Abstract
Objective
To review the literature regarding the efficacy and safety of mirabegron for the treatment of overactive bladder (OAB).
Data Sources
A literature search was performed using MEDLINE (PubMed) prior to 12/31/2013 using the terms “mirabegron” and “randomized-controlled trial.”
Study Selection/Data Extraction
All published, double-blind, randomized controlled trials assessing mirabegron were included. Articles were reviewed and included if mirabegron was used as monotherapy and if the primary outcome analyzed drug efficacy.
Data Synthesis
The efficacy of mirabegron for the treatment of OAB has been demonstrated in the selected five randomized, placebo-controlled trials. The majority of these trials lasted 12 weeks in duration and compared various doses of mirabegron to placebo and/or tolterodine extended release (ER). Primary efficacy outcomes for the trials included mean number of micturitions per 24 hours and mean number of incontinence episodes per 24 hours. Included trials showed statistically significant reductions in both efficacy outcomes for various doses of mirabegron when compared to placebo.
Conclusion
Based on the trials reviewed, mirabegron has been efficacious in reducing mean number of micturitions and incontinence episodes per 24 hours, as well as improved other secondary outcomes like OAB symptoms and quality of life measures. Common adverse drug events seen with mirabegron include: hypertension, nasopharyngitis, urinary tract infections, headache, constipation, upper respiratory tract infection, arthralgia, diarrhea, tachycardia, abdominal pain, and fatigue. Given the efficacy and safety data currently available, mirabegron represents a reasonable alternative to antimuscarinics for patients with OAB.Future studies are needed to determine the utility of mirabegron for OAB in a variety of demographics.
Keywords: beta-3 agonists, new FDA medication, overactive bladder, urge urinary incontinence, urology
Introduction
Overactive bladder (OAB) is a bothersome urological condition that can affect both men and women. In epidemiological studies the comparative prevalence of OAB increases with age.1–3 Based on a cross-sectional survey, frequency, urgency, and urge incontinence affects 13.7%, 7.6%, and 4% of the overall male population, respectively, while it affects 14.6%, 9.7%, and 7.4% of the overall female population, respectively.1 The highest incidence of these symptoms is in both men and women 75 years or older. Medical attention is often not sought by patients with OAB symptoms as patients often attribute the symptoms to an inevitable outcome of aging, a belief there is no effective treatment available, or have a history of previous failure with OAB medications due to poor efficacy or adverse events.4 Because of these factors, only about 20% of patients with OAB symptoms are treated with pharmacotherapy.3–5
Another reason why this condition may be undertreated is that the diagnosis of overactive bladder is very subjective, as the definitions of the hallmark symptoms differ from person-to-person and among studies.6 The definition of OAB is the presence of urinary urgency, increased frequency (8 or more micturition per waking hours), and nocturia (awaking to urinate one or more times), with or without urinary leakage.6–8 In addition, there are a variety of confounders that can affect these definitions such as number of hours slept, fluid intake, and other medical conditions such as diabetes and diuretic use in congestive heart failure. Because these symptoms are subjective, the effect on quality of life typically dictates treatment. One way to identify these subjective symptoms is by using a variety of questionnaires that assess severity of OAB symptoms.6
Current Management
There are several assessments that need to be considered prior to starting pharmacotherapy. A physical exam and laboratory testing need to be performed in order to rule out infection, vaginal atrophy, stool impaction, and diabetes mellitus. Current medications need to be reviewed to determine if symptoms are associated with medications such as diuretics and acetylcholinesterase inhibitors.9 Prior to the use of any oral agents for the treatment of OAB, a non-pharmacological approach should be utilized.6 Behavioral therapies such as bladder training and pelvic floor exercises can improve symptoms without use of medications. Pharmacotherapy should ultimately be dictated by the subjective symptoms of the patient and can be used in conjunction with these non-pharmacological interventions.6,10
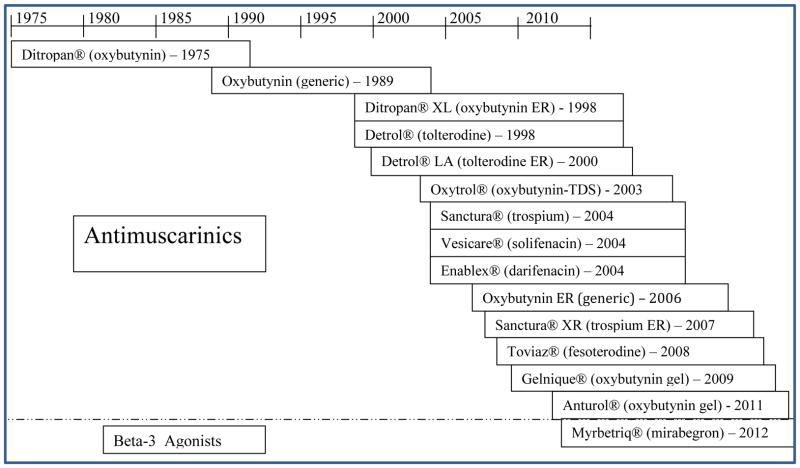
Use of Food and Drug Administration (FDA)-approved antimuscarinics dates back to 1975 with the approval of oxybutynin immediate release (IR).11 Since then, five new chemical entities with several formulations each have been approved (Figure 1). The United States American Urological Association (AUA) guidelines do not recommend one antimuscarinic therapy over another. If an antimuscarinic fails or a patient has an adverse drug reaction to a particular antimuscarinic, another can be tried.6
Figure 1. History of OAB Medications FDA Approvals.
Source: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
Chapple, et al performed a meta-analysis which evaluated the safety and efficacy of all FDA-approved antimuscarinic medications used to treat OAB.12 Across the various studies, there was a significant reduction in incontinence episodes by 0.5 to 1.08 per day from baseline in all antimuscarinics excluding tolterodine IR, which was no different from placebo. Frequency was significantly reduced by 0.54 to 1.3 episodes per day from baseline in all antimuscarinic modalities. Urgency episodes were also significantly reduced by 0.65 to 1.56 episodes per day. Adverse drug reactions (ADRs) typically associated with antimuscarinics include constipation, dry mouth, and blurry vision due to antagonism of the muscarinic (M3) receptor.13 In this meta-analysis, patients taking oxybutynin IR were more likely to withdraw from treatment and had a significantly higher incidence of dry mouth compared to other antimuscarinics.12 Additionally, solifenacin and darifenacin were more likely to cause constipation, and solifenacin was more likely to cause blurred vision compared to other antimuscarinics. There are also concerns for using antimuscarinics in the elderly due to their potential for cognitive dysfunction.14 Contraindications also exist for using antimuscarinics in patients with acute urinary retention.
Beta-3 agonists for OAB
The mechanism of micturition is a complicated process involving both voluntary and involuntary actions in the urinary tract through innervation of the parasympathetic, sympathetic, and somatic nerve pathways. Additionally, there are several neurohormonal pathways which control the filling and voiding phases. As the bladder fills with urine, it normally is able to maintain a low intravesical pressure via sympathetic nerve stimulation despite the increasing volume.15 Acetylcholine, which is normally released during the voiding phase, binds to the muscarinic receptors on the detrusor. Of the five known subtypes of muscarinic receptors, M3 receptors are responsible for causing involuntary contractions during normal micturition.13,16 When the bladder is full acetylcholine stimulates involuntary bladder contractions, which reduces sympathetic beta-3 activity.17 Until recently, antimuscarinics, were the only medications approved for the treatment of OAB.13,16
In 2012, the FDA approved the first non-antimuscarinic oral medication, mirabegron, to treat patients with OAB symptoms. Although mirabegron is a relatively new medication, the pathway involved in its mechanism was discovered approximately 20 years ago; however, the process of approving this medication was delayed due to potential cardiac side effects.18–19 Mirabegron works via the sympathetic nerve pathway and stimulates beta-3 receptors, causing smooth muscle relaxation in the bladder.18,20 The use of a beta-3 agonist is specific to the bladder as 97% of the beta adrenergic receptor subtypes are the beta-3 subtype.17 As mirabegron does not cause antagonism of M3 receptors, it is postulated that there is a lower risk for urinary retention compared to antimuscarinic agents.17,20
Dosing and Administration
The initial dose of Mirabegron (Myrbetriq®) is 25mg by mouth taken at approximately the same time every day, swallowed whole, with or without food. After eight weeks, the dose can be titrated to 50mg by mouth daily based on an individual’s response. It is not recommended to exceed 25mg by mouth daily in patients with severe renal disease (estimated creatinine clearance <30mL/min) or moderate hepatic impairment (Child-Pugh Class B). In addition, it should be avoided altogether in patients with end stage renal disease or severe hepatic impairment (Child-Pugh Class C). Mirabegron is a moderate cytochrome P (CYP) 450 inhibitor of 2D6 and should be used in caution with 2D6 substrates (i.e. metoprolol, desipramine, flecainide, and propafenone).21
Clinical Trials
A MEDLINE (PubMed) search was performed for studies prior to December 31st, 2013. For this review, the terms “mirabegron” and “randomized controlled trial” were used to identify publications. Only published, randomized, controlled trials (RCTs) with OAB efficacy endpoints as the primary outcome were included. Our search resulted in 10 trials, of which three met the criteria for inclusion. Other search strategies (i.e. reference lists of primary papers searched) identified two additional relevant studies. The five trials included are described in detail in Table 1 and results are listed in Table 2. The patient population included in these trials consisted of >65% females and >85% Caucasians. The average age ranged from 55 to 61 years of age, with >80% of patients under the age of 75. Of the trials that reported the following criteria, 40–50% of patients had taken a prior OAB medication and patients averaged approximately 11 micturition per 24 hours at baseline.
Table 1.
Summary of Trials of Mirabegron for Overactive Bladder
| Study Design | Inclusiona | Exclusiona | Intervention |
|---|---|---|---|
| BLOSSOM22 Multinational, multicenter, randomized, double-blind, double-dummy, parallel group, placebo and active-controlled Phase II proof-of-concept study |
Patients ≥18 years who had symptoms of OAB (urinary frequency and urgency with or without urgency incontinence) for ≥3 months Frequency of micturition average ≥8 times/24 h and ≥3 episodes or urgency (grade 3 or 4), with or without incontinence during 3-day micturition diary period |
Clinically significant outflow obstruction, significant PVR volume (>200 ml), stress predominant incontinence, indwelling catheters, neurological causes for abnormal detrusor activity, diabetic neuropathy, symptomatic bladder disorders, bladder stones | 2 week, single-blind, placebo run-in period; then randomized to placebo (n=66), mirabegron 100mg twice daily (n=65), mirabegron 150mg twice daily (n=65), or tolterodine ER 4mg once daily (n=66) for 4 weeks |
| DRAGON23 Multinational, multicenter, randomized, double-blind, double-dummy, parallel-group placebo- and active-controlled phase II study |
Patients ≥18 years who had OAB for ≥3 months Eligible for randomization if during a 3 day micturition diary period experienced frequency of micturition on average ≥8 times per 24 h and at least 3 episodes of urgency (grade 3 or 4) with or without incontinence |
Clinically significant bladder outflow obstruction, significant PVR volume (>200 ml), stress predominant incontinence, indwelling catheters, UTI, interstitial cystitis, diabetic neuropathy, clinically significant cardiovascular (including ECG abnormalities) or cerebrovascular disease | 2 week, placebo run-in; eligible patients were randomized to receive placebo (n=169), mirabegron 25mg (n=169), mirabegron 50mg (n=169), mirabegron 100mg (n=168), mirabegron 200mg (n=167), or tolterodine ER 4mg (n=85) orally once daily for 12 weeks |
| ARIES24 Randomized, double-blind, parallel-group, placebo-controlled trial; Phase III |
Patients ≥18 years with symptoms of OAB for ≥3 months Eligible for randomization if during a 3 day micturition diary period experienced ≥8 micturitions/24 h and ≥3 urgency episodes (grade 3 or 4) with or without incontinence |
Clinically relevant stress incontinence or mixed stress/urgency incontinence with stress as the predominant factor; an indwelling catheter; evidence of a symptomatic urinary tract infection, bladder stones, previous or current malignant disease of the pelvic organs; or severe HTN (sitting average SBP ≥180 mmHg and/or average DBP ≥110 mmHg); use of OAB medication which could not be stopped safely at screening | 2 week, single-blind, placebo run-in; then randomized in a 1:1:1 ratio to receive placebo (n=453), mirabegron 50mg (n=442) or 100mg (n=433) once daily for 12 weeks |
| SCORPIO25 Multicenter randomized, double-blind, parallel group placebo and active controlled phase III trial |
Patients ≥18 years who had OAB for ≥3 months Eligible for randomization if during a 3 day micturition diary period experienced ≥8 micturitions/24 h and ≥3 urgency episodes (with or without incontinence) |
Stress incontinence or stress-predominant mixed incontinence at screening, or an average total daily urine volume >3000 ml as recorded in a 3-day micturition diary period, clinically significant bladder outflow obstruction at risk of urinary retention, an indwelling catheter, severe HTN (SBP ≥180 and/or DBP ≥110), UTI, interstitial cystitis, bladder stones, patients receiving treatment with CYP3A4 inhibitors | 2 week, single blind placebo run in period; eligible patients were randomized in a 1:1:1:1 ratio to receive placebo (n=497), mirabegron 50mg (n=497), mirabegron 100mg (n=498), or tolterodine ER 4mg (n=495) orally once daily for 12 weeks |
| Herschorn et al26 Multicenter, randomized, double-blind, parallel-group, placebo-controlled, phase III trial |
Patients ≥18 years who had OAB for ≥3 months Eligible for randomization if during a 3 day micturition diary period experienced ≥8 micturitions/24 h and ≥3 urgency episodes (with or without incontinence) |
Average total daily urine volume >3000 mL during the diary period and stress incontinence or stress predominant mixed stress or urge incontinence | 2 week, single blind placebo run in period; eligible patients were randomized in a 1:1:1 ratio to receive placebo (n=433), mirabegron 25mg (n=432), or mirabegron 50mg (n=440) orally once daily for 12 weeks |
Abbreviations: DBP = diastolic blood pressure; ECG = electrocardiogram; ER = extended release; H = hours; HTN = hypertension; OAB = overactive bladder; PVR = post-void residual, SBP = systolic blood pressure; UTI = urinary tract infection
Only selected criteria are listed
Table 2.
Summary of Trial Results of Mirabegron for Overactive Bladder
| Study | End Points | Primary Endpoint Results | Secondary Endpoints Resultsa |
|---|---|---|---|
| BLOSSOM22 | Primary endpoint: change from baseline to treatment-end in mean number of micturitions/24 h Secondary endpoints: changes from baseline to treatment-end in mean volume voided per micturition, mean number of urinary incontinence and urgency urinary incontinence episodes, and urgency episodes/24 h, severity of urgency, nocturia, and patients’ perception of bladder condition and treatment benefit |
Change in mean number of micturitions/24 h (compared to placebo) Mirabegron 100mg BID: − 1.02 (95% CI −1.67 to − 0.36, p≤0.01) Mirabegron 150mg BID: − 1.03 (95% CI −1.69 to − 0.37, p≤0.01) Tolterodine ER 4mg: − 0.4(95% CI −1.06 to 0.26, p>0.05) |
Mean volume voided/micturition (mL) (compared to placebo) Mirabegron 100mg BID: 15.56 (95% CI −0.14 to 31.25, p>0.05) Mirabegron 150mg BID: 22.25 (95% CI 6.48 to 38.00, p≤0.05) Tolterodine ER 4mg: 13.10 (95% CI −2.71 to 28.91, p>0.05) Incontinence episodes/24 h (compared to placebo) Mirabegron 100mg BID: −1.16 (95% CI −1.93 to −0.38, p≤0.05) Mirabegron 150mg BID: −0.57 (95% CI −1.34 to 0.2, p>0.05) Tolterodine ER 4mg: −0.61 (95% CI −1.34 to 0.12, p>0.05) Nocturia episodes/24 h (compared to placebo) Mirabegron 100mg BID: −0.39 (95% CI −0.65 to −0.12, p≤0.05) Mirabegron 150mg BID: −0.171 (95% CI −0.44 to 0.1, p>0.05) Tolterodine ER 4mg: −0.2 (95% CI −0.47 to 0.08, p>0.05) |
| DRAGON23 | Primary endpoint: change from baseline to treatment-end in the mean number of micturitions/24 h Secondary outcomes: changes in mean volume voided per micturition, mean number of urinary incontinence, urgency urinary incontinence, and urgency episodes/24 h, severity of urgency, number of nocturia episodes, changes in ICIQ-OAB and ICIQ-OABqol symptom scores, and in patients’ perception of treatment benefit |
Change in mean number of micturitions/24 h (compared to placebo) Mirabegron 25mg: −0.45 (95% CI −0.99 to 0.10, p>0.05) Mirabegron 50mg: −0.64 (95% CI −1.19 to −0.10, p≤0.05) Mirabegron 100mg: −0.68 (95% CI −1.22 to −0.13, p≤0.05) Mirabegron 200mg: −0.80 (95% CI −1.34 to −0.25, p≤0.01) Tolterodine ER 4mg: − 0.52 (95% CI −1.18 to 0.15, p>0.05) |
Mean volume voided/micturition (mL) (compared to placebo) Mirabegron 25mg: 8.03 (95% CI −1.54 to 17.60, p>0.05) Mirabegron 50mg: 20.05 (95% CI 10.48 to 29.63, p≤0.001) Mirabegron 100mg: 18.28 (95% CI 8.66 to 27.89, p≤0.001) Mirabegron 200mg: 26.06 (95% CI 16.49 to 36.62, p≤0.001) Tolterodine ER 4mg: 16.81 (95% CI 5.09 to 28.5, p≤0.05) Incontinence episodes/24 h (compared to placebo) Mirabegron 25mg: −0.84 (95% CI −1.45 to −0.23, p≤0.01) Mirabegron 50mg: −0.62 (95% CI −1.22 to −0.02, p≤0.05) Mirabegron 100mg: −0.53 (95% CI −1.12 to 0.06, p>0.05) Mirabegron 200mg: −0.58 (95% CI −1.16 to 0.01, p>0.05) Tolterodine ER 4mg: −0.28 (95% CI −1.01 to 0.45, p>0.05) Nocturia episodes/24 h (compared to placebo) Mirabegron 25mg: −0.15 (95% CI −0.36 to 0.07, p>0.05) Mirabegron 50mg: −0.22 (95% CI −0.44 to −0.01, p≤0.05) Mirabegron 100mg: −0.04 (95% CI −0.26 to 0.17, p>0.05) Mirabegron 200mg: −0.21 (95% CI −0.43 to 0.00, p>0.05) Tolterodine ER 4mg: −0.21 (95% CI −0.47 to 0.05, p>0.05) ICIQ-OAB (compared to placebo) Mirabegron 25mg: −0.58 (95% CI −1.13 to −0.02, p≤0.05) Mirabegron 50mg: −0.69 (95% CI −1.24 to −0.13, p≤0.05) Mirabegron 100mg: −0.90 (95% CI −1.45 to −0.34, p≤0.01) Mirabegron 200mg: −1.20 (95% CI −1.76 to −0.65, p≤0.001) Tolterodine ER 4mg: −0.37 (95% CI −1.05 to 0.31, p>0.05) ICIQ-OABqol (compared to placebo) Mirabegron 25mg: −0.98 (95% CI −5.88 to 3.92, p>0.05) Mirabegron 50mg: −4.25 (95% CI −9.13 to 0.62, p>0.05) Mirabegron 100mg: −4.46 (95% CI −9.37 to 0.46, p>0.05) Mirabegron 200mg: −6.08 (95% CI −11.0 to −1.19, p≤0.05) Tolterodine ER 4mg: −1.32 (95% CI −7.32 to 4.69, p>0.05) |
| ARIES24 | Co-primary endpoints: changes from baseline to final visit in the mean number of incontinence episodes and micturitions/24 h Secondary endpoints: change from baseline to final visit in mean volume voided per micturition and changes from baseline to week 4 in mean numbers of incontinence episodes/24 h and micturitions/24 h, effect of treatment on urgency symptoms (mean levels of urgency, number of urgency incontinence episodes/24 h, and number of grade 3/4 urgency episodes/24 h) and HRQoL measures- - OAB-q, TS-VAS and PPBC |
Change in incontinence episodes/24 h (from baseline) Mirabegron 50mg: −1.47 (95% CI, −1.69 to −1.25) Mirabegron 100mg: −1.63 (95% CI −1.86 to −1.4) Change in number of micturitions/24 h (from baseline) Mirabegron 50mg: −1.66 (95% CI −1.92 to −1.4) Mirabegron 100mg: −1.75 (95% CI −2.01 to −1.48) |
Change in volume voided/micturition from baseline at final visit Placebo: 7.0 ml (95% CI 2.3 to 11.7) Mirabegron 50mg: 18.2 ml (95% CI 13.4 to 22.9) Mirabegron 100mg: 18.0 ml (95% CI 13.1 to 22.8) Level of urgency from baseline to final visit Placebo: −0.08 (95% CI −0.13 to −0.03) Mirabegron 50mg: −0.19 (95% CI −0.24 to −0.13) Mirabegron 100mg: −0.21 (95% −0.26 to −0.15) Number of nocturia episodes/24 h at final visit Placebo: −0.38 (95% CI −0.51 to −0.26) Mirabegron 50mg: −0.57 (95% CI −0.70 to −0.44) Mirabegron 100mg: −0.57 (95% CI −0.70 to −0.45) Treatment-satisfaction (TS-VAS) Placebo: 0.70 (95% CI 0.4 to 1.0) Mirabegron 50mg: 1.55 (95% CI 1.2 to 1.9) Mirabegron 100mg: 2.09 (95% CI 1.8 to 2.4) Symptom bother (OAB-q) Placebo: −10.8 (95% CI −12.7 to −8.9) Mirabegron 50mg: −17.0 (95% CI −18.9 to −15.1) Mirabegron 100mg: −20.2 (95% CI −22.1 to −18.2) HRQoL total score (OAB-q) Placebo: 10.7 (95% CI .0 to 12.5) Mirabegron 50mg: 14.8 (95% CI 13.1 to 16.6) Mirabegron 100mg: 17.3 (95% CI 15.5 to 19.0) PPBC Placebo: −0.5 (95% CI −0.6 to −0.4) Mirabegron 50mg: −0.7 (95% CI −0.8 to −0.6) Mirabegron 100mg: −0.8 (95% CI −0.9 to −0.7) |
| SCORPIO25 | Co-primary end points: change from baseline to final visit in the mean number of incontinence episodes and micturitions/24 h Secondary outcomes: change from baseline to final visit in mean volume voided per micturition, and changes from baseline to week 4 in mean number of incontinence episodes and micturitions/24 h, assess patient perception of improvement in HRQoL—including OAB-q, the PPBC, and the TS-VAS |
Change in incontinence episodes/24 h (compared to placebo) Mirabegron 50mg: −0.41 (95% CI, − 0.72 to −0.099, p=0.003) Mirabegron 100mg: −0.29 (95% CI −0.61 to 0.03, p=0.01) (after multiplicity adjustments) Tolterodine ER 4mg: −0.1 (95% CI −0.42 to 0.21; p=0.11) Change in number of micturitions/24 h (compared to placebo) Mirabegron 50mg: −0.6 (95% CI −0.9 to −0.29, p<0.001) Mirabegron 100mg: −0.44 (95% CI −0.74 to −0.13, p=0.005) Tolterodine ER 4mg: − 0.25 (95% CI −0.55 to 0.06, p=0.11) |
Change in incontinence episodes/24 h (from baseline) Mirabegron 50mg: −1.57 (95% CI −1.79 to −1.35) Mirabegron 100mg: −1.46, (95% CI −1.68 to −1.23) Tolterodine ER 4mg: −1.27 (95% CI −1.49 to −1.05) Change in number of micturitions/24 h (from baseline) Mirabegron 50mg: −1.93 (95% CI −2.15 to −1.7) Mirabegron 100mg: −1.77 (95% CI −1.99 to −1.56) Tolterodine ER 4mg: −1.59 (95% CI −1.8 to −1.37) Responder analysis for reduction in incontinence episodes at final visit (compared to placebo) Mirabegron 50mg: 1.75 (95% CI 1.23 to 2.49, p=0.002) Mirabegron 100mg: 1.45 (95% CI 1.02 to 2.05, p=0.037) Tolterodine ER 4mg: 1.44 (95% CI 1.02 to 2.03, p=0.037) Change from baseline to final visit in TS-VAS (compared to placebo) Mirabegron 50mg: 0.66 (95% CI 0.25 to 1.07, p=0.001) Mirabegron 100mg: 0.77 (95% CI 0.36 to 1.17, p<0.001) Tolterodine ER 4mg: 0.55 (95% CI 0.14 to 0.95, p=0.008) Change from baseline to final visit in symptom bother scale as assessed by the OAB-q (compared to placebo) Mirabegron 50mg: −4.7 (95% CI −7.1 to −2.4, p<0.001) Mirabegron 100mg: −5.0 (95% CI −7.3 to −2.6, p<0.001) Tolterodine ER 4mg: −3.5 (95% CI −5.9 to −1.2, p<0.003) Change from baseline to final visit in PPBC (compared to placebo) Mirabegron 50mg: −0.2 (95% CI −0.3 to −0.0, p=0.045) Mirabegron 100mg: −0.2 (95% CI −0.4 to −0.1, p=0.001) Tolterodine ER 4mg: −0.2 (95% CI −0.3 to −0.0, p=0.023) |
| Herschorn et al26 | Co-primary endpoints: change from baseline to final visit in mean number of incontinence episodes and micturitions per 24 h Secondary endpoints: change from baseline to final visit in mean volume voided per micturition, changes from baseline to week 4 in mean number of incontinence episodes and micturitions per 24 h, changes from baseline to final visit in mean level of urgency, number of urgency incontinence episodes, and urgency episodes per 24 h, change from baseline to each study visit scores for OAB-q, PPBC, and TS-VAS |
Change in mean number of incontinence episodes per 24 h (compared to placebo) Mirabegron 25mg: −0.40 (95% CI −0.74 to −0.06, p=0.005) Mirabegron 50mg: −0.42 (95% CI −0.76 to −0.08, p=0.001) Change in mean number of micturitions per 24 h (compared to placebo) Mirabegron 25mg: −0.47 (95% CI −0.82 to −0.13, p=0.007) Mirabegron 50mg: −0.42 (95% CI −0.76 to −0.08, p=0.015) |
Change from baseline to final visit in mean volume voided per micturition (compared to placebo) Mirabegron 25mg: 4.6 (95% CI −1.6 to 10.8, p=0.15) Mirabegron 50mg: 12.4 (95% CI 6.3 to 18.6, p<0.001) Change from baseline to week 4 in mean number of incontinence episodes per 24 h (compared to placebo) Mirabegron 25mg: −0.34 (95% CI −0.68 to −0.01, p=0.039) Mirabegron 50mg: −0.51 (95% CI −0.85 to −0.17, p<0.0016) Change from baseline to week 4 in mean number of micturitions per 24 h (compared to placebo) Mirabegron 25mg: −0.18 (95% CI −0.53 to 0.16, p=0.3) Mirabegron 50mg: −0.37 (95% CI −0.71 to −0.03, p=0.035) Change from baseline to week 4 in mean number of urgency incontinence episodes per 24 h (compared to placebo) Mirabegron 25mg: −0.36 (95% CI −0.67 to −0.05, p=0.004) Mirabegron 50mg: −0.39 (95% CI −0.69 to −0.08, p=0.002) Change from baseline to final visit for OAB-q symptom bother (compared to placebo) Mirabegron 25mg: −1.8 (95% CI −4.3 to 0.7, p=0.15) Mirabegron 50mg: −2.8 (95% CI −5.3 to −0.3, p=0.028) Change from baseline to final visit for HRQoL total score (compared to placebo) Mirabegron 25mg:1.3 (95% CI −0.9 to 3.5, p=0.26) Mirabegron 50mg: 1.2 (95% CI −1.0 to 3.4, p=0.28) Change from baseline to final visit in PPBC score (compared to placebo) Mirabegron 25mg: −0.1 (95% CI −0.2 to 0.1, p=0.49) Mirabegron 50mg: −0.0 (95% CI −0.2 to 0.1, p=0.64) Change from baseline to final visit in TS−VAS (compared to placebo) Mirabegron 25mg: 0.49 (95% CI 0.07 to 0.91, p=0.024) Mirabegron 50mg: 0.83 (95% CI 0.41 to 1.25, p<0.001) |
Abbreviations:BID = twice daily; CI = confidence interval; ER = extended release; H = hours; HRQoL= health related quality of life; ICIQ-OAB = international consultation on incontinence questionnaire-overactive bladder; ICIQ-OABqol= international consultation on incontinence questionnaire- overactive bladder quality of life;mL = milliliter; OAB-q= overactive bladder questionnaire; PPBC= patient perception of bladder condition; TS-VAS= treatment satisfaction-visual analog scale
Only selected secondary outcomes included
Statistically significantly superior compared with placebo at the 0.05 level with multiplicity adjustments
There are two Phase II trials performed by Chapple, et al: the BLOSSOM trial and the DRAGON trial. The BLOSSOM trial showed a statistically significant reduction in the mean number of micturition per 24 hours when compared to placebo for mirabegron 100mg BID (−1.02, 95% Confidence Interval (CI), −1.67 to −0.36) and mirabegron 150mg BID (−1.03, 95% CI, −1.69 to −0.372); however, there was no statistically significant improvement with tolterodine ER 4mg (−0.4, 95% CI, −1.06 to 0.26).22
In the DRAGON trial there was a statistically significant reduction in the mean number of micturition episodes per 24 hours compared to placebo for mirabegron 50mg (−0.64, 95% CI, −1.19 to −0.1), mirabegron 100mg (−0.68, 95% CI, −1.22 to −0.13), and mirabegron 200mg (−0.8, 95% CI, −1.34 to −0.25); however there was not a significant reduction with mirabegron 25mg (−0.45, 95% CI, −0.99 to 0.1) or tolterodine ER 4mg (−0.52, 95% CI, −1.18 to 0.15).23
In the study by Nitti, et al there were significant improvements from baseline in incontinence episodes per 24 hours for mirabegron 50mg (−1.47, 95% CI, −1.69 to −1.255) and mirabegron 100mg (−1.63, 95% CI −1.86 to −1.4). Additionally, there were significant reductions in the number of micturition per 24 hours from baseline for mirabegron 50mg (−1.66, 95% CI −1.92 to −1.4) and mirabegron 100mg (−1.75, 95% CI −2.01 to −1.48).24
In the evaluation by Khullar, et al there were significant improvements from baseline in incontinence episodes per 24 hours for mirabegron 50mg (−0.41, 95% CI, −0.72 to −0.09) and mirabegron 100mg (−0.29, 95% CI −0.61 to 0.03; p=0.01 after multiplice adjustments); however, this was not seen in tolterodine ER 4mg (−0.1, 95% CI −0.42 to 0.21). Additionally, there were statistically significant reductions in the number of micturition per 24 hours for mirabegron 50mg (−0.6, 95% CI −0.9 to −0.29) and mirabegron 100mg (−0.44, 95% CI −0.74 to −0.13); however, there was no difference when tolterodine ER 4mg (−0.25, 95% CI −0.55 to 0.06) was compared to placebo.25
In a study by Herschorn, et al mirabegron 25mg (−0.40, 95% CI −0.74 to −0.06) and 50mg (−0.42, 95% CI −0.76 to −0.08) showed a statistically significant decrease in the mean number of incontinence episodes per 24 hours when compared to placebo. There was also a statistically significant decrease in the mean number of micturition per 24 hours for both mirabegron 25mg (−0.47, 95% CI −0.82 to −0.13) and mirabegron 50mg (−0.42, 95% CI −0.76 to −0.08) when compared to placebo.26
An additional study evaluating mirabegron was not included above as the primary outcome was safety. Chapple, et al performed a randomized, double-blind, active-controlled phase III trial (TAURUS trial).27 Patients ≥18 years of age with OAB symptoms for ≥3 months were enrolled in a 2 week single-blind, placebo run-in. Patients were randomized (n=2452) 1:1:1 to mirabegron 50mg (n=815), mirabegron 100mg (n=824), or tolterodine ER 4mg (n=813) once daily for 12 months. The primary variable was the incidence and severity of treatment emergent adverse events (TEAEs) starting from the first double-blind study drug intake until 30 days after the last study drug dose. Efficacy end points consisted of the change in key OAB symptoms recorded in the 3-day micturition diary from baseline to months 1, 3, 6, and 12. There was no statistically significant difference between mirabegron and tolterodine in the efficacy assessment.
A recent pooled analysis incorporating data from three of the previously discussed phase III studies evaluated the efficacy of mirabegron 50mg (n=1324) and 100mg (n=890) once daily.28 The co-primary outcomes included change from baseline to final visit in the mean number of incontinence episodes per 24 hours and mean number of micturition per 24 hours. This pooled analysis found a statistically significant decrease for both co-primary outcomes for both doses of mirabegron. There was a reduction in the mean number of incontinence episodes per 24 hours compared to placebo for mirabegron 50mg (−0.40, 95% CI −0.58 to −0.21) and mirabegron 100mg (−0.41, 95% CI −0.62 to −0.19) and in the change in the mean number of micturition per 24 hours compared to placebo for mirabegron 50mg (−0.55, 95% CI −0.75 to −0.36) and mirabegron 100mg (−0.54, 95% CI −0.77 to −0.31).
Secondary Efficacy Outcomes
The above five trials assessed mirabegron using primary or co-primary efficacy end points. However, other secondary end points were evaluated using objective data endpoints such as mean volume voided per micturition; mean number of urinary incontinence and urgency episodes per 24 hours; severity of urgency; and nocturia, along with subjective improvements in OAB symptoms and quality of life measurements using questionnaires (Table 2). These assessments include the OAB Questionnaire (OAB-q), the Patient Perception of Bladder Condition (PPBC), and the Treatment Satisfaction-Visual Analog Scale (TS-VAS). The OAB-q involves multiple questions assessing the patient’s symptom bother, coping, concern, sleep, and social aspects related to overactive bladder. The PPBC asks patients to rate their perceived bladder condition on a scale from 1 (indicating no problems) to 6 (indicating severe problems), and the TS-VAS is a measurement of current health status.
Limitations
While all of the trials demonstrated improvements in OAB symptoms and patient-reported outcomes, several limitations exist in these studies. Many of the trials included patients who had already participated in earlier phase studies of mirabegron, and approximately 80% of the participants in TAURUS had completed a recent mirabegron trial.27 While the previously discussed studies were all blinded, prior participant exposure to the study drug may confound the results.
Another limitation is that differing doses of mirabegron were used in each of the trials, which makes a consistent assessment of all studies difficult. However, all doses studied did show similar improvements in OAB symptoms and patient-reported outcomes. In addition, many of the data in these trials are patient-reported via micturition diaries and questionnaires which have not been standardized. This subjective data in the trials could be considered a confounder; however, this method of utilizing subjective data is consistent with previous studies assessing OAB medications.
Patients in these trials may not be representative of all patients affected by OAB. The majority of patients in each study were female with an age range of 55–61 years old, which does not encompass older males who suffer from OAB and may limit the external validity of these studies. For example, most of the study populations were comprised of >65% females, with >80% of the population younger than 75 years old.2–27 The durations of the trials were also short and may not allow for a full assessment of adverse effects and maintenance of efficacy throughout a prolonged period of time. The TAURUS trial was 12 months long and it concluded that the safety and efficacy profile of mirabegron identified in shorter trials was maintained throughout its trial period, with no new relevant safety concerns that could be attributed to the drug and no decrease in efficacy.27
An overall limitation of these trials is that they have not directly compared mirabegron to the current standard of therapy. While tolterodine ER was included in some studies, there was not a direct statistical comparison of non-inferiority or superiority made between mirabegron and tolterodine ER as these trials only demonstrated efficacy versus placebo or compared to baseline OAB symptoms.22–27
Adverse Effects
Prevalence of Adverse Effects
Overall, the trials discussed above demonstrated the tolerability of mirabegron and no life-threatening adverse events were noted in these particular studies. The main adverse drug reactions from mirabegron in these trials were hypertension, dry mouth, constipation, and headache.
TEAEs were consistent for mirabegron throughout the 5 studies and the TAURUS study. The BLOSSOM trial had similar rates in the mirabegron groups – 18.5%–24.6% compared to tolterodine ER 4mg – 26.6% (n=17).22 The DRAGON trial had lower TEAEs in the tolterodine cohort – 15.3% (n=13) compared to the mirabegron combination group – 20.1%–22.5%.23 The TEAEs were similar across the treatment groups in the SCORPIO trial for mirabegron 50mg/day, 100mg/day, and tolterodine ER 4mg/day at 42.8% (n=211), 40.1% (n=199), 46.7% (n=231), respectively.25 The ARIES trial did not report total TEAEs for each treatment group.24 Additionally, the TAURUS trial, an evaluation of 12 month use of medications, established that TEASs occurred at a similar rate between mirabegron 50mg, 100mg, and tolterodine ER 4mg, with total prevalence of adverse effects at 59.7% (n=485) for mirabegron 50mg, 61.3% (n=503) for mirabegron 100mg, and 62.6% (n=508) for tolterodine ER 4mg/day, respectively. Discontinuation rates were similar in active-treatment groups within each study.23,25,27
Increases in Heart Rate/Blood Pressure
There appears to be a dose dependent increase in heart rate and blood pressure with the use of mirabegron. There was no significant increase in heart rate in mirabegron 25mg/day in either the morning or afternoon, 0.34 bpm and 0.49 bpm, respectively (p>0.05),23 or in mirabegron 50mg/day in the morning or afternoon, 0.8–1.64 bpm and 0.7–1.12 bpm, respectively (p>0.05).23,25 In the BLOSSOM trial, subjects taking mirabegron 150mg BID had a 5bpm increase compared to no change in mirabegron 100mg BID and tolterodine ER 4mg/day.22 In trials of higher doses there were significant increases in heart rate. There was an increase of 1.6–2.5 bpm in the morning and 2.0–2.71 bpm in the afternoon in mirabegron 100mg/day (p<0.05).23,25 There was also a 4.63–4.66 bpm increase in the mirabegron 200mg/day group (p<0.001); however, there were no reported cardiac adverse effects.23 Changes in heart rate were not reported in the ARIES trial.24
When evaluating the impact on blood pressure, the TAURUS trial showed minimal changes from baseline for each group. The systolic and diastolic blood pressure (SBP/DBP) changes for mirabegron 50mg/day were +0.2/−0.3 mmHg, +0.4/+0.4 mmHg for mirabegron 100mg/day, and −0.5/+0.1 mmHg for tolterodine ER 4mg/day.27 This small increase in blood pressure would likely not be considered clinically significant by most practitioners. The incidence of blood pressure increases in the other trials described was similar between the groups (4.9% to 6.1%).24–25 Caution still should be taken as it was reported that 5–6% of patients taking mirabegron have experienced an increase in blood pressure of 15/10 mmHg or more.21 Overall, the approved doses of mirabegron 25mg/day and 50mg/day have yet to yield significant increases in heart rate or blood pressure; however, it would be suggested to monitor closely.
Anticholinergic Adverse Effects
One potential difference between mirabegron and antimuscarinics are anticholinergic side effects, including dry mouth and constipation. The SCORPIO trial and TAURUS trial did see an increased risk for dry mouth in the tolterodine group (8.6–10.1%) compared to mirabegron 50mg/day and 100mg/day (2.3 – 2.8%).25–27 However, rates of dry mouth were similar in the DRAGON and BLOSSOM trials between the tolterodine groups (3.5 – 4.7%) and the mirabegron groups (0 – 6.2%).23–23 Across these studies, there were similar rates of constipation in the tolterodine ER groups (1.2 – 2.7%) and the mirabegron groups (1.2 – 3.0%).22–23,25,27
Other Adverse Effects
Overall there were low incidences (<1%) of elevated post-void residual volumes in any of the treatment groups.22–23,25 Incidence of headache was similar in tolterodine ER groups and mirabegron groups (1.2 – 6.3% vs. 1.8 – 4.6%), respectively.22–24,25
Drug Interactions
Mirabegron is a minor substrate of CYP2D6, CYP3A4, and P-glycoprotein. It is a moderate inhibitor of CYP2D6 and a weak inhibitor of CYP3A4. While no dose adjustments are recommended during co-administration with the majority of CYP2D6 substrates, appropriate monitoring should occur since mirabegron can significantly increase the concentrations of these agents.21 A study with metoprolol and mirabegron showed that the combination of multiple doses of mirabegron 160mg/day and a single dose of metoprolol 100mg/day showed an increase in the area under the curve (AUC) of metoprolol by 3.29 fold and mean increase in half-life from 2.96 hours to 4.11 hours.29 Similar to metoprolol, there was a 3.41 fold increase in AUC of desipramine and mean increase in half-life from 19.5 hours to 35.8 hours after a 50mg single dose of desipramine was administered to patients having taken multiple doses of mirabegron 100mg/day.29 Monitoring should also be employed with CYP2D6 substrates with narrow therapeutic indices such as thioridazine, flecainide, and propafenone. A dose adjustment is recommended when initiating digoxin with mirabegron. The lowest digoxin dose should be used initially and serum digoxin concentrations should be utilized to titrate the digoxin to the therapeutic range. Although the combination of warfarin and mirabegron requires further investigation, multiples doses of warfarin with mirabegron 100mg have resulted in an increased AUC and Cmax of warfarin with a potential increase in INR.21 Overall, mirabegron does not appear to have any significant drug interactions in patients who are regular metabolizers of CYP2D6. However, diligence is necessary especially in elderly patients taking multiple medications that may be metabolized by enzymes involved with mirabegron.
Anticholinergic agents may increase the effects of mirabegron based on its side effect profile. Although no dose adjustment is recommended, mirabegron should be used with caution with antimuscarinic drugs such as solifenacin for the treatment of OAB due to the risk of urinary retention.30 In terms of food interactions, low-fat meals have been shown to decrease the AUC of mirabegron more than high-fat meals.21 One randomized crossover study by Lee and colleagues showed a decrease in mirabegron plasma exposure that was dependent on meal composition but not dose, resulting in a greater decrease in mirabegron exposure after a low-fat versus high-fat meal. When mirabegron 50 or 100mg was combined with a high-fat meal, the Cmax was decreased by 39–45% and the AUC was decreased by 17–18%. When these doses were combined with a low-fat meal, the Cmax was decreased by 64–75% and the AUC was decreased by 47–51%. In both groups, the 90% CI fell below the limits for bioequivalence of 80–125%, which indicates an effect of food on mirabegron. However, all other phase 3 mirabegron trials, in which mirabegron was administered irrespective of food intake, demonstrated similar efficacy and tolerability. Overall, safety and efficacy were not clinically affected by food and mirabegron may be administered without regard to food.31
Future Directions/Place in Therapy
Recent evidence suggests mirabegron as a viable alternative to antimuscarinic therapy. First, antimuscarinic therapy in older adults can contribute to anticholinergic side effects such as constipation, dry eyes, and confusion. Therefore, having a non-antimuscarinic alternative may be useful for treating OAB without contributing to these symptoms. Although most studies looked at non-elderly adults, a published abstract of pooled results from subjects 65 or older compared to subjects less than 65 years of age suggest similar efficacy between both groups.32 Safety was not assessed and future studies are needed to determine safety endpoints in the older adult population. Second, there are no published studies assessing combination antimuscarinic therapy with beta-3 agonists; however, there are active studies assessing this question.
Currently, the AUA Guidelines (2012) on OAB indicate that the use of a beta-3 agonist shows promise, but do not include specific recommendations for mirabegron in the treatment algorithms.6 It can be postulated that mirabegron may be recommended as second line treatment after antimuscarinic failure due to poor efficacy or ADRs. As it has been recently approved, consideration of cost and insurance coverage may prevent it from being routinely used as a first-line therapy for OAB.
Conclusion
To date, mirabegron has demonstrated data in both safety and efficacy for patients with OAB. In 2012, mirabegron was approved by the FDA at the dose of 25mg by mouth daily, which can be titrated to 50mg by mouth daily. The most common adverse effects when used for this indication include hypertension, constipation, and headache. This medication has shown improvement in symptoms based on reductions in frequency, urgency, and incontinence episodes, along with other subjective OAB tests. It is a new option for patients with OAB which avoids potential anticholinergic adverse effects. Although some literature suggests it may be safe and effective, future studies are needed to determine its utility for OAB in a variety of demographics.
Abbreviations
- ADRs
Adverse drug reactions
- AUA
American Urological Association
- AUC
Area under the curve
- BID
Twice daily
- cAMP
cyclic adenosine monophosphate
- CI
Confidence interval
- CYP
Cytochrome P
- DBP
Diastolic blood pressure
- ER
Extended release
- FDA
Food and Drug Administration
- IR
Immediate release
- M3
Muscarinic 3 receptor
- OAB
Overactive bladder
- OAB-Q
Overactive Bladder Questionnaire
- PPBC
Patient perception of bladder condition
- RCT
Randomized controlled trial
- SBP
Systolic blood pressure
- TEAs
Treatment emergent adverse events
- TS-VAS
Treatment Satisfaction-Visual Analog Scale
Footnotes
Disclosure: This manuscript was supported through the Washington University in St. Louis grant ICTS UL1 TR000448. The authors have no potential conflicts of interest.
Contributor Information
Rebecca Bragg, PGY2 Ambulatory Care Resident, St. Louis College of Pharmacy/Department of Health.
Danielle Hebel, PGY2 Ambulatory Care Resident, St. Louis College of Pharmacy/St. Mary’s Health Center.
Scott Martin Vouri, Assistant Professor of Pharmacy Practice, St. Louis College of Pharmacy.
Jamie M. Pitlick, Email: Jamie.pitlick@stlcop.edu, Assistant Professor of Pharmacy Practice, St. Louis College of Pharmacy, 4588 Parkview Place, St. Louis, MO 63110, Tel: 314-768-8339, Fax: 314-446-8500.
References
- 1.Milsom I, Stewart W, Thuroff JW. The prevalence of overactive bladder. Am J Manag Care. 2000;6(11 Suppl):S565–73. [PubMed] [Google Scholar]
- 2.Tubaro A. Defining overactive bladder: epidemiology and burden of disease. Urology. 2004;64(Suppl 6):2–6. doi: 10.1016/j.urology.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Gomelsky A. Urinary incontinence in the elderly female. Ann Longterm Care. 2009;17(10):41–5. [Google Scholar]
- 4.Milsom I, Abrams P, Cardozo L, et al. How widespread are the symptoms of overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–6. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 5.Nitti V, Taneja S. Overactive bladder: achieving a differential diagnosis from other lower urinary tract conditions. Int J ClinPrac. 2005;59(7):825–30. doi: 10.1111/j.1742-1241.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 6.American Urological Guidelines. [Accessed January 24, 2013];Diagnosis and treatment of overactive bladder (non-neurogenic) in adults. 2012 Available at http://www.auanet.org/content/media/OAB_guideline.pdf.
- 7.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association/International Continence Society joint report on the terminology for female pelvic floor dysfunction. IntUrogynecol J. 2010;21:5–26. doi: 10.1007/s00192-009-0976-9. [DOI] [PubMed] [Google Scholar]
- 8.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function. NeurourolUrodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 9.Lavelle JP, Karram M, Chu FM, et al. Management of incontinence for family practice physicians. Am J Med. 2006;119(3A):37S–40S. doi: 10.1016/j.amjmed.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency incontinence. Int J ClinPract. 2009;63(8):1177–91. doi: 10.1111/j.1742-1241.2009.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marinkovic SP, Rovner ES, Moldwin RM, et al. The management of overactive bladder syndrome. BMJ. 2012;344:38–44. doi: 10.1136/bmj.e2365. [DOI] [PubMed] [Google Scholar]
- 12.Chapple CR, Khullar V, Gabriel Z, et al. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008;54:543–62. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 13.Abrams P, Andersson KE. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007;100:987–1006. doi: 10.1111/j.1464-410X.2007.07205.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagg A. Treating overactive bladder in the elderly. Can Urol Assoc J. 2011;5(5Suppl2):S149–51. doi: 10.5489/cuaj.11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler CJ. Integrated control of lower urinary tract – clinical perspective. Brit J Pharmacol. 2006;147:S14–24. doi: 10.1038/sj.bjp.0706629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansfield KJ, Chandran JJ, Vaux KJ. Comparison of receptor binding characteristics of commonly used muscarinic antagonists in human bladder detrusor and mucosa. J Pharmacol Exp Ther. 2009;328(3):893–9. doi: 10.1124/jpet.108.145508. [DOI] [PubMed] [Google Scholar]
- 17.Igawa Y, Aizawa N, Homma Y. Beta3-adrenoceptor agonists: possible role in the treatment of overactive bladder. Korean J Urol. 2010;51:811–8. doi: 10.4111/kju.2010.51.12.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson KE, Hedlund P. Pharmacologic perspective on the physiology of the lower urinary tract. Urology. 2002;605(Suppl 1):13–21. doi: 10.1016/s0090-4295(02)01786-7. [DOI] [PubMed] [Google Scholar]
- 19.Andersson KE. Prospective pharmacologic therapies for the overactive bladder. Ther Adv Urol. 2009;1(2):71–83. doi: 10.1177/1756287209103937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouslander J. Management of overactive bladder. N Engl J Med. 2004;350(8):785–99. doi: 10.1056/NEJMra032662. [DOI] [PubMed] [Google Scholar]
- 21.Myrbetriq (mirabegron) [prescribing information] AstellasPharma US, Inc; Northbrook, IL: Jun, 2012. [Google Scholar]
- 22.Chapple CR, Amarenco G, Lopez MA, et al. A proof-of-concept study: mirabegron, a new therapy for overactive bladder (BLOSSOM trial) Neurourol Urodynam. 2013;32(8):1116–22. doi: 10.1002/nau.22373. [DOI] [PubMed] [Google Scholar]
- 23.Chapple CR, Dvorak V, Radziszewski P, et al. A phase II dose-ranging study of mirabegron in patients with overactive bladder (DRAGON trial) Int Urogynecol. 2013;24(9):1447–58. doi: 10.1007/s00192-013-2042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitti VW, Auerbach S, Martin N, et al. Results of a randomized phase III trial of mirabegron in patients with overactive bladder (ARIES trial) J Urol. 2013;189(4):1388–95. doi: 10.1016/j.juro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Khullar V, Amarenco G, Angulo JC, et al. Efficacy and tolerability of mirabegron, a β(3)-adrenoceptor agonist, in patients with overactive bladders: results from a randomized European-Australian phase 3 trial (SCORPIO trial) Eur Urol. 2013;63(2):283–95. doi: 10.1016/j.eururo.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Herschorn S, Barkin J, Castro-Diaz D, et al. A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicenter study to assess the efficacy and safety of the beta-3 adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology. 2013;82(2):313–20. doi: 10.1016/j.urology.2013.02.077. [DOI] [PubMed] [Google Scholar]
- 27.Chapple C, Kaplan S, Mitcheson H, et al. Randomized, double-blind, active-controlled phase III study to assess 12-month safety and efficacy of mirabegron, a β3-adrenoceptor agonist, in overactive bladder (TAURUS trial) EurUrol. 2013;63:296–305. doi: 10.1016/j.eururo.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Nitti VW, Khullar V, van Kerrebroeck P, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomized, double-blind, placebo-controlled, phase III studies. Int J Clin Pract. 2013;67(7):619–32. doi: 10.1111/ijcp.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krauwinkel W, Dickinson J, Schaddelee M, et al. The effect of mirabegron, a potent and selective beta3-adrenoceptor agonist, on the pharmacokinetics of CYP2D6 substrates desipramine and metoprolol. Eur J Drug Metab Pharmacokinet. 2014;39(1):43–52. doi: 10.1007/s13318-013-0133-1. [DOI] [PubMed] [Google Scholar]
- 30.Tyagi P, Tyagi V, Chancellor M. Mirabegron: a safety review. Expert Opin Drug Saf. 2011;10(2):287–94. doi: 10.1517/14740338.2011.542146. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Zhang W, Moy S, et al. Effects of food intake on the pharmacokinetic properties of mirabegron oral controlled absorption system: a single-dose, randomized, crossover study in healthy adults. ClinTher. 2013;35(3):333–41. doi: 10.1016/j.clinthera.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Khullar V, Cambronero J, Angulo J, et al. Age-related efficacy of the selective b3-adrenoceptor agonist mirabegron for the treatment of overactive bladder: pooled analysis of three prospective, randomized phase III studies in patients aged ≥ 65 years. International Continence Society. 2013:Abstract 331. Accessed at: http://www.ics.org/Abstracts/Publish/134/000331.pdf.