Abstract
The recent resurgence of ballistocardiogram (BCG) measurement and interpretation technologies has led to a wide range of powerful tools available for unobtrusively assessing mechanical aspects of cardiovascular health at home. Researchers have demonstrated a multitude of modern BCG measurement modalities, including beds, chairs, weighing scales, and wearable approaches. However, many modalities produce significant variations in the morphology of the measured BCG, creating confusion in the analysis and interpretation of the signals. This paper creates a framework for comparing wearable BCG measurements to whole body measurements—such as taken with a weighing scale system—to eventually allow the same analysis and interpretation tools that have been developed for whole body systems to be applied in the future to wearable systems. To the best of our knowledge, it represents the first attempt to morphologically compare vertical acceleration recordings measured on different locations on the torso to whole body displacements measured by BCG instrumentation.
I. Introduction
Ballistocardiography (BCG) is a non-invasive tool for assessing the mechanical aspects of cardiovascular function [1]. BCG was first discovered more than 100 years ago [2], and in the mid-1900s myriad studies demonstrated the clinical potential of the measurement technique (e.g., [3, 4]); however with the advent of powerful imaging modalities in the 1960s-1970s, confusion regarding BCG instrumentation, and high inter-subject variability in the signal shape, the BCG was largely abandoned from research and clinical use [5].
Recently, with the need for better techniques for assessing mechanical aspects of cardiovascular health outside of the clinic, BCG has resurfaced as a tool of interest for many researchers [6]. Particularly appealing is the potential for improving the availability of clinically relevant information regarding cardiac output [7], cardiac contractility [8], and beat-by-beat left ventricular function [9]. The level of information provided by the BCG is thus comparable to more obtrusive or expensive alternatives such as finger-cuff arterial blood pressure measurement [10] or impedance cardiography [11].
Accordingly, many modalities of modern BCG measurement have been implemented leveraging recent advances in embedded systems technology and sensor miniaturization. He, et al. mounted a three-axis accelerometer behind the ear together with electrodes to measure BCG and electrocardiogram (ECG) signals at the head [12]. Using similar techniques as previous BCG studies [8, 13], changes in the time interval between the ECG R-wave and the BCG J-wave were shown to be correlated to changes in the preejection period (PEP) of the heart. However, the measured R-J interval values were found to be between 150-180 ms, while in the existing literature the typical R-J interval for a healthy adult is 250 ms [13], and for 92 healthy subjects studied in Inan's dissertation, the mean, minimum, and maximum R-J interval were found to be 203, 245, and 290 ms, respectively [14]. Wiard, et al. also found an unusually short R-J interval with their accelerometer-based BCG system of 133 ms, but found in microgravity that the R-J interval for the same subject increased to 246 ms; simultaneously measured R-J interval values for BCG measurements from the scale were 191 and 200 ms, respectively, terrestrially and at microgravity [15].
An important scientific question remains in analyzing the vibrations of the body in response to the heartbeat: How do surface vibrations compare to whole body vibrations, and can the same analysis techniques and human subjects findings be applied to both of these signals? We posit that each point on the body that is rigidly coupled to the skeletal system – most notably, the ribcage or spine – will, to the first order, displace together with the center of mass (COM), causing accelerations that are proportional to the second-derivative of this displacement. Accordingly, we believe that a scaled version of the acceleration signal in the vertical direction will capture a significant portion of the BCG signal information. This paper presents a systematic approach to addressing this important topic with preliminary results, and lays the groundwork for a future human subjects study aimed at confirming these initial, promising findings on a wide range of body sizes and types.
II. Methods
A. Hardware and Data Collection
This study was conducted under a protocol reviewed and approved by the Georgia Institute of Technology (GT) Institutional Review Board (IRB). All subjects provided written informed consent before experimentation. Five subjects of similar anthropometrics were recruited for this preliminary study. The subjects were chosen as healthy, young adult males, with similar height and weight, to understand the inter-subject variability in a reasonably uniform study population.
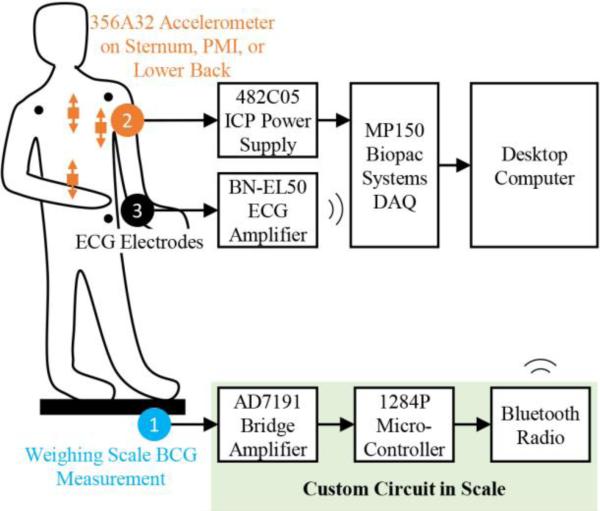
As depicted in Figure 1, the whole body displacement BCG and ECG signals were measured as previously described [16], with the strain gauges and handlebar electrodes from the scale being interfaced to custom electronics, including the AD7191 integrated bridge amplifier and analog-to-digital converter (Analog Devices, Norwood, MA). A microcontroller (1284P, Atmel Corporation, San Jose, CA) and Bluetooth radio were also used to send the signals wirelessly to a laptop for storage. The BCG signals were sampled at 120 Hz and the ECG at 1 kHz.
Figure 1.
Block diagram showing measurement setup and instrumentation. Commercially available devices and systems were used for the electrocardiogram (ECG) and acceleration measurements, and a custom circuit was used for the ballistocardiogram (BCG) measurements. The signals were recorded on a desktop computer for post-processing and analysis.
While each subject was standing on the scale, a small, ultra-low noise accelerometer (356A32, PCB Piezotronics, Depew, NY) was attached to different locations of the body, while a Lead II ECG was measured with three surface electrodes connected to a data acquisition system with 1 kHz sampling rate (MP150WSW, BIOPAC Systems, Inc., Goleta, CA). This accelerometer was chosen for its relatively small footprint (11.4 × 11.4 × 11.4 mm), low weight (5.4 g), ultra-low spot noise (20 μg/√Hz at 10 Hz) and total noise (300 μgrms, BW: 1-10,000 Hz), and wide bandwidth (0.7–5 kHz, +/− 1 dB). For this study, the accelerometer was always placed such that one axis was parallel to the vertical direction (head-to-foot), the direction that was analyzed, and compared to the BCG signals measured from the scale in the same direction.
B. Signal Processing and Mechanical Assumptions
As a subject stands on the weighing scale, and his heart contracts, a force is imparted on the blood and vasculature. The reaction forces experienced by the body then cause the center of mass (COM) to displace upward and downward with the cardiac cycle: this displacement is sensed by the strain gauges in the scale and results in the BCG signal.
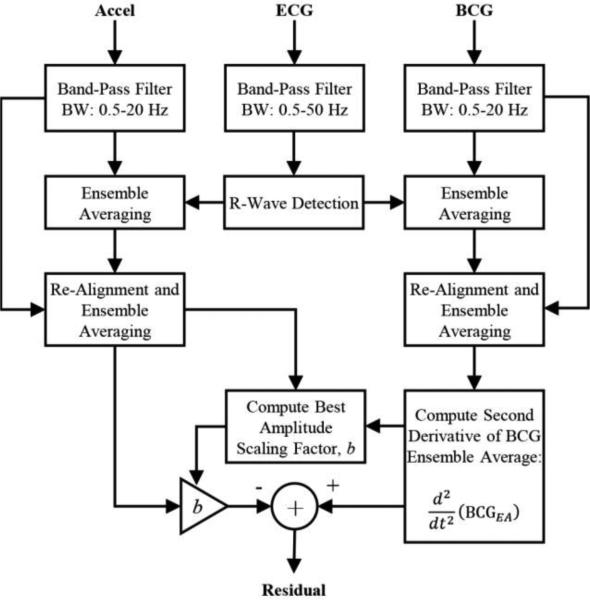
To quantify this, we used the signal pre-processing, ensemble averaging, and signal comparison methods illustrated in Figure 2. The BCG and acceleration signals were band-pass filtered, then segmented into an array of heartbeats using the ECG signal R-wave peaks as a fiducial point. These segmented beats were then ensemble averaged, re-aligned using cross-correlation with the original ensemble average, and then re-averaged. These methods are described in detail in previous work [17, 18]. The second-derivative of the ensemble averaged BCG was then computed, and a simple scaling factor was found to normalize signal amplitude between this second-derivative BCG and the vertical acceleration ensemble averages. A residual was then computed as the difference between the scaled acceleration signal, and the second-derivative of the BCG. To determine the quality of match between these two resultant waveforms, the mean-square of the residual normalized to the mean-square of the BCG second derivative, and the correlation coefficient were computed.
Figure 2.
Block diagram summarizing signal processing steps for quantitatively comparing the second derivative of the displacement BCG waveforms measured using the weighing scale to the acceleration measurements from various points on the body. The residual signal was computed for all positions and all subjects to quantify the degree to which the acceleration matched the second derivative of the BCG.
III. Results
A. Example Results for One Subject
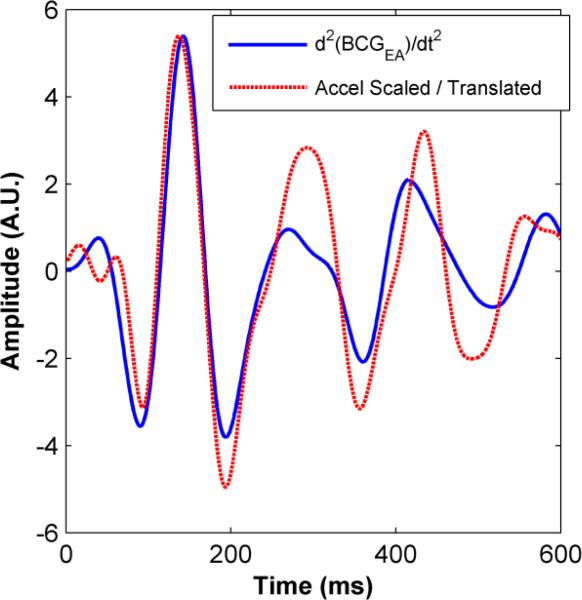
Figure 3 shows the second derivative of the ensemble averaged BCG waveform alongside the scaled acceleration waveform (measured from the sternum) ensemble average. This represents the best case in terms of the quantitative matching between the second derivative of the BCG and acceleration signal among all of the subjects tested. For all of the subjects, the second derivative of the BCG matched the acceleration signals much more closely both visually and quantitatively compared to the BCG signal itself, further supporting the assumption that the BCG measured on the scale is proportional to body displacement.
Figure 3.
Second-derivative of the BCG signal (ensemble averaged) measured from the weighing scale shown simultaneously with the vertical acceleration waveform (ensemble averaged) taken from the sternum after scaling (1/160 ×) and translation (20 ms). The correlation coefficient between the waveforms was 0.70, and the mean-square normalized residual was 0.71.
B. Waveforms for All Subjects
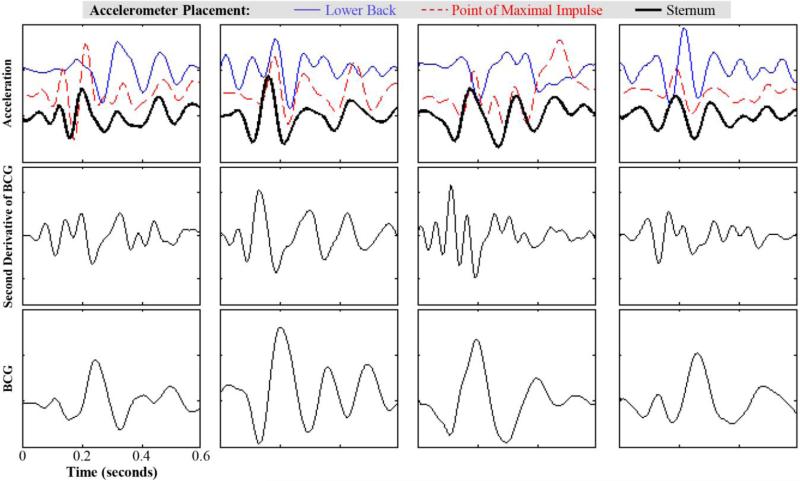
The waveforms measured from all subjects in this preliminary study are shown in Figure 4. For all subjects, the sternal acceleration measurements preceded the point of maximal impulse (PMI) and lower back measurements in time. Furthermore, the waveform morphology was significantly different at the lower back in particular compared to the PMI and sternum. The acceleration waveforms visually appear to more closely resemble the second derivative of the BCG, rather than the BCG itself; however, the specific location at which the acceleration in the vertical direction most closely resembles the BCG is subject-dependent.
Figure 4.
The ensemble averaged acceleration (top), second derivative of BCG (middle), and BCG (bottom) waveforms are shown for all four subjects. For all subjects, the acceleration signals measured on the sternum had the largest peak earlier than at the point of maximal impulse (PMI) and the lower back. The second derivative of the BCG resembles the morphology of the accelerometer signal more closely than the BCG itself; however, the location on the body where the acceleration waveform most closely resembles the BCG second derivative varies from one subject to another, despite the fact that the subjects were very similar anthropometrically.
Quantitatively, the normalized residual (Resid.) and correlation coefficient (r) are shown in Table 1 for all subjects and all locations of measurement. On average, the lower back provided the closest match to the second derivative of the BCG, with the lowest normalized residual; however, the results at this point are still preliminary due to the small subject population for this pilot study. Additionally, the correlation was significant (p < 0.05) for all of the locations, suggesting that the acceleration signal in the vertical direction does match the second derivative of the BCG waveform in morphology.
Table 1.
Summary of Results from All Subjects
| Subj. | Lower Back | PMI | Sternum | |||
|---|---|---|---|---|---|---|
| Resid. | r | Resid. | r | Resid. | r | |
| 1 | 0.86 | 0.79 | 5.3 | 0.48 | 1.02 | 0.79 |
| 2 | 1.05 | 0.62 | 1.05 | 0.86 | 0.71 | 0.70 |
| 3 | 1.80 | 0.37 | 3.24 | 0.38 | 2.27 | 0.35 |
| 4 | 1.96 | 0.61 | 1.62 | 0.62 | 4.20 | 0.56 |
| Avg | 1.42 | 0.60 | 2.80 | 0.59 | 2.05 | 0.59 |
| Stdev | 0.54 | 0.17 | 1.91 | 0.21 | 1.59 | 0.17 |
IV. Discussion and Future Work
Wearable “continuous” BCG measurements offer multiple potential advantages over stationary “snapshot” measurements (such as with a weighing scale, chair, or other fixed apparatus): continuously monitoring the cardiovascular system throughout the day and night would allow trends to be determined, responses of the system to normal activities of daily living such as exercise to be quantified, and effects of environmental stressors, such as heat or altitude, to be assessed. However, several challenges need to be addressed to further the understanding of how to interpret these measurements using BCG analysis principles: primarily, the significant differences in signal morphology between wearable and stationary BCG measurements, and the differences in wearable measurements depending on the location on the body where they are taken.
Additionally, while the BCG is fundamentally a measure of the hemodynamics, wearable BCG measurements can also include other phenomena such as the heart sounds and the movement of the heart itself. This has led to scientific questions as to what measurements should be considered “BCG” and which should be considered “SCG” (seismocardiogram) instead. Regardless of the naming, better understanding and quantifying the relationship between cardiogenic forces measured from the whole body compared to locally interrogated representations is an important step towards unification of these fields and eventual clinical acceptance.
With this objective in mind, this paper presents some key preliminary findings in this area that should be considered in future work:
Acceleration waveforms in the vertical direction measured on the body more closely resemble the second derivative of the BCG measured on the weighing scale, not the BCG signal itself.
Accelerometer signals in the vertical direction measured from the chest cannot always be directly transformed into displacement BCG signals using double integration in time.
For even a small population of subjects, the accelerometer position on the body (sternum, point of maximal impulse, lower back) providing the closest match to the second derivative of the BCG was subject-dependent.
In future work, we plan to expand this study with a greater population of subjects, preferably with a wide range of body types and ages, and with representation from both genders, and attempt to extract information regarding changes in cardiac output from the wearable signals. We believe that the body shape and type differences will further increase the differences between body-worn vertical acceleration measurements and the second derivative of the BCG from the scale, particularly when measurements are taken from the chest (sternum or point of maximal impulse). While on the lower back the sensor is closely coupled to the skeletal system and spine, on the chest the viscous damping and spring constant associated with the fat and muscle may dampen the acceleration signal compared to the COM acceleration.
Contributor Information
Andrew Wiens, School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA 30308 USA.
Mozziyar Etemadi, Department of Bioengineering and Therapeutic Sciences at the University of California, San Francisco and the Department of Electrical Engineering and Computer Science at the University of California, Berkeley, Berkeley, CA 94720 USA (mozziyar.etemadi@ucsf.edu)..
Liviu Klein, School of Medicine, University of California, San Francisco, San Francisco, CA 94143 (lklein@medicine.ucsf.edu)..
Shuvo Roy, Department of Bioengineering and Therapeutic Sciences at the University of California, San Francisco, San Francisco, CA 94158 USA (shuvo.roy@ucsf.edu)..
Omer T. Inan, School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, GA 30308 USA.
REFERENCES
- 1.Starr I, Rawson AJ, Schroeder HA, Joseph NR. Studies on the estimation of cardiac output in man, and of abnormalities in cardiac function, from the heart's recoil and the blood's impacts; the ballistocardiogram. American Journal of Physiology. 1939;127:1–28. [Google Scholar]
- 2.Gordon JW. Certain Molar Movements of the Human Body produced by the Circulation of the Blood. J Anat Physiol. 1877;11:533–536. [PMC free article] [PubMed] [Google Scholar]
- 3.Mandelbaum H, Mandelbaum RA. Studies Utilizing the Portable Electromagnetic Ballistocardiograph: IV. The Clinical Significance of Serial Ballistocardiograms Following Acute Myocardial Infarction. Circulation. 1953;7:910–915. doi: 10.1161/01.cir.7.6.910. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson JL, Warren JV, Brannon ES. The Cardiac Output in Man: Studies with the Low Frequency, Critically-Damped Ballistocardiography, and the Method of Right Atrial Catheterization. J Clin Invest. 1947;26:1–10. doi: 10.1172/JCI101780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovangrandi L, Inan OT, Wiard RM, Etemadi M, Kovacs GTA. Ballistocardiography: A method worth revisiting. Engineering in Medicine and Biology Society,EMBC, 2011 Annual International Conference of the IEEE. 2011:4279–4282. doi: 10.1109/IEMBS.2011.6091062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inan OT. Recent advances in cardiovascular monitoring using ballistocardiography. Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE. 2012:5038–5041. doi: 10.1109/EMBC.2012.6347125. [DOI] [PubMed] [Google Scholar]
- 7.Inan OT, Etemadi M, Paloma A, Giovangrandi L, Kovacs GTA. Non-invasive cardiac output trending during exercise recovery on a bathroom-scale-based ballistocardiograph. Physiological Measurement. 2009;30:261. doi: 10.1088/0967-3334/30/3/003. [DOI] [PubMed] [Google Scholar]
- 8.Etemadi M, Inan OT, Giovangrandi L, Kovacs GTA. Rapid Assessment of Cardiac Contractility on a Home Bathroom Scale. Information Technology in Biomedicine, IEEE Transactions on. 2011;15:864–869. doi: 10.1109/TITB.2011.2161998. [DOI] [PubMed] [Google Scholar]
- 9.Inan OT, Etemadi M, Wiard RM, Giovangrandi L, Kovacs GTA. Robust ballistocardiogram acquisition for home monitoring. Physiological Measurement. 2009;30:169. doi: 10.1088/0967-3334/30/2/005. [DOI] [PubMed] [Google Scholar]
- 10.Jones RDM, Kornberg JP, Roulson CJ, Visram AR, Irwin MG. The Finapres 2300e finger cuff. Anaesthesia. 1993;48:611–615. doi: 10.1111/j.1365-2044.1993.tb07129.x. [DOI] [PubMed] [Google Scholar]
- 11.Patterson RP. Fundamentals of Impedance Cardiography. IEEE Engineering in Medicine and Biology Magazine. 1989;8:35–38. doi: 10.1109/51.32403. [DOI] [PubMed] [Google Scholar]
- 12.He DD, Winokur ES, Sodini CG. A continuous, wearable, and wireless heart monitor using head ballistocardiogram (BCG) and head electrocardiogram (ECG) Engineering in Medicine and Biology Society,EMBC, 2011 Annual International Conference of the IEEE. 2011:4729–4732. doi: 10.1109/IEMBS.2011.6091171. [DOI] [PubMed] [Google Scholar]
- 13.Lindqvist A, Pihlajamäki K, Jalonen J, Laaksonen V, Alihanka J. Static-charge-sensitive bed ballistocardiography in cardiovascular monitoring. Clinical Physiology. 1996;16:23–30. doi: 10.1111/j.1475-097x.1996.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 14.Inan OT. PhD, Electrical Engineering. Stanford University; 2009. Novel Technologies for Cardiovascular Monitoring using Ballistocardiography and Electrocardiography. [Google Scholar]
- 15.Wiard RM, Inan OT, Giovangrandi L, Cuttino CM, Kovacs GTA. Preliminary results from standing ballistocardiography measurements in microgravity. Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE. 2013:7290–7293. doi: 10.1109/EMBC.2013.6611241. [DOI] [PubMed] [Google Scholar]
- 16.Inan OT, Dookun P, Giovangrandi L, Kovacs GTA. Noninvasive Measurement of Physiological Signals on a Modified Home Bathroom Scale. Biomedical Engineering, IEEE Transactions on. 2012;59:2137–2143. doi: 10.1109/TBME.2012.2186809. [DOI] [PubMed] [Google Scholar]
- 17.Inan OT, Pandia K, Giovangrandi L, Zamanian RT, Kovacs GTA. A preliminary study investigating the quantification of beat-to-beat in seismocardiogram signals. Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE. 2013:7286–7289. doi: 10.1109/EMBC.2013.6611240. [DOI] [PubMed] [Google Scholar]
- 18.Inan OT, Etemadi M, Wiard RM, Kovacs GTA, Giovangrandi L. Novel methods for estimating the ballistocardiogram signal using a simultaneously acquired electrocardiogram. Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE. 2009:5334–5347. doi: 10.1109/IEMBS.2009.5333709. [DOI] [PubMed] [Google Scholar]