Abstract
A cellular membrane affinity chromatography (CMAC) column has been created using cellular membrane fragments from a 1321N1 cell line stably transfected with the P2Y1 receptor. The CMAC(1321N1P2Y1) column contained functional P2Y1 and histamine 1 receptors, which independently bound receptor-specific ligands. The data obtained with the CMAC(1321N1P2Y1) column demonstrate that multiple-G-protein coupled receptor (GPCR) columns can be developed and used to probe interactions with the immobilized receptors and that endogenously expressed GPCRs can be used to create CMAC columns. The results also establish that the histamine 1 receptor can be immobilized with retention of ligand-specific binding.
Keywords: Affinity chromatography, Drug discovery, Receptor screening, Anti-histamines, Membrane receptors
1. Introduction
Cellular membrane affinity chromatography (CMAC) is an experimental approach based upon the creation of chromatographic columns packed with liquid chromatographic stationary phases that contain immobilized cellular membrane fragments. It has been demonstrated that CMAC columns can be used to characterize the binding of small molecules to receptors, ion channels and transporters contained within the immobilized membranes including the determination of binding affinities (Kd), the number of active binding sites on the column (Bmax) and functional properties (EC50 and IC50 values). The preparation and use of the CMAC approach has been recently reviewed [1,2].
We have reported the use of the CMAC approach to study ligand binding to G-protein coupled receptors (GPCRs). GPCRs are the largest class of cell surface receptors involved in intracellular signaling pathways and are key targets in drug discovery programs [3]. We have previously described CMAC columns containing functional GPCRs (CMAC(GPCR)) columns developed using stably transfected cells lines expressing δ-opioid and κ-opioid receptors [4], the β2-adrenergic receptor [5] and the P2Y1 purinergic receptor [6]. It was demonstrated that the CMAC(GPCR) columns could be used for the online screening for GPCR agonists and competitive antagonists using frontal displacement chromatography techniques [4–6]. In addition, Massolini and co-workers have recently demonstrated that a CMAC column containing a P2Y-like receptor, GPR17, transiently expressed in 1321N1 cells can be employed with rapid frontal affinity chromatography to simultaneously screen mixtures of compounds for potential ligands to this newly identified purinergic receptor [7].
The CMAC column used for the initial studies of the P2Y1 receptor was prepared using membrane fragments obtained from a stably transfected 1321N1 astrocytoma cell line, the CMAC(1321N1P2Y1) column [6]. This column was only used to characterize ligand binding to the P2Y1. However, it is reasonable to assume that the immobilized 1321N1P2Y1 membranes contained other endogenously expressed GPCRs, which had also been functionally immobilized within the CMAC(1321N1P2Y1) column. This possibility was suggested by recent data that demonstrated that CMAC columns prepared from native, i.e. non-transfected, 1321N1 and A172 astrocytoma cell lines contained four functional and independently active ligand-gated ion channels [8]. In order to test this hypothesis, we have investigated whether a second GPCR, the histamine subtype 1 receptor (H1R), was also present in the CMAC(1321N1P2Y1) column, if the column could be used to identify H1R ligands and if the expression of the P2Y1 receptor in the 1321N1 cell line altered the characteristics of the H1R. The H1R was chosen for study based upon previous studies demonstrating that membranes obtained from the 1321N1 cell line contained functional H1Rs [9].
2. Experimental
2.1. Materials
Radio-labeled marker ligands [3H]-mepyramine, [3H]-2-MeSADP and [3H]-4-methylhistamne were obtained from GE Healthcare (Buckinghamshire, UK) and histamine, 4-methylhistamine, mepyramine and 2-MeSADP were obtained from Fisher Scientific/Tocris Bioscience (Ellisville, MO). HEPES, NaCl, 2-mercaptoethanol, Tris–HCl, benzamidine, MgCl2, CaCl2, MgSO4, aprotinin, leupeptin, tosyl-amido-2-phenylethyl-chloromethyl ketone (TPCK), phenyl methyl sulfonyl fluoride (PMSF), adenosine triphosphate (ATP), n-octyl-β-D-glucopyranoside, glycerol, ethylenediaminetetraacetic acid (EDTA) and Trizma were purchased from Sigma-Aldrich Co. (St. Louis, MO). The immobilized artificial membrane PC stationary phase (IAM-PC, 12 μm, 300 Å) was purchased from Regis Chemicals, Grove, IL).
2.2. Cell lines
The human 1321N1 astrocytoma cell line was obtained from European Collection of Cell Cultures (Sigma-Aldrich) and the 1321N1 astrocytoma cell line stably transfected with P2Y1 {1321N1P2Y1} was donated by K. Harden (Department of Medicine, University of North Carolina). The cells were seeded in T-150 culture flasks (Fisher Scientific) with Dulbecco’s modified Eagle’s medium (DMEM) containing 4 mM glutamine and 4.5 g/l glucose (Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum (Fetal Clone III, Hyclone, Logan, UT), penicillin (100 U/ml, Mediatech), streptomycin (100 μg/ml, Mediatech) and maintained at 37 °C in a humidified atmosphere containing 5% CO2. The cells were sub-cultured or harvested for experiments at ~90% confluence and the medium was replaced at 3–4 day intervals. Cell pellets were stored frozen at −80 °C until use.
2.3. Preparation of CMAC(1321N1P2Y1) and CMAC(1321N1) columns
The CMAC columns were prepared following previously described procedures [2,6]. In brief, about 107 cells from the 1321N1 or 1321N1P2Y1 cell line were homogenized in 10 ml of HEPES buffer [20 mM, pH 8.0] containing 500 mM NaCl, 5 mM 2-mercaptoethanol, 100 μM benzamidine, 10 μg/ml aprotin, 10μg/ml leupeptin, 50 μg/ml TCPK, 100 μg/ml PMSF and 100 μM ATP for 3 × 30 s at a setting of 11 on a model PT-2100 homogenizer (Kinematica, Luzern, Switzerland). The resulting homogenate was centrifuged at 700 × g at 4 °C for 5 min and the pellet discarded. The supernatant was further centrifuged at 27,000 rpm at 4 °C for 30 min. The supernatant was discarded and the pellet re-suspended in 10 ml of HEPES buffer [20 mM, pH 8.0] containing 2% (w/v) n-octyl-β-D-glucopyranoside, 500 mM NaCl, 5 mM 2-mercaptoethanol, 100 μM benzamidine, 10 μg/ml aprotin, 10 μg/ml leupeptin, 50 μg/ml TCPK, 100 μg/ml PMSF, 100 μM ATP and 10% glycerol. The mixture was rotated in an orbit shaker at 150 rpm for 18 h at 4 °C and then centrifuged at 27,000 rpm at 4 °C for 25 min. The resultant supernatant was mixed with 200 mg of IAM-PC and rotated in an orbit shaker at 150 rpm at room temperature for 1 h and then dialyzed against HEPES buffer [20 mM, pH 8.0] containing 500 mM NaCl and 1 mM EDTA buffer for 24 h at 4 °C. The mixture was then centrifuged at 700 × g at 4 °C for 3 min, the resulting pellet was washed with Tris–HCl [10 mM, pH 7.5] containing 1 mM MgCl2, centrifuged at 400 rpm at 4 °C for 3 min and the stationary phase was packed into a Tricorn 5/20 column (GE Health-care, UK) yielding a 150 mm × 5 mm (i.d.) chromatographic bed.
2.4. Chromatographic studies
The CMAC(1321N1P2Y1) and CMAC(1321N1) columns were placed in a frontal affinity chromatography system and competitive displacement studies were carried out using previously described techniques, cf. [2,6]. Briefly the system consisted of a manual FPLC injection valve, 50-ml superloop both obtained from (Amersham Biotechnology), CMAC column, LC-10AD HPLC pump (Shimadzu Inc.) and an online radioactive/scintillation flow detector (IN/US, Tampa, FL). Solutions of the marker and test ligands were prepared in the running buffer, Tris–HCl [10 mM, pH 7.5] containing 1 mM MgCl2, and 10 ml samples were placed in the superloop, pumped across the CMAC column at a flow rate of 0.2 ml/min and monitored through a 250 μl flow cell with the radioflow detector. The scintillation flow rate was 0.8 ml/min. The breakthrough volume of the marker was calculated using the retention times at the midpoint of the chromatographic curves and the effect on the breakthrough volumes produced by increasing displacer concentrations was used to calculate the dissociation constant (Kd) and number of binding sites (Bmax) of the displacer as previously described, c.f. [2]. The marker concentrations were 50 pM for [3H]-mepyramine and 1 nM for [3H]-2-MeSADP and 100 pM [3H]-4-Methyl Histamine. The displacer concentrations for unlabeled mepyramine were 100 nM, 5 nM, 2 nM, and 0.5 nM while for unlabeled 2-MeSADP were 200 nM, 150 nM, 100 nM and 50 nM, and for unlabeled 4-Methyl histamine were 500 pM, 1 nM, 50 nM and for Histamine were 0.5 μM, 1 μM, 5 μM, 50 μM and 100 μM.
2.5. Membrane binding studies
Equilibrium binding of [3H]-mepyramine and [3H]-4-methylhistamne and Histamine to 1321N1 cell membranes was determined using previously described filtration binding assay with slight modifications [9]. Briefly, GF/C filters were pre-soaked for 30 min in a 0.5% solution of Polyethyleneimine (PEI). Filters were placed on a Millipore filtration unit (XX2702550, Millipore Corporation, Bedford, MA) and a gentle (15 mmHg) vacuum was applied to remove excess moisture. 1321N1 cell membranes were suspended in HEPES buffer [10 mM, pH 7.4] containing 5 mM MgCl2 (final concentration 4 mg protein/ml, final volume 400 μl) and incubated for 30 min at room temperature with increasing concentrations of [3H]-mepyramine (final concentrations 0.5 nM, 2 nM, 5 nM, 10 nM, 20 nM and 40 nM) or [3H]-4-methylhistamne (final concentrations 5 nM, 10 nM, 25 nM, 50 nM, 75 nM, and 150 nM) or 10 nM [3H]-Mepyramine with varying concentrations of Histamine (1 nM, 50 nM, 100 nM, 250 nM, 500 nM, 1 μM, 5 μM, 10 μM) pre-incubated for 10 min. A 400 μl aliquot of the solution was distributed over the surface of the filter, a vacuum was reapplied to allow the solution to permeate the filter and the filters were washed with 2 × 3 ml Tris–HCl [10 mM, pH 7.5] containing 5 mM MgCl2. The radioactivity retained by the filters was counted in a liquid scintillation counter. Non-specific binding was determined in the presence of 2 μM tripolidine hydrochloride for Mepyramine and 2 μM 4-Methyl Histamine for 4-Methyl Histamine and 1 mM Histamine for Histamine. Curve fitting and parameter estimation were performed using Graph pad Prism v4.0 software (San Diego, CA). All assays were carried out in triplicate.
3. Results and discussion
The presence of functional P2Y1 receptors on the CMAC(1321N1P2Y1) column used in this study was established by passing a 1 nM concentration of the characterized P2Y1 agonist [3H]-2-MeSADP through the column. As has been previously reported, the experiment produced the expected frontal chromatographic curve that contained an initial relatively flat section, representing binding to specific and non-specific sites on the membrane fragment, followed by vertical breakthrough, representing the process of saturating the receptor, and a plateau region, representing saturation of the receptor [2,6]. The columns were stable for 6–8 weeks. The addition of increasing concentrations of 2-MeSADP, from 50 nM to 200 nM, produced corresponding reductions in the breakthrough volumes which were used to calculate the observed affinity, Kd value, for 2-MeSADP. The calculated value was 88.5 nM, Table 1, which was consistent with the previously reported Kd of 50 nM [11] and 186.4 nM [6] calculated using standard membrane binding and CMAC approaches, respectively.
Table 1.
The binding affinities (Kd values) for 2-MeSADP, mepyramine and histamine determined on the CMAC(1321N1P2Y1 ) and CMAC(1321N1) columns using frontal affinity chromatography and filtration assays.
| Ligand/Receptor | CMAC(1321N1P2Y1 ) Kd (nM) | CMAC(1321N1) Kd (nM) | Filtration assay Ki (nM) |
|---|---|---|---|
| 2-MeSADP (P2Y1 ) | 88.5 | No binding | 50 [6] |
| Mepyramine (H1 R) | 2.9 | 7.7 | 16.1 |
| Histamine (H1 R) | 7400 | 5500 | 1300 |
The expression of H1R in the 1321N1P2Y1 and 1321N1 cell lines was confirmed by Western blot and confocal microscopy studies (data not shown). The presence of functional H1Rs on the CMAC(1321N1P2Y1) column was established by passing a 100 pM concentration of the characterized H1R antagonist [3H]-mepyramine through the column. The expected frontal chromatographic curve was observed, Fig. 1, and the addition of increasing concentrations of mepyramine, from 0.5 nM to 20 nM, produced corresponding reductions in the breakthrough volumes, Fig. 1. The calculated Kd value of mepyramine was 2.9 nM, Table 1, which was consistent with the Kd value of 16.1 nM obtained by filtration assay using tripolidine to determine non-specific binding [9]. When histamine was used as the displacer, the calculated affinity of histamine for the immobilized H1R was 7.4 μM which was consistent with the Ki value obtained of 1.30 μM determined from filtration assays using 1 mM Histamine for non-specific binding [9], Table 1. The results indicate that the CMAC(1321N1P2Y1) column contained functional H1R, which could be used to determine the binding affinities of H1R agonists (histamine) and antagonists (mepyramine).
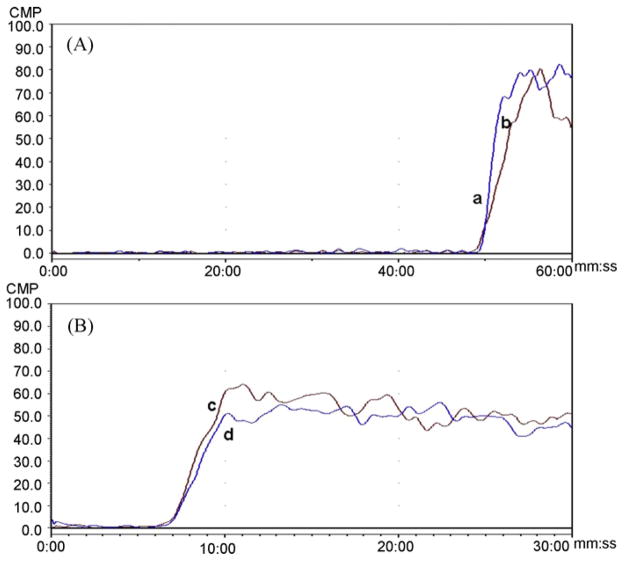
Fig. 1.
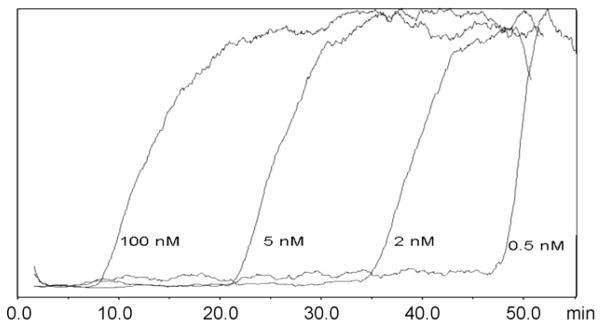
The chromatographic traces produced by [3H]-mepyramine [50 pM] on the CMAC(1321N1P2Y1) column after the addition of mepyramine to the running buffer in 0.5 nM, 2 nM, 5 nM and 100 nM concentrations.
To determine if the observed binding interactions with the P2Y1 and H1R represented independent functioning of the two GPCRs, the ability of the P2Y1 marker 2-MeSADP to displace the H1R marker mepyramine and the ability of mepyramine to displace 2-MeSADP was investigated using the CMAC(1321N1P2Y1) column. When 50 pM [3H]-mepyramine was used as the marker ligand, the addition of 10 μM 2-MeSADP to the mobile phase had no effect on the breakthrough volume of mepyramine, Fig. 2A. The same effect was observed when the marker ligand was 1 nM [3H]-2-MeSADP and 1 μM mepyramine was added to the mobile phase, Fig. 2B. The results indicate that on the CMAC(1321N1P2Y1) column, the immobilized P2Y1 and H1R receptors bind receptor specific ligands independently of each other.
Fig. 2.
The chromatographic traces observed on the CMAC(1321N1P2Y1 ) column in which: A. The frontal curves produced by [3H]-mepyramine [1 nM] alone, curve a, and after the addition of 2-MeSADP [10 μM] to the running buffer, curve b; B. The frontal curves produced by [3H]-2-MeSADP [1 nM] alone, curve c, and after the addition of mepyramine [1 μM] to the running buffer, curve d.
In order to determine if the expression of H1R was affected by the transfection of the cDNA encoding the P2Y1 receptor, a CMAC(1321N1) column was created using membrane fragments obtained from native 1321N1 cells and the same solubilization and immobilization buffers used to create the CMAC(1321N1P2Y1) column. When [3H]-2-MeSADP was added to the mobile phase, no specific retention was observed indicating that the P2Y1 receptor was not present in the non-transfected 1321N1 cell line nor functioning on the column, which is consistent with previous data [6,10]. The addition of [3H]-mepyramine to the mobile phase produced the expected frontal chromatographic curve and competitive displacement studies were used to calculate the Kd values for the binding of mepyramine and histamine to the H1R, 7.7 nM and 5.5 μM, respectively, Table 1. The histamine subtype selectivity of the CMAC(1321N1) column was established by chromatography of the H4-histamine receptor-selective agonist 4-methylhistamine [11]. No specific retention was observed indicating that the column could distinguish between H1R and H4R ligands (data not shown). This result was confirmed by cellular membrane binding studies which demonstrated 4-mehtylhistamine did not specifically bind to 1321N1 cellular membranes (data not shown).
The data from the frontal affinity chromatography studies with mepyramine were used to determine the number (Bmax) of active H1R receptors on the CMAC(1321N1P2Y1) and CMAC(1321N1) columns, and the calculated Bmax values were 4 and 15 pmol, respectively. The results from the chromatographic studies demonstrate that there were no significant differences between the Kd and Bmax values determined using the CMAC(1321N1) and CMAC(1321N1P2Y1) columns and indicate that the expression and activity of the H1R endogenously expressed in the 1321N1 cell line was not affected by the presence of the transfected P2Y1 receptor. The same results were obtained when the CMAC(1321N1) column was prepared using solubilization and immobilization buffers that did not contain 100 μM ATP (data not shown), indicating that the presence of ATP had no effect on the immobilization and activity of the H1R.
4. Conclusions
The results from this study demonstrate that for the first time the co-immobilization of two GPCRs on the CMAC(1321N1P2Y1) column has been carried out. And both the immobilized P2Y1 and H1 receptors retained their ability to independently bind receptor-selective ligands. The data establish that multiple-GPCR columns can be created as online screens with the ability to probe multiple targets in a single chromatographic assay. The results also establish that functional CMAC columns can be created using endogenously expressed GPCRs and is the initial report of the functional immobilization of the H1R within a chromatographic system. The ability to create CMAC columns from endogenously expressed GPCRs also presents the possibility that the CMAC approach can be used to study the expression, function and interrelationship of multiple GPCRs in target cell lines.
Acknowledgments
This work was supported by funds from the Intramural Research Program of the National Institute on Aging/NIH.
References
- 1.Moaddel R, Wainer IW. Development of immobilized membrane-based affinity columns for use in the online characterization of membrane bound proteins and for targeted affinity isolations. Anal Chim Acta. 2006;546:97–105. doi: 10.1016/j.aca.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Moaddel R, Wainer IW. The preparation and development of cellular membrane affinity chromatography columns. Nat Protoc. 2009;4:197–205. doi: 10.1038/nprot.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–375. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 4.Beigi F, Wainer IW. Syntheses of immobilized G protein-coupled receptor chromatographic stationary phases: characterization of immobilized μ and κ opioid receptors. Anal Chem. 2003;75:4480–4485. doi: 10.1021/ac034385q. [DOI] [PubMed] [Google Scholar]
- 5.Beigi F, Chakir K, Xiao R, Wainer IW. G-Protein-coupled receptor chromatographic stationary phases. 2. Ligand-induced conformational mobility in an immobilized β2-adrenergic receptor. Anal Chem. 2004;76:7187–7193. doi: 10.1021/ac048910c. [DOI] [PubMed] [Google Scholar]
- 6.Moaddel R, Calleri E, Massolini G, Frazier CR, Wainer IW. The synthesis and initial characterization of an immobilized purinergic receptor (P2Y1 ) liquid chromatography stationary phase. Anal Biochem. 2007;364:216–218. doi: 10.1016/j.ab.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temporini C, Ceruti S, Calleri E, Ferrario S, Moaddel R, Abbracchio MP, Massolini G. Development of an immobilized GRP17 receptor stationary phase for binding determination using frontal affinity chromatography coupled to mass spectrometry. Anal Biochem. 2009;384:123–129. doi: 10.1016/j.ab.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Katabatake T, Moaddel R, Cole R, Gandhari M, Frazier C, Hartenstein J, Rosenberg A, Bernier M, Wainer IW. Characterization of a multiple ligand-gated ion channel cellular membrane affinity chromatography column and identification of endogenously expressed receptors in astrocytoma cell lines. Anal Chem. 2008;80:8673–8680. doi: 10.1021/ac8016407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakahata N, Martin MW, Hughes AR, Hepler JR, Harden TK. H1-Histamine receptors on human astrocytoma cells. Mol Pharmacol. 1986;29:188–195. [PubMed] [Google Scholar]
- 10.Schachter JL, Li Q, Boyer L, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of human P2Y1 receptor. Br J Phamacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Venable JD, Thurmond RL. The histamine H4 receptor in autoimmune disease. Expert Opin Investig Drugs. 2006;15:1443–1452. doi: 10.1517/13543784.15.11.1443. [DOI] [PubMed] [Google Scholar]