Abstract
Since 2004, the Pan American Health Organization (PAHO) has carried out rotavirus surveillance in Latin America and the Caribbean. Here we report the characterization of human rotavirus with the novel G-P combination of G4P[14], detected through PAHO surveillance in Barbados. Full genome sequencing of strain RVA/Human-wt/BRB/CDC1133/2012/G4P[14] revealed that its genotype is G4-P[14]-I1-R1-C1-M1-A8-N1-T1-E1-H1. The possession of a Genogroup 1 (Wa-like) backbone distinguishes this strain from other P[14] rotavirus strains. Phylogenetic analyses suggested that this strain was likely generated by genetic reassortment between human, porcine and possibly other animal rotavirus strains and identified 7 lineages within the P[14] genotype. The results of this study reinforce the potential role of interspecies transmission in generating human rotavirus diversity through reassortment. Continued surveillance is important to determine if rotavirus vaccines will protect against strains that express the P[14] rotavirus genotype.
Keywords: rotavirus, reassortment, P[14], human, porcine
Group A rotaviruses (RVA) are the most frequently detected viral agents associated with acute gastroenteritis in infants and young children worldwide (Parashar et al., 2006). Globally, an estimated 453,000 children die of RVA every year (Tate et al., 2012) and most deaths occur in low- to middle-income countries in sub-Saharan Africa, South- and SouthEast Asia, and parts of the Americas (Parashar et al., 2009). In 2004, the Pan American Health Organization (PAHO), initiated regional RVA surveillance in Latin America and the Caribbean to obtain useful comprehensive disease-burden data for policy makers to help implement the RVA vaccine program (de Oliveira et al., 2009). The Caribbean Epidemiology Center (CAREC) and its successor, the Caribbean Public Health Agency (CARPHA), provide laboratory reference and epidemiology services to member countries of the Caribbean community [(Hamilton and Diggory, 1979); http://carpha.org/]. As part of the surveillance program, strain monitoring is being conducted at the U.S. Centers of Disease Control and Prevention (CDC) to determine if changes occurred in strain prevalence after introduction of the vaccine.
RVA are members of the Reoviridae family. The RVA genome consists of 11 double-stranded RNA gene segments that encode six structural (VP1-4 and VP6-7) and five or six non-structural (NSP1-5/6) proteins (Estes, 2007). The traditional binomial RVA classification system was based on the two outer capsid proteins, VP7 and VP4 and at least 27 G-types and 35 P-types have been identified (Matthijnssens et al., 2011). Extending the classical binomial genotyping system using the VP4 and VP7 segments, a new RVA classification system was proposed in which all 11 segments are considered (Matthijnssens et al., 2011). Following this new classification system, the notations of Gx-P[x]-Ix-Rx-Cx-Mx-Ax-Nx-Tx-Ex-Hx are used for the VP7-VP4-VP6-VP1-VP2-VP3-NSP1-NSP2-NSP3-NSP4-NSP5/6 encoding genes, respectively.
In humans, there are at least five common G types (G1-G4 and G9), and three common P types (P[4], P[6] and P[8]) circulating worldwide (Banyai et al., 2012; Gentsch et al., 2005; Patel et al., 2011; Santos and Hoshino, 2005) Other previously uncommon G and P types (e.g., G5, G8, G12, P[14], P[19]) have been more frequently associated with human disease in the last decade (Chitambar et al., 2011; Cilla et al., 2012; Cunliffe et al., 2001; Esona et al., 2009a; Esona et al., 2009b; Matthijnssens et al., 2009a; Saikruang et al., 2013). These reports highlighted reassortment based interspecies and zoonotic transmission of RVAs (Gentsch et al., 2005; Martella et al., 2010). Genetic reassortment is common due to the segmented nature of RVA. Following mixed infections, chimeric progeny viruses with novel constellation of segments and unusual phenotypes can be formed (Estes, 2007).
Here we report the genetic characterization of a G4P[14] reassortant RVA strain, representing a novel VP7-VP4 genotype combination, detected through the PAHO RVA Surveillance program in Barbados.
In 2012, a 6-year-old boy presented to a physician in St. Peter Parish, Barbados with vomiting, diarrhea, and a dry cough. A stool sample was collected when the patient returned to the doctor’s office 3 days later with abdominal pain. The sample was forwarded to the CDC for genotyping along with 20 other surveillance samples from Barbados that year. RNA was extracted from the sample using the MagMax 96 Viral RNA Isolation kit (Applied Biosystems, Inc., Foster City, CA) on KingFisher Flex Magnetic Particle Processor (Thermo Fisher Scientific, Pittsburgh, PA). The extracted dsRNA was denatured at 97°C for 4 min, RT-PCR and DNA cycle sequencing were carried out as previously described (Esona et al., 2009b). Previously published primers were used for the amplification of VP2, VP3, VP6, VP7, NSP2, NSP3, NSP4, and NSP5 gene segments (Das et al., 1994; Gouvea et al., 1990; Iturriza-Gomara et al., 2001; Matthijnssens et al., 2006; Mijatovic-Rustempasic et al., 2011; Tsugawa and Hoshino, 2008). New primers were also designed for amplification of VP1, VP4, and NSP1 genes based on initial sequencing results, some with M13 sequencing tails (Table 1).
Table 1.
Newly designed primers used in the study.
| Gene | Primer | Sequence (5′-3′) | Nt position, strand |
|---|---|---|---|
| VP1 | 2012821133-M13-VP1F | TGT AAA ACG ACG GCC AGT GGC TAT TAA AGC TAT ACA TGT AAA ACG ACG GCC AGT GGT CAC ATC | 1–18,+ |
| 2012821133-M13-VPR | TAA GCG | 3288–3302,− | |
| 2012821133-VP1intF1 | GGA GTT CCA AGA CAT AGC GAA A | 559–580,+ | |
| 2012821133-VP1intF2 | GCG TGG ACA GAG TAC CCA AT | 2118–2137,+ | |
| 2012821133-VP1intR1 | ACT CAC CGT TTG AGG CTA AT | 1212–1231,− | |
| 2012821133-VP1intR2 | GCG CGT ACG AAT TCA ATT TT | 2529–2548,− | |
| VP4 | 2012821133-M13-VP4F | TGT AAA ACG ACG GCC AGT GGC TAT AAA | 1–9,+ |
| 2012821133-M13-VP4R | TGT AAA ACG ACG GCC AGT GGT CAC ATC TTG AAA CAG | 2345–2362,− | |
| 2012821133-VP4intF1 | CAC ACA CGA GCA CAA ATG AA | 742–761,+ | |
| 2012821133-VP4intF2 | TTG CTA AAC TTG TAA CGA ATT CTC | 2132–2155,+ | |
| 2012821133-VP4intR1 | TCT TGC CTC ACC GTT ACA GA | 1471–1488,− | |
| NSP1 | 2012821133-M13-NSP1F | TGT AAA ACG ACG GCC AGT GGC TTT TTT TTA TGA AAA | 1–18,+ |
| 2012821133-M13-NSP1R | TGT AAA ACG ACG GCC AGT GGT CAC ATT TTA TGC TGC CT | 1549–1568,− | |
| 2012821133-NSP1intF | CTA TGC TGG GCA AAT GGA AT | 784–803,+ | |
| 2012821133-NSP1intR | ATT CCA TTT GCC CAG CAT AG | 784–803,− |
Sequences were aligned using the MUSCLE program within MEGA version 5 (Tamura et al., 2011). Once aligned, the JModelTest 2 program (Posada, 2008) was used to identify the optimal evolutionary model that best fitted the sequence datasets. Using corrected Akaike Information Criterion (AICc) the following models; GTR+I+G (NSP1 and VP7), TRM+G (NSP2), TIM3+G (NSP3), GTR+G (VP1 and VP4), HKY+G (NSP4), TIM1+G (NSP5), TIMI+I+G (VP2), GTR+I (VP3), and GTR+I (VP6) were found to best fit the sequence data for the different genes. Using these models, maximum likelihood trees were constructed using PhyML 3.0 along with aLRT statistics for branch support (Guindon et al., 2010). The evolutionary rate for VP4 gene was calculated as described previously (Matthijnssens et al., 2010) using BEAST (Drummond and Rambaut, 2007). We used the GTR evolutionary model (based on jModeltest) along with a log normal relaxed clock. The MCMC chains length was set at 10,000,000 with sampling every 10,000 runs. The location was compiled from GenBank and added as a discrete trait. GenBank accession numbers (NSP1-5, VP1-4, VP6 and VP7) for each individual genomic segment are: KF035102-12, respectively.
The complete open reading frames for all eleven genes were sequenced and revealed that this strain, designated RVA/Human-wt/BRB/CDC1133/2012/G4P[14], possesses a novel G and P combination of G4P[14] and a full genome constellation of G4-P[14]-I1-R1-C1-M1-A8-N1-T1-E1-H1. This is the first P[14] strain shown to possess a Genogroup 1 (Wa-like) genomic backbone. This was the only G4P[14] strain detected in Barbados in 2012. Of 21 samples genotyped that season, G3P[8] was the predominant genotype, comprising 90.5% of all strains, and there was a single G1P[8] detection.
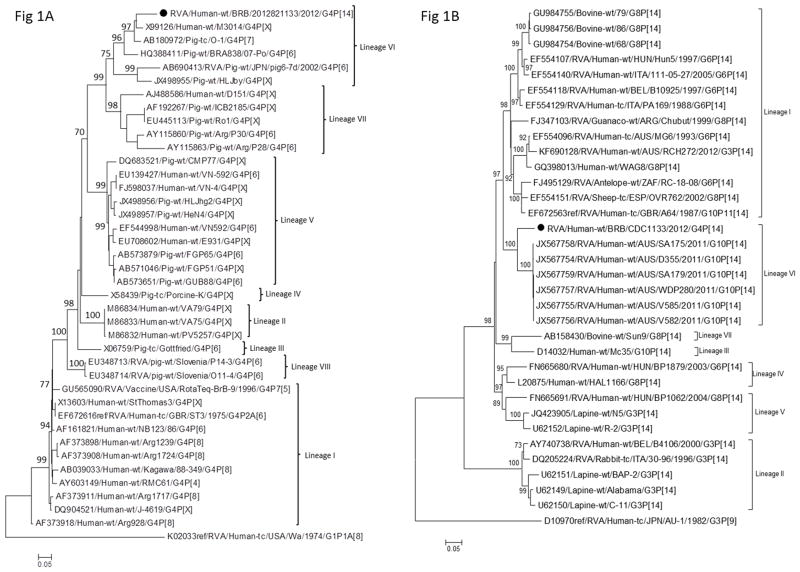
For VP7, we compared the sequence of our G4P[14] strain to those of published human and porcine RVAs with known G4 specificities and found that the Barbados strain VP7 gene clusters with genes of other human and porcine G4 strains from lineage VI (McDonald et al., 2011) (Figure 1A). The strain RVA/Human-wt/BRB/CDC1133/2012/G4P[14] VP7 gene exhibits 92% homology to human strain M3014 and porcine strain O-1, which has previously been shown to have a combination of antigenic regions showing significant homology to more than one serotype (G9 and G4) (Hoshino et al., 2005; Palombo et al., 1997). G4 porcine RVAs are believed to share common origin with human Wa-like strains (Matthijnssens et al., 2008a).
Figure 1.
Phylogenetic trees based on nucleotide sequences of complete open reading frames of (a) VP7 and (b) VP4 rotavirus genes. The maximum likelihood trees were constructed using PhyML 3.0 with best model identified by JModelTest 2 program along with aLRT statistics for branch support shown at the node (<70% not shown). The GenBank accession numbers, strain names, and G and P-type associated are shown where available.
For the P[14] VP4 gene, we performed phylogenetic analysis of 33 P[14] strains available in GenBank (Figure 1B). These P[14] strains exhibit notable genetic variation with an overall mean diversity of 86.4% (range 79.3–100% identity). The VP4 gene of strain RVA/Human-wt/BRB/CDC1133/2012/G4P[14] shared the highest degree of nucleotide sequence identity with P[14] strains from an human outbreak in Northern Territory, Australia, in 2011 (Cowley et al., 2013) with 91.2% identity and 88.2% or less with other P[14] strains. Within P[14] strain genes, we identified 7 lineages with strong bootstrap support and a minimum of 10% genetic distance between lineages (Figure 1B). Previous studies suggested that human P[14] strains have common origins with those of the even-toed ungulates belonging to the mammalian order Artiodactyla (Matthijnssens et al., 2009b). All lineages contain strains that were detected in humans. Lineage II and V exhibit the greatest genetic diversity of the lineages and contain G3 strains from rabbits. Lineages I, III, IV and VII contain G6, G8 and G10 strains isolated from bovines, guanacos, sheep, and other ungulates. Strain RVA/Human-wt/BRB/CDC1133/2012/G4P[14] VP4 gene occupies lineage VI shared with the aforementioned P[14] strains from Australia (Figure 1B) (Cowley et al., 2013). Unlike the Barbados strain, the Australian P[14] strains possess a G10-P[14]-I2-R2-C2-M2-A11-N2-T6-E2-H3 Genogroup 2 (DS-1 like) genome constellation which is consistent with G6P[14] and G8P[14] strains identified globally (Matthijnssens et al., 2009b). In recent years, more P[14] strains have been associated with gastroenteritis in humans and most of the reported P[14] strains have the Genogroup 2 gene constellation, with one or more gene segments that are of bovine origin (Banyai et al., 2009; Banyai et al., 2010; Cowley et al., 2013; Donato et al., 2014; El Sherif et al., 2011; Ghosh et al., 2007; Matthijnssens et al., 2009b; Medici et al., 2008; Mullick et al., 2012). The VP8* region of the P[14] VP4 protein has been shown to interact with the type A histo blood group antigens of humans (Hu et al., 2012; Liu et al., 2012) as well as bovine and porcine mucins (Liu et al., 2012) and this is thought to play a role in cross-species transmission of P[14] RVAs.
Through BEAST analysis of 33 P[14] sequences, we estimated that the evolutionary rate for P[14] genes to be 2.04 × 10−3 nucleotide substitutions/site/year (confidence interval, 4.98 × 10−3 to 2.52 × 10−4) which was comparatively higher than that reported previously for the RVA VP4 gene (0.58 × 10−3 nucleotide substitutions/site/year) (Jenkins et al., 2002). At the calculated rate of evolution, the P[14] gene of strain RVA/Human-wt/BRB/CDC1133/2012/G4P[14] is estimated to diverged from other lineage VI P[14] strains approximately 36 years previously (1976) and the estimated lineage VI-lineage I split occurred around the year 1911. Phylogeographic analysis of the P[14] strains suggests Italy as the root of P[14] evolution whereas our strain (G4P[14] has recently evolved from an Australian lineage (data not shown).
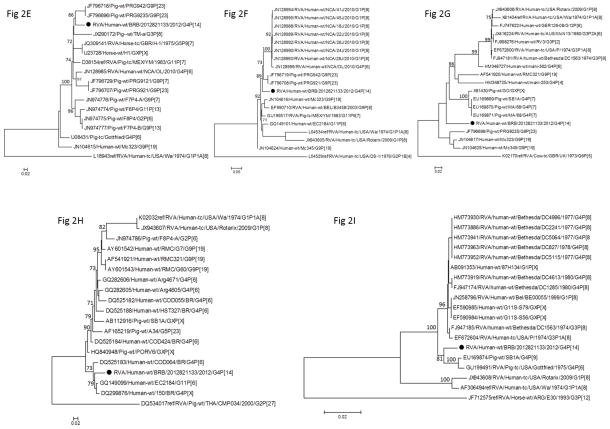
The VP1-3, VP6-7, and NSP1-4 genes of strain RVA/Human-wt/BRB/CDC1133/2012/G4P[14] share 90–95% nucleotide identity with cognate sequences in GenBank and the NSP5 gene shares 98.7% homology with human strain BP271 (GenBank accession KF835969). For all genes except VP4, the Barbados strain genome follows the gene constellation of the porcine Gottfried strain, G4-P[6]-I1-R1-C1-M1-A8-N1-T1-E1-H1, (Matthijnssens et al., 2008b) though all genes are genetically distinct. The A8 NSP1 genotype found in both strain RVA/Human-wt/BRB/CDC1133/2012/G4P[14] and Gottfried has been reported previously from human RVA cases from Bulgaria, Hungary, India, and Nicaragua and is thought to be of porcine origin (Bucardo et al., 2012; Mladenova et al., 2012; Mukherjee et al., 2011; Papp et al., 2013). For all genes except VP4, NSP3, and NSP4, the Barbados strain genes cluster within supported clades containing both human and porcine RVA strains and, in the cases of VP1, VP2, VP3, and NSP1, a small number of equine and bovine strains as well (Figures 1A and 2A–F, 2I). In phylogenetic estimates of the VP4, NSP3 and NSP4 genes (Figures 1B, 2G and 2H), strain RVA/Human-wt/BRB/CDC1133/2012/G4P[14] occupies sublineages containing human strains only suggesting that these genes may now be established in human RVA populations. It is difficult, however, to ascertain the host species of origin for this strain since its genes may have been derived from human, porcine, equine, or bovine RVAs.
Figure 2.
Phylogenetic trees based on nucleotide sequences of the complete open reading frame of (a) VP1, (b) VP2, (c) VP3, (d) VP6, (e) NSP1, (f) NSP2, (g)NSP3, (h) NSP4, and (i) NSP5 rotavirus genes. The maximum likelihood trees were constructed using PhyML 3.0 with best model identified by JModelTest 2 program along with aLRT statistics for branch support shown at the node (<70% not shown). The GenBank accession numbers, strain names, and G and P-type associated are shown where available.
In summary, the study describes the genetic characterization of a novel human RVA with the G and P combination of G4P[14] and identification of 7 lineages within the P[14] genotype. The natural history of this strain probably involved one or more reassortment events involving multiple gene segments and interspecies transmission. Continued surveillance of RVA strains will be important to determine the extent of similar strains in the community which is important in the context of whether or not vaccine-induced heterotypic immunity is sufficient to protect against strains that express the P[14] RVA genotype.
Acknowledgments
We wish to thank Rashi Gautam and Slavica Mijatovic-Rustempasic for their review of the manuscript and helpful comments. We also wish to thank the Ministry of Health, Barbados, the Pan American Health Organization, and the World Health Organization for their support of this study.
Footnotes
Conflict of interest statement
The authors of this study declare that they have no conflict of interest, financial or otherwise, related to this article.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
References
- Banyai K, Laszlo B, Duque J, Steele AD, Nelson EA, Gentsch JR, Parashar UD. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine. 2012;30(Suppl 1):A122–130. doi: 10.1016/j.vaccine.2011.09.111. [DOI] [PubMed] [Google Scholar]
- Banyai K, Martella V, Molnar P, Mihaly I, Van Ranst M, Matthijnssens J. Genetic heterogeneity in human G6P[14] rotavirus strains detected in Hungary suggests independent zoonotic origin. The Journal of infection. 2009;59:213–215. doi: 10.1016/j.jinf.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Banyai K, Papp H, Dandar E, Molnar P, Mihaly I, Van Ranst M, Martella V, Matthijnssens J. Whole genome sequencing and phylogenetic analysis of a zoonotic human G8P[14] rotavirus strain. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2010;10:1140–1144. doi: 10.1016/j.meegid.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Bucardo F, Rippinger CM, Svensson L, Patton JT. Vaccine-derived NSP2 segment in rotaviruses from vaccinated children with gastroenteritis in Nicaragua. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;12:1282–1294. doi: 10.1016/j.meegid.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar SD, Arora R, Kolpe AB, Yadav MM, Raut CG. Molecular characterization of unusual bovine group A rotavirus G8P[14] strains identified in western India: emergence of P[14] genotype. Veterinary microbiology. 2011;148:384–388. doi: 10.1016/j.vetmic.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Cilla G, Montes M, Gomariz M, Alkorta M, Iturzaeta A, Perez-Yarza EG, Perez-Trallero E. Rotavirus genotypes in children in the Basque Country (North of Spain): rapid and intense emergence of the G12[P8] genotype. Epidemiology and infection. 2012:1–7. doi: 10.1017/S0950268812001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley D, Donato CM, Roczo-Farkas S, Kirkwood CD. Novel G10P[14] rotavirus strain, northern territory, Australia. Emerging infectious diseases. 2013;19:1324–1327. doi: 10.3201/eid.1908.121653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe NA, Gondwe JS, Graham SM, Thindwa BD, Dove W, Broadhead RL, Molyneux ME, Hart CA. Rotavirus strain diversity in Blantyre, Malawi, from 1997 to 1999. Journal of clinical microbiology. 2001;39:836–843. doi: 10.1128/JCM.39.3.836-843.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das BK, Gentsch JR, Cicirello HG, Woods PA, Gupta A, Ramachandran M, Kumar R, Bhan MK, Glass RI. Characterization of rotavirus strains from newborns in New Delhi, India. Journal of clinical microbiology. 1994;32:1820–1822. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira LH, Danovaro-Holliday MC, Andrus JK, de Fillipis AM, Gentsch J, Matus CR, Widdowson MA Rotavirus Surveillance, N. Sentinel hospital surveillance for rotavirus in latin american and Caribbean countries. The Journal of infectious diseases. 2009;200(Suppl 1):S131–139. doi: 10.1086/605060. [DOI] [PubMed] [Google Scholar]
- Donato CM, Manuelpillai NM, Cowley D, Roczo-Farkas S, Buttery JP, Crawford NW, Kirkwood CD. Genetic characterization of a novel G3P[14] rotavirus strain causing gastroenteritis in 12year old Australian child. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2014;25C:97–109. doi: 10.1016/j.meegid.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC evolutionary biology. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sherif M, Esona MD, Wang Y, Gentsch JR, Jiang B, Glass RI, Abou Baker S, Klena JD. Detection of the first G6P[14] human rotavirus strain from a child with diarrhea in Egypt. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11:1436–1442. doi: 10.1016/j.meegid.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Esona MD, Geyer A, Banyai K, Page N, Aminu M, Armah GE, Hull J, Steele DA, Glass RI, Gentsch JR. Novel human rotavirus genotype G5P[7] from child with diarrhea, Cameroon. Emerging infectious diseases. 2009a;15:83–86. doi: 10.3201/eid1501.080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esona MD, Geyer A, Page N, Trabelsi A, Fodha I, Aminu M, Agbaya VA, Tsion B, Kerin TK, Armah GE, Steele AD, Glass RI, Gentsch JR. Genomic characterization of human rotavirus G8 strains from the African rotavirus network: relationship to animal rotaviruses. Journal of medical virology. 2009b;81:937–951. doi: 10.1002/jmv.21468. [DOI] [PubMed] [Google Scholar]
- Estes M, Kapikian A. Rotaviruses. In: Knipe DMHP, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. 5. Kluwer/Lippincott, Williams and Wilkins; Philadelphia, PA: 2007. pp. 1917–1974. [Google Scholar]
- Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, Jain V, Cunliffe NA, Nakagomi O, Kirkwood CD, Fischer TK, Parashar UD, Bresee JS, Jiang B, Glass RI. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. The Journal of infectious diseases. 2005;192(Suppl 1):S146–159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Varghese V, Samajdar S, Sinha M, Naik TN, Kobayashi N. Evidence for bovine origin of VP4 and VP7 genes of human group A rotavirus G6P[14] and G10P[14] strains. Journal of clinical microbiology. 2007;45:2751–2753. doi: 10.1128/JCM.00230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, Fang ZY. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. Journal of clinical microbiology. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic biology. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Hamilton P, Diggory P. The Caribbean Epidemiology Centre (CAREC) Bulletin of the Pan American Health Organization. 1979;13:187–194. [PubMed] [Google Scholar]
- Hoshino Y, Honma S, Jones RW, Ross J, Santos N, Gentsch JR, Kapikian AZ, Hesse RA. A porcine G9 rotavirus strain shares neutralization and VP7 phylogenetic sequence lineage 3 characteristics with contemporary human G9 rotavirus strains. Virology. 2005;332:177–188. doi: 10.1016/j.virol.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Hu L, Crawford SE, Czako R, Cortes-Penfield NW, Smith DF, Le Pendu J, Estes MK, Prasad BV. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 2012;485:256–259. doi: 10.1038/nature10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gomara M, Isherwood B, Desselberger U, Gray J. Reassortment in vivo: driving force for diversity of human rotavirus strains isolated in the United Kingdom between 1995 and 1999. Journal of virology. 2001;75:3696–3705. doi: 10.1128/JVI.75.8.3696-3705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins GM, Rambaut A, Pybus OG, Holmes EC. Rates of molecular evolution in RNA viruses: a quantitative phylogenetic analysis. Journal of molecular evolution. 2002;54:156–165. doi: 10.1007/s00239-001-0064-3. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang P, Tan M, Liu Y, Biesiada J, Meller J, Castello AA, Jiang B, Jiang X. Rotavirus VP8*: phylogeny, host range, and interaction with histo-blood group antigens. Journal of virology. 2012;86:9899–9910. doi: 10.1128/JVI.00979-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V, Banyai K, Matthijnssens J, Buonavoglia C, Ciarlet M. Zoonotic aspects of rotaviruses. Veterinary microbiology. 2010;140:246–255. doi: 10.1016/j.vetmic.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Bilcke J, Ciarlet M, Martella V, Banyai K, Rahman M, Zeller M, Beutels P, Van Damme P, Van Ranst M. Rotavirus disease and vaccination: impact on genotype diversity. Future microbiology. 2009a;4:1303–1316. doi: 10.2217/fmb.09.96. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, Patton JT, Rahman M, Van Ranst M. Full genome-based classification of rotaviruses reveals a common origin between human Wa-Like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. Journal of virology. 2008a;82:3204–3219. doi: 10.1128/JVI.02257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, Buesa J, Esona MD, Estes MK, Gentsch JR, Iturriza-Gomara M, Johne R, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Parreno V, Rahman M, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Patton JT, Desselberger U, Van Ranst M. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG) Archives of virology. 2011;156:1397–1413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Banyai K, Estes MK, Gentsch JR, Iturriza-Gomara M, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Patton JT, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Desselberger U, Van Ranst M. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Archives of virology. 2008b;153:1621–1629. doi: 10.1007/s00705-008-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Heylen E, Zeller M, Rahman M, Lemey P, Van Ranst M. Phylodynamic analyses of rotavirus genotypes G9 and G12 underscore their potential for swift global spread. Molecular biology and evolution. 2010;27:2431–2436. doi: 10.1093/molbev/msq137. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J, Potgieter CA, Ciarlet M, Parreno V, Martella V, Banyai K, Garaicoechea L, Palombo EA, Novo L, Zeller M, Arista S, Gerna G, Rahman M, Van Ranst M. Are human P[14] rotavirus strains the result of interspecies transmissions from sheep or other ungulates that belong to the mammalian order Artiodactyla? Journal of virology. 2009b;83:2917–2929. doi: 10.1128/JVI.02246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Rahman M, Martella V, Xuelei Y, De Vos S, De Leener K, Ciarlet M, Buonavoglia C, Van Ranst M. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. Journal of virology. 2006;80:3801–3810. doi: 10.1128/JVI.80.8.3801-3810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Davis K, McAllen JK, Spiro DJ, Patton JT. Intra-genotypic diversity of archival G4P[8] human rotaviruses from Washington, DC. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11:1586–1594. doi: 10.1016/j.meegid.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici MC, Abelli LA, Martinelli M, Dettori G, Chezzi C. Molecular characterization of VP4, VP6 and VP7 genes of a rare G8P[14] rotavirus strain detected in an infant with gastroenteritis in Italy. Virus research. 2008;137:163–167. doi: 10.1016/j.virusres.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Mijatovic-Rustempasic S, Banyai K, Esona MD, Foytich K, Bowen MD, Gentsch JR. Genome sequence based molecular epidemiology of unusual US Rotavirus A G9 strains isolated from Omaha, USA between 1997 and 2000. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11:522–527. doi: 10.1016/j.meegid.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Mladenova Z, Papp H, Lengyel G, Kisfali P, Steyer A, Steyer AF, Esona MD, Iturriza-Gomara M, Banyai K. Detection of rare reassortant G5P[6] rotavirus, Bulgaria. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;12:1676–1684. doi: 10.1016/j.meegid.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Ghosh S, Bagchi P, Dutta D, Chattopadhyay S, Kobayashi N, Chawla-Sarkar M. Full genomic analyses of human rotavirus G4P[4], G4P[6], G9P[19] and G10P[6] strains from North-eastern India: evidence for interspecies transmission and complex reassortment events. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17:1343–1346. doi: 10.1111/j.1469-0691.2010.03383.x. [DOI] [PubMed] [Google Scholar]
- Mullick S, Mukherjee A, Ghosh S, Pazhani GP, Sur D, Manna B, Nataro JP, Levine MM, Ramamurthy T, Chawla-Sarkar M. Genomic analysis of human rotavirus strains G6P[14] and G11P[25] isolated from Kolkata in 2009 reveals interspecies transmission and complex reassortment events. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;14:15–21. doi: 10.1016/j.meegid.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Palombo EA, Bugg HC, Masendycz PJ, Bishop RF. Sequence of the VP7 gene of an atypical human rotavirus: evidence for genetic and antigenic drift. DNA sequence : the journal of DNA sequencing and mapping. 1997;7:307–311. doi: 10.3109/10425179709034050. [DOI] [PubMed] [Google Scholar]
- Papp H, Borzak R, Farkas S, Kisfali P, Lengyel G, Molnar P, Melegh B, Matthijnssens J, Jakab F, Martella V, Banyai K. Zoonotic transmission of reassortant porcine G4P[6] rotaviruses in Hungarian pediatric patients identified sporadically over a 15 year period. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2013;19:71–80. doi: 10.1016/j.meegid.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. The Journal of infectious diseases. 2009;200(Suppl 1):S9–S15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerging infectious diseases. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. The Pediatric infectious disease journal. 2011;30:S1–5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Molecular biology and evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Saikruang W, Khamrin P, Chaimongkol N, Suantai B, Kongkaew A, Kongkaew S, Ushijima H, Maneekarn N. Genetic diversity and novel combinations of G4P[19] and G9P[19] porcine rotavirus strains in Thailand. Veterinary microbiology. 2013;161:255–262. doi: 10.1016/j.vetmic.2012.07.036. [DOI] [PubMed] [Google Scholar]
- Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Reviews in medical virology. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD Network W.H.-c.G.R.S. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- Tsugawa T, Hoshino Y. Whole genome sequence and phylogenetic analyses reveal human rotavirus G3P[3] strains Ro1845 and HCR3A are examples of direct virion transmission of canine/feline rotaviruses to humans. Virology. 2008;380:344–353. doi: 10.1016/j.virol.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]