trials enrolled 2,167 patients age 60 and older.(4-6) Two trials restricted enrollment above age 80 and the other enrolled 16 patients age 80 and older. Consequently, the optimal management of DLBCL remains unclear in those age 80 years and older, the fastest growing age subgroup within the elderly population.(7) Treatment is often limited by comorbidities and concerns about frailty and treatment-related toxicity.(8-11) In addition, the life expectancy for an 80 year old man in the United States is 7.9 years compared to 20.9 years for a 60 year old man,(12) therefore raising concerns about the risk-benefit ratio of curative-intent therapy in this population. Without treatment, however, DLBCL is rapidly fatal.
Anthracycline-based combination chemotherapy has been a cornerstone of treatment for DLBCL for several decades.(13) With the addition of rituximab in 2002, the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen became the standard of care.(4) In practice, however, a majority of patients age 80 and older do not receive doxorubicin, and many do not receive any treatment.(14, 15) One study at a single tertiary care center in patients age 75 and over demonstrated improved survival associated with doxorubicin use, but these results may have been biased by better baseline health status in those given doxorubicin.(16) Even if the decision is made to use doxorubicin, there is little guidance regarding appropriate dosing in this population.(17)
Previous retrospective studies across all ages have shown that in patients treated with CHOP, maintaining chemotherapy dose intensity >70% - 90% is associated with improved overall survival.(18, 19) However, in a nationwide study performed in the United States, only 40% of patients over age 60 were able to achieve dose intensity ≥ 85%.(20) We examined the associations between treatment selection, anthracycline dose intensity, and overall survival in the largest described cohort of DLBCL patients age 80 and older at diagnosis with detailed treatment information. In light of the growing size of this age group, determining the optimal management of older patients with DLBCL is critical.
MATERIALS AND METHODS
Study Cohort
Patients age 80 and older with newly diagnosed with DLBCL between October 1, 1998 and December 31, 2008 were identified in the Veteran's Health Administration cancer registry based on the InterLymph classification system (International Classification of Diseases-O3 codes 9680 or 9684), which allows conversion of tumor registry codes into the diagnoses recognized by the World Health Organization.(21) Information obtained from the cancer registry included: date of birth, date of diagnosis, stage, and the presence or absence of B-symptoms (fevers, night sweats, or unintentional weight loss). The study was approved by the Veterans Affairs St. Louis Health Care System and Washington University Institutional Review Boards prior to cohort assembly.
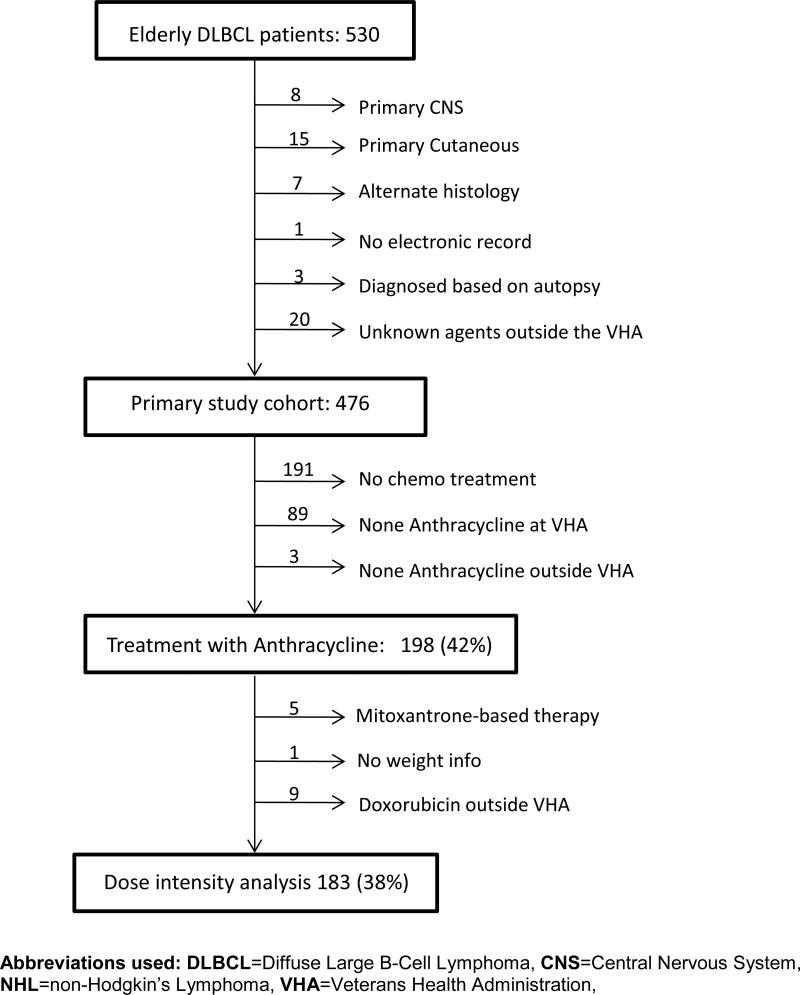
Patient records were linked to anthropomorphic data (height and weight) and International Classification of Diseases-9 codes for comorbid conditions at the time of diagnosis. The Compensation and Pension Records Interchange software system was used to determine baseline performance status and to identify the following: chemotherapy drug, dose, date of administration, myeloid growth factor use, lactate dehydrogenase (LDH) level and pathologic diagnosis. Patients were excluded for the following reasons: primary central nervous system or cutaneous disease, alternate lymphoma histology, lack of electronic records, diagnosis made after death, or treatment outside the Veterans Affairs Health Care System using an unspecified regimen (Figure 1).
Figure 1.
CONSORT diagram
Study Measurements and Outcomes
The primary outcome of interest was overall survival, which was measured from diagnosis until death. Patients without death information were assumed to be alive at the time of last recorded death within the cohort (January 2012), an assumption supported by previous studies demonstrating that > 97% of veteran death events are captured in Vital Status files.(22, 23)
Secondary outcomes of interest were treatment-related mortality and doxorubicin dose intensity. Treatment-related mortality was defined as death within 30 days of last chemotherapy cycle, since there is no uniform definition of treatment related mortality.(24) The expected doxorubicin dose was 50 mg/m2 administered at twenty-one day intervals. Doxorubicin dose intensity was calculated based upon date and dose of doxorubicin administered, divided by expected dose and interval.(25) Patients with stage I or II disease who received radiotherapy were expected to receive a minimum of 3 treatment cycles. All other patients were expected to receive a minimum of 6 treatment cycles.(26) In patients experiencing treatment-related mortality, treatment dose intensity up to the time of death was utilized. Consistent with previous literature, doxorubicin dose intensity of ≥ 85% was considered full dose intensity, while < 85% was considered reduced dose intensity.(20)
Patients receiving at least one dose of doxorubicin or the anthracenedione mitoxantrone (n=5) were included in the anthracycline treatment group, similar to an intention-to-treat analysis. Those receiving systemic chemotherapy or immunotherapy without doxorubicin or mitoxantrone were analyzed in the non-anthracycline treatment group. Rituximab use was considered as a dichotomous variable. Patients who did not have any recorded systemic treatment were analyzed in the no systemic treatment group, though palliative radiotherapy was permitted in these patients.
Body Surface Area (BSA) was calculated according to the DuBois formula,(27) using weight measured within one month of treatment initiation and consistently recorded height data. Weight measurements were screened for extreme values using the Rosner outlier detection algorithm,(28) and none were observed. Age at diagnosis (years) was included as a continuous variable in all statistical models. Myeloid growth factor use was considered as a dichotomous variable, regardless of use in primary or secondary prophylaxis. The Romano adaptation of the Charlson comorbidity index was calculated using diagnostic codes for comorbid conditions.(29)
Eastern Cooperative Oncology Group (ECOG) performance status(30) at the time of diagnosis was obtained from physician notes, converted from Karnofsky score,(31) or estimated based on physician and other notes describing ambulation and performance of activities of daily living.(32) Performance status was dichotomized as 0-1 or 2-4, consistent with the age-adjusted International Prognostic Index.(33) Assessments of performance status were made by two independent and blinded physician reviewers (P.R .and R.L.). Discrepancies in performance status assessments were adjudicated by a third physician reviewer (R.R.). The Cohen's kappa statistic for agreement between the primary reviewers was 0.55.
Statistical Analyses
Fisher's exact, Cochrane-Mantel-Haenszel, and one-way analysis of variance tests were used for univariate analyses, where appropriate. For non-parametric variables the Kruskal-Wallis test was used. Survival was estimated using the methods of Kaplan and Meier, with statistical comparisons using the log-rank test. Cox modeling was used to estimate hazard ratios for death while controlling for potential confounding variables. The proportional hazards assumption was tested using Schoenfeld residuals and by insertion of time-dependent covariates. Interaction terms were tested using the Wald Chi-Square test, and none were found to be significant. Logistic regression was used to estimate odds-ratios for treatment-related mortality. Sensitivity analysis was performed using the inverse probability weighting approach.(34) A two-tailed alpha significance level of 0.05 was considered statistically significant. SAS version 9.2 (Cary, NC) was used to perform all statistical analyses.
RESULTS
We identified 530 patients age 80 or older with newly diagnosed DLBCL. Fifty-four patients were excluded for the reasons delineated in Figure 1. Patients with primary cutaneous DLBCL, central nervous system involvement at the time of diagnosis, or lymphoma diagnosis other than DLBCL were excluded due to the associated differences in prognosis. In addition, patients who receive treatment outside of the VHA with unknown agents were excluded as the association between treatment and survival could not be determined. Baseline characteristics of the remaining 476 patients are presented in Table 1. Across the entire cohort, median age was 83, mean 83.5, range 80 – 100.
Table 1.
Age-standardized baseline characteristics of elderly US veterans diagnosed with DLBCL 1998-2008, stratified by treatment group (N=476)
| AT | Non-AT | NT | ||
|---|---|---|---|---|
| Demographic and Clinical Characteristics | n=198 | n=87 | n=191 | p-value |
| Age at Diagnosis (mean years) | 83.1 | 83.6 | 83.8 | 0.048d |
| Sex (Male %) | 99 | 95.4 | 97.9 | 0.196b |
| Race (%) | 0.322b | |||
| White | 94.4 | 89.7 | 92.2 | |
| Black | 3.5 | 5.8 | 5.2 | |
| Others | 2 | 4.6 | 2.6 | |
| Stage (%) | 0.598c | |||
| Stage I/II | 43.9 | 37.9 | 41.9 | |
| Stage III/IV | 56.1 | 62.1 | 56 | |
| Unknown | 0 | 0 | 2.1 | |
| LDH (%) | 0.23c | |||
| Elevated | 50 | 49.4 | 32.5 | |
| Not Elevated | 41.9 | 43.7 | 18.3 | |
| Unknown | 8.1 | 6.9 | 49.2 | |
| ECOG Performance status (%) | <.001c | |||
| 0-1 | 52 | 37.9 | 10 | |
| 2-4 | 46.5 | 60.9 | 86.4 | |
| Unknown | 1.5 | 1.2 | 3.7 | |
| Age adjusted IPI (%)a | 0.145b | |||
| 0 | 16.2 | 8.1 | ||
| 1 | 27.8 | 31 | ||
| 2 | 31.8 | 26.4 | ||
| 3 | 14.7 | 27.6 | ||
| Unknown | 9.6 | 6.9 | ||
| Co-morbidity (mean Charlson score) | 1.8 | 2.3 | 2.5 | <0.001d |
| B-symptom( %) | 0.037b | |||
| Yes | 42.4 | 55.2 | 50.8 | |
| No | 55.1 | 43.7 | 41.4 | |
| Unknown | 2.5 | 1.2 | 7.9 | |
| Myeloid growth factor use (%)a | 78.8 | 45.2 | <.001b | |
| Rituximab use (%)a | 80.3 | 77 | 0.501b | |
| Year of diagnosis (Median) | 2005 | 2005 | 2004 | 0.114e |
| Treatment Regimens a | ||||
| CHOP/R-CHOP | 190 | - | ||
| CNOP/R-CNOP | 5 | - | ||
| CVP/R-CVP | - | 53 | ||
| R-EPOCH | 1 | - | ||
| Rituximab | - | 21 | ||
| Other | 2 | 13 |
Footnote:
Comparison between doxorubicin and non-doxorubicin groups only
Chi-Square or Fisher's exact test
Row mean score test
One way ANOVA
Kruskal-Wallis Test
Abbreviations used: DLBCL=Diffuse Large B-cell Lymphoma; N=number; AT= Anthracycline based therapy; non-AT= Non-anthracycline based therapy; NT= No treatment; LDH= Lactate dehydrogenase; ECOG=Eastern Cooperative Oncology Group; IPI=International Prognostic Index; CHOP/R-CHOP=Cyclophosphamide, Hydroxydaunorubicin, Oncovin, Prednisone, Rituximab; CNOP/R-CNOP=Cyclophosphamide, Novantrone, Oncovin, Prednisone, Rituximab; CVP/R-CVP=Cyclophosphamide, Vincristine, Prednisone, Rituximab; R-EPOCH=Rituximab, Etoposide, Prednisone, Oncovin, Cyclophosphamide, Hydroxydaunorubicin.
Treatment selection and survival
Anthracycline-based therapy was given to 198 (42%) patients and 87 (18%) patients received systemic treatment without an anthracycline. Among the 191 (40%) patients who received no systemic treatment, 31 received palliative radiotherapy. Age, performance status, comorbidity score, B-symptoms, and myeloid growth factor use were significantly different across groups (Table 1). In a Kaplan-Meier analysis stratified by treatment, median overall survival was 1.9 months for no systemic treatment, 13.1 months for treatment without an anthracycline, and 28.1 months for the anthracycline treatment group.
To understand whether the survival differences observed above were primarily associated with anthracycline use or differences in baseline patient characteristics and supportive care, we performed Cox analysis to evaluate the association of anthracycline treatment and overall survival among those who received systemic therapy. This analysis was controlled for variables that were significantly associated with overall survival on univariate analyses (data not shown): age, LDH, comorbidities, B-symptoms, and performance status; given that these are known risk factors affecting overall survival. While disease stage is a known prognostic factor in DLBCL, it was not significantly associated with survival during the univariate analysis, and was thus not included in subsequent analyses. Myeloid growth factor use was also explored in the model, given its association with a reduction in febrile neutropenia and infectious complications. Furthermore, rituximab use was included in the Cox model because of its clear overall survival benefit.(4) In the multivariable analysis, rituximab was associated with reduced mortality (hazard ratio [HR], 0.65; 95% confidence interval [CI]: 0.46 - 0.93), while myeloid growth factor use demonstrated a statistically non-significant trend towards reduced mortality (HR, 0.74; 95% CI: 0.54 – 1.01). Comorbidities (HR, 1.09; 95% CI: 1.01 – 1.18) and elevated serum LDH at the time of diagnosis (HR, 2.24; 95% CI: 1.67 – 3.01) were associated with increased mortality. Patients with a performance status of 2-4 were at substantially increased risk of death within 60 days of diagnosis (HR, 17.0; 95% CI: 2.28- 126.26), which then attenuated after 60 days from diagnosis (HR, 1.73; 95% CI: 1.27 -2.35). After controlling for the other variables, anthracycline use was no longer significantly associated with overall survival (HR, 0.82; 95% CI: 0.59 – 1.13); nor was age (HR, 0.99; 95% CI: 0.95 – 1.05). B-symptoms were also not significantly associated with survival. The inclusion or exclusion of B-symptoms in the Cox model did not alter the significance or effect size of the other variables; therefore B-symptoms were not included in the final Cox model.
Sensitivity analysis was performed using inverse probability weighting to adjust for differences in baseline performance status, co-morbidity score, and the presence of B-symptoms. Similar to the Cox analysis, there was no significant association between anthracycline use and overall survival (HR, 0.93; 95% CI: 0.70 - 1.23). Performance status of 2 – 4 remained strongly associated with death within the first 60 days after diagnosis (HR, 6.81; 95% CI: 2.20 – 21.08), again attenuating after 60 days (HR, 1.81; 95% CI: 1.35 – 2.41). Co-morbidity score was also associated with increased risk of death (HR, 1.09; 95% CI: 1.01 – 1.18). Rituximab (HR, 0.63; 95% CI: 0.45 – 0.88) and myeloid growth factor (HR, 0.70; 95% CI: 0.53 – 0.93) use were also associated with a decreased risk of death.
Treatment-related mortality
Among the 273 patients who received any systemic treatment and who had treatment date information, 48 (18%) experienced treatment-related mortality. Thirty-two of the 48 deaths (67%) were associated with the first cycle of therapy. Infectious complications were the proximate cause of death in 28 (58%), 8 (17%) were cardiac or respiratory in nature and the remaining 12 (25%) were unknown or other etiology. Although not statistically significant, there was a trend towards a higher rate of treatment-related mortality in the patients who did not receive an anthracycline compared to those who did (23% vs. 15%, p = 0.15), on univariate analysis. Furthermore, baseline performance status 2 -4 was strongly associated with increased treatment-related mortality compared with performance status 0-1 (TRM = 27% vs. 8%, respectively, p< .001). After analyzing anthracycline use while controlling performance status and myeloid growth factor use in a logistic regression model, performance status remained a significant predictor of treatment-related mortality (odds ratio [OR], 3.87; 95% CI: 1.86 – 8.03), while anthracycline (OR, 0.86; 95% CI: 0.42 – 1.77) and myeloid growth factor use (OR, 0.67; 95% CI: 0.33 – 1.36) were not significant. This suggests that the difference in treatment-related mortality noted between the anthracycline and non-anthracycline groups on univariate analysis was largely due to differences in baseline performance status.
Doxorubicin dose intensity and completion of therapy
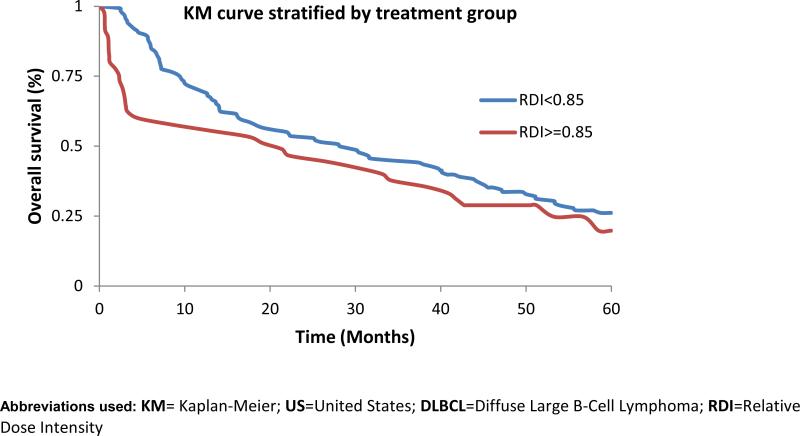
Doxorubicin dose intensity was next evaluated in the doxorubicin treated patients who had height, weight, and dosing information. As demonstrated in Table 1, anthracycline treated patients were younger, had better performance status and fewer comorbidities compared to the remainder of the cohort. Of the 183 patients doxorubicin, only 26 (14%) completed therapy at full (≥ 85%) dose intensity without treatment-related mortality; 9 of whom had early stage disease and were able to stop systemic therapy after three cycles. Thirty-three patients (18%) either died or discontinued treatment after a single dose of doxurubicin. Patients treated with a doxorubicin dose ≥ 85% of expected in the first cycle demonstrated a trend towards higher treatment-related mortality than those who did not (11% vs. 2%, p = 0.07). Largely because of this difference, a Kaplan-Meier analysis stratified by dose intensity demonstrates poorer near-term survival associated with full doxorubicin dose intensity (Figure 2). Median survival was 21.8 months in those receiving full doxorubicin dose intensity and 28.1 months in those receiving reduced doxorubicin dose intensity. At one year, 59% of those treated at full dose intensity were alive, while 70% of those treated at reduced dose intensity were alive (Log-rank p =0.029).
Figure 2.
KM curves of elderly US veterans diagnosed with DLBCL 1998-2008 doxorubicin dose-intensity and overall survival
DISCUSSION
In this, the largest reported cohort of newly diagnosed DLBCL age 80 and older with detailed treatment data: 42% received anthracycline-based treatment, 18% received treatment without an anthracycline, and 40% received no systemic treatment at all. Even among those deemed healthy enough to receive an anthracycline, only 26 (14%) were able to complete treatment at full dose intensity. When the 26 patients completing full dose therapy are compared to 476 patients in the primary study cohort, only 5% of these patients completed full dose, standard of care therapy. Treatment-related mortality among patients receiving an anthracycline was 14%, far exceeding the 3% to 8% mortality seen in clinical trials of doxorubicin-based regimens in patients with DLBCL over age 60.(4-6) Overall, these findings suggest that the standard 3 - 6 cycles of full dose R-CHOP chemotherapy is an unrealistic therapeutic goal for most patients in this age group.
Multivariable analysis further suggested that the survival difference observed between patients treated with and without an anthracycline is largely explained by better baseline performance status. At the same time, use of the well-tolerated monoclonal antibody rituximab was strongly associated with survival. This suggests that patients in this age group benefit from therapy, but the long-term survival benefits associated with an anthracycline may not outweigh the toxicity for some. This is understandable given the shorter average life expectancy of patients age 80 and older due to competing risks of death such as heart disease and the high risk of treatment-related mortality. Undoubtedly, some patients over age 80 do benefit from anthracycline treatment. However, these benefits were not statistically significant when considered across this patient sample. This suggests the decision to administer anthracyclines in patients over age 80 should be undertaken with careful consideration of performance status and other variables that may predict how well this treatment will be tolerated.
Among patients receiving an anthracycline, full dose intensity was associated with considerable treatment-related mortality and reduced survival at one year, compared to the decreased dose intensity group. Although the exact reasons for this association cannot be determined in this analysis, possible hypotheses include age-related physiologic changes including decreased bone marrow reserve, which could put elderly patients at increased risk for severe myelosuppression and infectious complications. Additionally, decline in organ function may lead to alterations in drug metabolism putting patients at risk for further toxicity.
Although full dose intensity was associated with reduced survival at one year, the proportion of patients surviving in each group became similar by two years after diagnosis (53% reduced intensity, 48% full intensity). This may be due to a higher frequency of relapse and disease-related mortality in those treated at lower doxorubicin dose intensity. The improvement in near-term, but not long-term survival associated with reduced dose intensity doxorubicin could provide meaningful benefit in terms of both quality and quantity of life.
Strengths of this study should be highlighted. First, electronic data systems within the Veterans Affairs Health Care System allowed ascertainment of patient-level details not available in administrative databases such as Medicare. Second, the large number of untreated patients is consistent with other population-based studies looking at DLBCL patients of this age,(14, 35) suggesting our findings are generalizable. Finally, the diversity of Veterans Affairs hospitals resulted in diverse treatment patterns, likely reflecting community practice patterns across the United States.
Weaknesses of this study should also be noted. First, the patients were nearly all men. Second, performance status was not assessed systematically at the time of diagnosis in most patients. However, assessments were made using physician and allied healthcare provider notes, resulting in reasonable inter-rater agreement between the blinded physician assessors. Third, some patients may have received treatment outside of the Veterans Health Administration, potentially biasing results, though the dismal prognosis noted in the no treatment group suggests that most in this group actually did not receive treatment. Fourth, consistent with previous reports in the veteran population, co-morbidity scores were higher than often reported elsewhere, (36) which could enhance the toxicity of anthracycline-based therapy in this population. However, this represents the real-world experience in patients age 80 and older, who are seldom eligible for enrollment in a clinical trial. Finally, since treatments were not assigned to the patients randomly, it is possible that residual confounding has biased the results. We attempted to address this last issue by controlling for comorbidities, performance status, and other factors, though there may be unmeasured variables that could not be quantified.
In conclusion, this study of veterans with DLBCL age 80 and older suggests that current clinical practice guidelines in this population do not represent an achievable standard of care. The high risk of treatment-related mortality and decreased long-term survival benefit makes the role of full dose doxorubicin in the treatment of these patients unclear. Through a reduction in early mortality, either attenuated doses of doxorubicin or alternative treatment regimens may provide greater overall survival benefit across this patient population. Several strategies have previously been considered to reduce toxicity in older patients. Pre-phase treatment with corticosteroids followed by chemotherapy, in order to reduce disease burden and reduce complications such as tumor lysis syndrome, has been advocated by some.(7) Treatment with a reduced dose intensity form of R-CHOP (that cuts the doxorubicin dose in half), has also been shown to offer reasonable outcomes in patients over age 80.(17) Another potential option that could be considered is treatment regimens without doxorubicin, specifically cyclophosphamide, etoposide, vincristine, and prednisone (CEOP) or cyclophosphamide, etoposide, procarbazine, and prednisone (R-CEPP).(38, 39, 40) Ultimately, widespread application of clinical assessment tools, including the comprehensive geriatric assessment, may provide the best approach to identification of elderly patients who are most likely to benefit from curative intent treatment with standard dose chemotherapy. (9, 41, 42)
ACKNOWLEDGEMENTS
Funding: This study was funded in part by: The Barnes-Jewish Hospital Foundation, The American Cancer Society grant IRG 58-010-52, and the National Cancer Institute at the National Institutes of Health, grants U54CA155496 and KM1CA15608. The content is solely the responsibility of the authors and does not necessarily represent the official views of: The United States Department of Veterans Affairs, the National Cancer Institute or the National Institutes of Health.
Footnotes
Authors’ contributions: All authors have approved the final article. Conception and Design – KRC and GAC. Data Collection – PR, RL, RR, WL, KO, KMS, and AG. Analysis and Interpretation of data – KRC, WL, JL, CN, TMW, AG, KMS, KO, NLB, AC, NWJ, TAF, and GAC. Manuscript writing - KRC, WL, JL, CN, TMW, PR, RL, RR, AG, KMS, KO, NLB, AC, NWJ, TAF, and GAC. Approval of final article - KRC, WL, JL, CN, TMW, PR, RL, RR, AG, KMS, KO, NLB, AC, NWJ, TAF, and GAC.
Disclosure conflicts of interest: KRC has consulted and is a member of the speaker's bureau for Genentech, manufacturer of the drug rituximab. This relationship is tangential to this project as it is already established science that rituximab works. All other authors have no conflict of interest to disclose.
REFERENCES
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA, Smith BD, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J CLIN ONCOL. 2009;27(17):2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Goulding MR, Rogers ME, Smith SM. Public Health and Aging: Trends in Aging - United States and Worldwide. MMWR - Morbidity and Mortality Weekly Report. 2003;52(6):101–6. [PubMed] [Google Scholar]
- 3.SEER Non-Hodgkin Lymphoma: Incidence and Mortality rates by age. 2012 Feb 15; 2012 Available from: http://seer.cancer.gov/csr/1975_2008/browse_csr.php?section=19&page=sect_19_table.07.html.
- 4.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N ENGL J MED. 2002;346(4):235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). J LANCET ONCOL. 2008;9(2):105–16. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 6.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J CLIN ONCOL. 2006;24(19):3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 7.World Population Ageing: 1950-2050. United Nations, Department of Economic and Social Affairs PD; New York: 2001. [Google Scholar]
- 8.Janssen-Heijnen ML, van Spronsen DJ, Lemmens VE, Houterman S, Verheij KD, Coebergh JW, et al. A population-based study of severity of comorbidity among patients with non-Hodgkin's lymphoma: prognostic impact independent of International Prognostic Index. BR J HAEMATOL. 2005;129(5):597–606. doi: 10.1111/j.1365-2141.2005.05508.x. [DOI] [PubMed] [Google Scholar]
- 9.Nabhan C, Smith SM, Helenowski I, Parsons B, Karmali R, Feliciano J, et al. Analysis of very elderly non-hodgkin lymphoma: impact of functional status and comorbidities on outcome. BR J HAEMATOL. 2012;156:196–204. doi: 10.1111/j.1365-2141.2011.08934.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramsdale E, Smith S, Ramsdale E, Smith S. Old versus frail: why it matters in lymphoma. LEUK LYMPHOMA. 2011;52(6):938–40. doi: 10.3109/10428194.2011.582657. [DOI] [PubMed] [Google Scholar]
- 11.Hershman DL, McBride RB, Eisenberger A, Tsai WY, Grann VR, Jacobson JS, et al. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin's lymphoma. J CLIN ONCOL. 2008;26(19):3159–65. doi: 10.1200/JCO.2007.14.1242. [DOI] [PubMed] [Google Scholar]
- 12.Bureau USC. Life expectancy by Sex, Age, and Race. 2008 Nov 18; 2012 Available from: http://www.census.gov/compendia/statab/cats/births_deaths_marriages_divorces/life_expectancy.html.
- 13.McKelvey EM, Gottlieb JA, Wilson HE, Haut A, Talley RW, Stephens R, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. CANCER. 1976;38(4):1484–93. doi: 10.1002/1097-0142(197610)38:4<1484::aid-cncr2820380407>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Link BK, Brooks J, Wright K, Pan X, Voelker M, Chrischilles E, et al. Diffuse large B-cell lymphoma in the elderly: diffusion of treatment with rituximab and survival advances with and without anthracyclines. LEUK LYMPHOMA. 2011;52(6):994–1002. doi: 10.3109/10428194.2011.557167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thieblemont C, Grossoeuvre A, Houot R, Broussais-Guillaumont F, Salles G, Traulle C, et al. Non-Hodgkin's lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. ANN ONCOL. 2008;19(4):774–9. doi: 10.1093/annonc/mdm563. [DOI] [PubMed] [Google Scholar]
- 16.Toomey CE, Muzikanksy A, Lee AI, Barnes JA, Michaelson JS, Abramson JS, et al. Treatment Choice and Outcome In Diffuse Large B Cell Lymphoma (DLBCL) Patients 75 Years and Older. ASH Annual Meeting Abstracts. 2010;116(21):733. [Google Scholar]
- 17.Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile JF, Castaigne S, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. J LANCET ONCOL. 2011;12(5):460–8. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 18.Bosly A, Bron D, Van Hoof A, De Bock R, Berneman Z, Ferrant A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. ANN HEMATOL. 2008;87(4):277–83. doi: 10.1007/s00277-007-0399-y. [DOI] [PubMed] [Google Scholar]
- 19.Lepage E, Gisselbrecht C, Haioun C, Sebban C, Tilly H, Bosly A, et al. Prognostic significance of received relative dose intensity in non-Hodgkin's lymphoma patients: application to LNH-87 protocol. The GELA. (Groupe d'Etude des Lymphomes de l'Adulte). ANN ONCOL. 1993;4(8):651–6. doi: 10.1093/oxfordjournals.annonc.a058619. [DOI] [PubMed] [Google Scholar]
- 20.Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI, Lyman GH, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin's lymphoma: a nationwide study. J CLIN ONCOL. 2004;22(21):4302–11. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 21.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). BLOOD. 2007;110(2):695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savas L, del Junco D, Bastian L, Vernon S. Mortality Ascertainment of Women Veterans: A Comparison of Sources of Vital Status Information, 1979-2002. MED CARE. 2009;47(1):125–8. doi: 10.1097/MLR.0b013e3181809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn M, Arnold N, Maynard C, Hynes D. Accuracy and completeness of mortality data in the Department of Veterans Affairs. POPUL HEALTH METR. 2006;4(2) doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ethier MC, Blanco E, Lehrnbecher T, Sung L, Ethier M-C, Blanco E, et al. Lack of clarity in the definition of treatment-related mortality: pediatric acute leukemia and adult acute promyelocytic leukemia as examples. BLOOD. 2011;118(19):5080–3. doi: 10.1182/blood-2011-07-363333. [DOI] [PubMed] [Google Scholar]
- 25.Hryniuk WM, Goodyear M. The calculation of received dose intensity. J CLIN ONCOL. 1990;8(12):1935–7. doi: 10.1200/JCO.1990.8.12.1935. [DOI] [PubMed] [Google Scholar]
- 26.Zelenetz AD. Non-Hodgkin's Lymphomas. 2012 Oct 14; 2012 Available from: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
- 27.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. ARCH INTERN MED. 1916;17:863–71. [Google Scholar]
- 28.Rosner B. On the Detection of Many Outliers. TECHNOMETRICS. 1975;17(2):221–7. [Google Scholar]
- 29.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J CLIN EPIDEMIOL. 1993;46(10):1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion 81-90. [DOI] [PubMed] [Google Scholar]
- 30.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. AM J CLIN ONCOL. 1982;5(6):649–56. [PubMed] [Google Scholar]
- 31.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. EUR J CANCER. 1996;32A(7):1135–41. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 32.Kamby C, Sengelov L. Assessment of functional status in patients with invasive carcinoma of the urothelial tract. UROL ONCOL. 1996;2(2):43–51. doi: 10.1016/s1078-1439(96)00049-x. [DOI] [PubMed] [Google Scholar]
- 33.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N ENGL J MED. 1993;329(14):987–94. doi: 10.1056/NEJM199309303291402. Anonymous. [DOI] [PubMed] [Google Scholar]
- 34.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. PSYCHOL METHODS. 2010;15(3):234–49. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Schans SA, Wymenga AN, van Spronsen DJ, Schouten HC, Coebergh JW, Janssen-Heijnen ML. Two sides of the medallion: poor treatment tolerance but better survival by standard chemotherapy in elderly patients with advanced-stage diffuse large B-cell lymphoma. ANN ONCOL. 2012;23(5):1280–6. doi: 10.1093/annonc/mdr411. [DOI] [PubMed] [Google Scholar]
- 36.Landrum MB, Keating NL, Lamont EB, Bozeman SR, Krasnow SH, Shulman L, et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J CLIN ONCOL. 2012;30(10):1072–9. doi: 10.1200/JCO.2011.35.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. BLOOD. 2010;116(24):5103–10. doi: 10.1182/blood-2010-07-259333. [DOI] [PubMed] [Google Scholar]
- 38.Moccia AA, Schaff K, Hoskins P, Klasa R, Savage KJ, Shenkier T, et al. R-CHOP with Etoposide Substituted for Doxorubicin (R-CEOP): Excellent Outcome in Diffuse Large B Cell Lymphoma for Patients with a Contraindication to Anthracyclines. ASH Annual Meeting Abstracts. 2009;114(22):408. [Google Scholar]
- 39.Weidmann E, Neumann A, Fauth F, Atmaca A, Al-Batran SE, Pauligk C, et al. Phase II study of bendamustine in combination with rituximab as first-line treatment in patients 80 years or older with aggressive B-cell lymphomas. ANN ONCOL. 2011;22(8):1839–44. doi: 10.1093/annonc/mdq671. [DOI] [PubMed] [Google Scholar]
- 40.Chao NJ, Rosenberg SA, Horning SJ. CEPP(B): An effective and well-tolerated regimen in poor-risk, aggressive Non-Hodgkins lymphoma. BLOOD. 1990;76(7):1293–1298. [PubMed] [Google Scholar]
- 41.Maartense E, Kluin-Nelemans HC, Noordijk EM. Non-Hodgkin's lymphoma in the elderly. A review with emphasis on elderly patients, geriatric assessment, and future perspectives. ANN HEMATOL. 2003;82(11):661–70. doi: 10.1007/s00277-003-0722-1. [DOI] [PubMed] [Google Scholar]
- 42.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J CLIN ONCOL. 2007;25(14):1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]