Abstract
Neurophysiological recording of brain activity has been critically important to the field of neuroscience, but has contributed little to the field of developmental psychobiology. The reasons for this can be traced largely to methodological difficulties associated with recording neural activity in behaving newborn rats and mice. Over the last decade, however, the evolution of methods for recording from head-fixed newborns has heralded a new era in developmental neurophysiology. Here, we review these recent developments and provide a step-by-step primer for those interested in applying the head-fix method to their own research questions. Until now, this method has been used primarily to investigate spontaneous brain activity across sleep and wakefulness, the contributions of the sensory periphery to brain activity, or intrinsic network activity. Now, with some ingenuity, the uses of the head-fix method can be expanded to other domains to benefit our understanding of brain-behavior relations under normal and pathophysiological conditions across early development.
Keywords: infant, rat, mouse, neurophysiology, multiunit activity, local field potential, EEG, imaging, sleep, wake, spontaneous activity, plasticity, pathophysiology
INTRODUCTION
One of the core aims of developmental psychobiology is to understand the bidirectional relations of brain and behavior across periods of developmental change (Blumberg, Freeman, & Robinson, 2010; Michel & Moore, 1995). The field has adopted many standard neuroscience methods in pursuit of this aim, including brain lesions, neuropharmacological manipulations, electrical brain stimulation, tract tracing, autoradiography, and measurement of immediate early gene expression (e.g., fos). However, one prominent neuroscience method has been underrepresented until only very recently: direct measurement of brain activity in unanesthetized newborn animals, especially in rats over the first postnatal week.
What explains this relative absence of neurophysiological recording studies across the early postnatal period? Clearly, the answer rests largely with the fact that rats give birth to very small offspring with uncalcified skulls. As the skull hardens after the first postnatal week, it becomes more amenable to conventional neurophysiological approaches. Thus, numerous studies report measures of neural activity in freely moving rats beyond the first postnatal week, including measures of cortical activity at 7 days of age and older (Frank & Heller, 1997; Gramsbergen, 1976; Jouvet-Mounier, Astic, & Lacote, 1970; Mirmiran & Corner, 1982; Sarro, Wilson, & Sullivan, 2014; Seelke & Blumberg, 2008), hippocampal and parahippocampal activity at 11 days of age and older (Bjerknes, Langston, Kruge, Moser, & Moser, 2014; Langston et al., 2010; Wills, Cacucci, Burgess, & O’Keefe, 2010), and cerebellar interpositus activity at 17 days of age and older (Freeman & Nicholson, 2000). With respect to subcortical activity, only a handful of papers exist, including several from the 1980s reporting neural activity in the reticular formation of freely moving pups beginning on the day of birth (Tamásy & Korányi, 1980; Tamásy, Korányi, & Lissák, 1979; Tamásy, Korányi, & Lissák, 1980) or at 8 days of age (Corner & Bour, 1984) and a more recent investigation of hippocampal activity in freely moving 4- to 6-day-old rats (Leinekugel et al., 2002).
We can contrast this slow accumulation of developmental neurophysiological studies in freely moving pups with the sudden upsurge that has occurred over the past decade as methods of recording from head-fixed subjects were introduced and refined. This period began with investigations of cortical activity in 1- to 6-day-old head-fixed rats (Khazipov et al., 2004) and midbrain and medullary activity in 6- to 10-day-old rats (Karlsson & Blumberg, 2005; Karlsson, Gall, Mohns, Seelke, & Blumberg, 2005). Table 1 lists these and many other subsequent papers using head-fixed rats and, in some cases, mice. Reviews of various aspects of this work have been published (Blumberg, 2010; Blumberg, Marques, & Iida, 2013; Hanganu-Opatz, 2010; Khazipov & Buzsáki, 2010; Khazipov & Luhmann, 2006).
Table 1.
A of Neurophysiological Studies Conducted in Head-Fixed Infant Rats (or Mice) Organized by Brain Area and Published Between 2004 and 2014
A few studies that examined neural activity in more than one brain area are cited twice.
P, postnatal day; MUA, multiunit activity; LFP, local field potential; m, mice.
Intracellular recordings also performed.
The corpus of published work listed in Table 1 excludes in vitro studies using brain slices and in vivo studies using deeply anesthetized pups. However, in some of the studies listed in Table 1, pups were “lightly anesthetized” during the recording sessions, although the use of anesthesia is declining. In our laboratory, as we developed methods for recording from the brainstem of head-fixed pups, we routinely performed precollicular decerebrations because we assumed that intact unanesthetized pups would not tolerate head restraint without extensive habituation (Karlsson et al., 2005; Karlsson & Blumberg, 2005); we soon learned, however, that intact animals habituated quickly to the head restraint. Also, in our initial head-fix set-up, the pups’ torso and limbs were wrapped in gauze because we had noted the calming effects of swaddling (Corner & Kwee, 1976); however, we later found that pups remained just as calm when their torso was wrapped in gauze and secured to a platform but their limbs were allowed to dangle freely on either side of the platform (Marcano-Reik & Blumberg, 2008); this modification gave us visual access to the limbs and allowed us to deliver tactile and proprioceptive stimulation. Similar evolutionary processes have undoubtedly occurred in other laboratories as questions changed and new methodological insights emerged.
Thus, although there are many reasons to value recordings of brain activity in freely moving subjects, there are also clear benefits to recording from head-fixed subjects. The increase in research productivity alone using the head-fix method testifies to its value. There are several reasons for this increased productivity: First, movement artifact is particularly problematic when recording from newborns, and head restraint helps to reduce noise in the neurophysiological signal. Second, the head-fix preparation provides experimenters with the flexibility to adjust electrode location while monitoring behavior, which greatly increases the likelihood of successfully recording from a brain area of interest. Moreover, this method allows investigators to record more easily from multiple brain areas and combine recording with other methods (e.g., neural stimulation, pharmacological manipulation, microdialysis, amperometry) in the same experimental session. The added flexibility inherent in this method is particularly valuable for neural network analysis, as well as for relating neural activity to spontaneous motor activity or peripheral stimulation (e.g., An, Kilb, & Luhmann, 2014; Mohns & Blumberg, 2010; Tiriac, Del Rio-Bermudez, & Blumberg, 2014; Tiriac, Uitermarkt, Fanning, Sokoloff, & Blumberg, 2012; Yang, Hanganu-Opatz, Sun, & Luhmann, 2009).
There are other advantages to the head-fix method, some which have not yet been fully exploited. For example, for some methods like 2-photon imaging (Golshani et al., 2009) and voltage-sensitive dye imaging (McVea, Mohajerani, & Murphy, 2012; Tiriac et al., 2012), and for studies using genetically modified mice, there may be no practical alternative to head restraint for studying brain activity in unanesthetized pups.
The many benefits of head restraint have inspired its use in adult rats and mice (e.g., Andermann, Kerlin, & Reid, 2010; Boissard, Fort, Gervasoni, Barbagli, & Luppi, 2003; Bryant, Roy, & Heck, 2009; Dombeck, Khabbaz, Collman, Adelman, & Tank, 2007; Komiyama et al., 2010; Zagha, Casale, Sachdev, McGinley, & McCormick, 2013). In adults, however, several days of recovery from surgery and several more days or weeks of daily habituation sessions are required before experimental testing can begin. In contrast, infant rats and mice recover from surgery and habituate to head restraint very quickly. For example, in our experiments, pups exhibit organized sleep-wake cycles within 2–3 hr from the end of surgery and tolerate the head restraint for at least 4–8 hr after that.
In the next section, we provide a step-by-step description of our method of recording brain activity in head-fixed infant rats and mice. We focus here on our own method, which was devised with an eye toward enabling the recording and manipulation of activity from any brain region in an unanesthetized pup exhibiting regular sleep-wake cycles with coherent expression of behavioral and electrographic measures. It should be noted, however, that other investigators have created their own methods for answering the questions of interest to them; the reader may wish to compare methods across laboratories to identify the most appropriate one for any given project.
RECORDING BRAIN ACTIVITY IN HEAD-FIXED NEWBORN RODENTS: A PRIMER
Using our head-fix method, we can easily record from P1 to P12 rats, although pups can be tested at later ages with appropriate modifications to the method and apparatus. Mice can also be tested through at least P12; however, they are much smaller than rats, thus posing additional challenges for maintenance of brain temperature and recording from deep brain areas.
The Head-Fix Device
We build the head-fix device using a large metal washer and associated materials (Fig. 1; Table 2). The size of the washer is determined by the age and size of the infant subject. Metal strips are glued or welded to opposite sides of the washer and bolts are inserted through holes drilled through the metal strips. Nuts are screwed in place and glue is applied for added stability. Each device can be cleaned and reused many times.
FIGURE 1.
Constructing a head-fix device for infant rats and mice. Left: Materials needed to construct the device, including a large metal washer (A), metal strips (B), bolts (C), small metal washers (D), and nuts (E). The part numbers, dimensions, and prices of each part are provided in Table 2. The dimensions indicated for the large washer and metal strips are appropriate for use with 6- to 8-day-old rats. Right: Step-by-step method for constructing the device. (1) A hole is drilled at one end of each metal strip. The location of the hole determines the elevation of the pup’s head. (2) The end the strip containing the hole is bent 90° using flat-nose pliers. These operations must be performed identically on the two metal strips. (3) A bolt is secured with a washer (D) and nut (E) in each metal strip. The bolt should be further secured using cyanoacrylate adhesive. (4) The two metal strips are glued to the top of the washer. When performing this step, the bolts attached to each metal strip should be accurately aligned.
Table 2.
Materials for Constructing the Head-Fix Device and Associated Costs
| Component | Source | Quantity/Price | Cost/Device |
|---|---|---|---|
| 18-8 stainless steel washer; ID: 15/16—; OD: 1/2—; thickness: 0.03–0.07— | McMaster Carr | 100/$3.89 | $0.08 |
| 18-8 stainless steel washer; for No. 0 screw size, ID 1/16—; OD: 5/32— | McMaster Carr | 100/$1.27 | $0.03 |
| 420 stainless steel hardened 6— × 6— sheet; thickness: 0.018— | McMaster Carr | 1/$48.24 | $1.34 |
| 18-8 Stainless steel slotted flat machine bolts, 2/56— × 5/16— | McMaster Carr | 100/$3.60 | $0.08 |
| Plain 18-8 stainless steel nuts | McMaster Carr | 100/$2.77 | $0.06 |
| Total price per device: | $1.59 |
These materials are associated with those illustrated in Figure 1.
The dimensions indicated for the large washer are appropriate for use with 6- to 8-day-old rats.
The threaded ends of the bolts are sized to screw into the ends of the ear bars that, in turn, are secured to the stereotaxic apparatus. When fixed in place, the device is sufficiently rigid such that the pup’s head is stably secured and movement artifacts in the neurophysiological record are minimal or absent. Depending on the target location in the brain, parts of the metal washer can be cut away to allow better access to the skull. Also, for recordings with infant mice, the device can be fashioned from lightweight but hard plastic instead of metal with little sacrifice in stability. Alternatively, using a 3-D printer, it is now possible to produce stiff, lightweight head-fix devices of any shape from a single piece of plastic.
Equipment and Apparatus
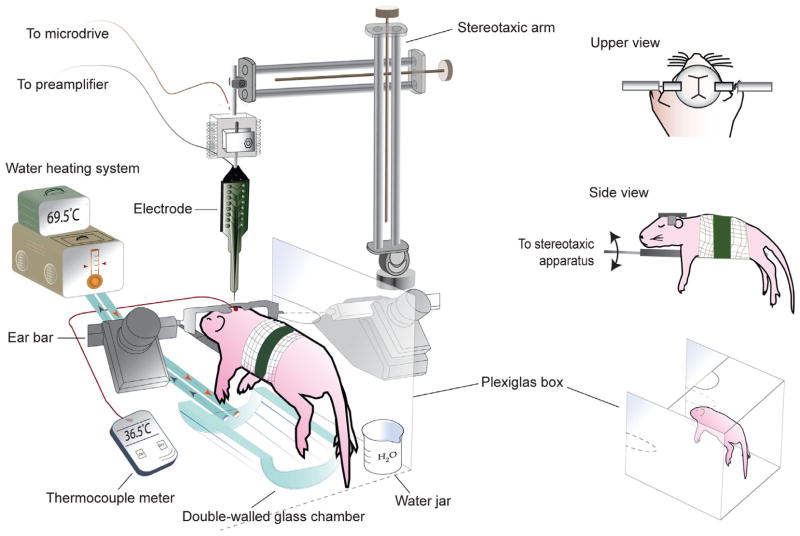
The basic experimental set-up is illustrated in Figure 2. It consists of a standard stereotaxic apparatus supported on a low-vibration table. To remove electrical noise, the stereotaxic apparatus sits inside a Faraday cage and all electrical equipment and metal devices inside the cage are grounded. The thermal environment is controlled using a small custom-blown glass chamber through which temperature-controlled water is circulated; we use circulating water to prevent electrical noise, but low-noise electrically heated mats can also be used. The chamber is placed beneath the pup during testing. A small jar of water is placed in proximity to the pup and a Plexiglas box around the pup helps to maintain temperature and humidity.
FIGURE 2.
Recording set-up for head-fixed infant rats and mice. The head-fixed pup is secured on an elevated platform by applying tape around the gauze-wrapped torso and the platform. Ear bars are attached to the head-fix device and are secured to the stereotaxic apparatus. The double-walled glass chamber, through which temperature-controlled water is circulated, is positioned under the pup. To monitor temperature, a thermocouple is inserted through pre-drilled hole in the skull distant from the recording site. A Plexiglas box surrounds the pup on three sides to help maintain air temperature. A water-filled jar near the pup helps to maintain adequate humidity. An electrode, attached to a headstage on a stereotaxic arm, can be lowered into the brain via a pre-drilled hole using a microdrive.
For neurophysiological recordings, electrodes connect through a headstage to a preamplifier, also located within the Faraday cage. All electrographic signals are then channeled to a data acquisition system. For some studies where precise behavioral or kinematic measures are needed, a video camera can be used to capture data in synchrony with the electrographic signals.
Preparation of the Subject
On the day of testing, an infant rat or mouse is removed from the home cage. Pups with visible milk bands and of the appropriate weight for their age are chosen to ensure adequate health, nutrition, and hydration. Depending on the length of the experiment, pups can be intubated with commercial half-and-half (50% cream–50% milk) or milk formula (3% of body weight in milliliters). We have found that P8 rats do not exhibit substantial changes in either heat production or sleep-wake durations even after 8 hr of separation from the mother (Seelke & Blumberg, 2005).
The pup is placed in an induction chamber filled with isoflurane (3–5%). When anesthesia is induced, the pup is removed from the chamber and placed in a prone position with an anesthesia mask over its snout; for maintenance of anesthesia, the concentration of isoflurane is reduced to 2–3%. The pup is kept warm throughout surgery using a heating pad and the pup is continually monitored to ensure that it is breathing regularly and pedal or pinnal reflexes are absent.
When appropriate, 27 g sterile needles are used to insert bipolar hook electrodes into select skeletal muscles for measurement of electromyographic (EMG) activity. These electrodes connect to a bank of micro-grabbers located inside the Faraday cage. A silver ground electrode is looped through the skin on the back. All EMG and ground electrodes are secured to the skin with collodion. Next, the skin overlying the skull is removed using surgical scissors or a scalpel. The exposed skull is cleaned and dried using either a hydrogen peroxide or bleach solution. Bupivacaine is applied topically to the wound for pain control and, for general analgesia, the pup is administered a subcutaneous injection of an NSAID (e.g., Rymadyl; 5 mg/kg). Gauze is then wrapped around the torso and secured with tape, leaving the limbs outside of the wrap. For some experiments where stereotaxic precision is not required (e.g., for cortical or hippocampal targets), it is possible to drill holes through the skull at this stage.
Cyanoacrylate adhesive gel is applied to the bottom of the metal washer approximately 15 min before it is fixed to the skull. When applied, the gel should be tacky. Before attaching the device to the skull, the application of Vetbond (3M, St Paul, MN) or superglue to the region around the skull and beneath the surface of the surrounding skin helps to ensure a strong attachment of the head-fix device to the skull. Before positioning on the skull, the metal washer must be aligned so that the midline of the skull matches the midline of the washer. Then, the device is gently placed on the skull and light pressure is applied for 5–10 s. Once attached to the skull, liquid cyanoacrylate adhesive can also be applied to the inside circumference of the washer to reinforce adhesion. When the surgery is complete, the pup’s body and limbs are wrapped temporarily in a second layer of gauze to restrain all movement while the pup recovers in an incubator maintained at thermoneutrality (e.g., 35°C for a P8 rat) for approximately 1 hr. The entire surgery, from induction to placement in the incubator, typically takes 10–15 min.
After 1 hr in the incubator, by which time the head-fix device should be fully secure, the pup is anesthetized again (if necessary) to prepare the skull for neurophysiological recording. To do this, the head-fix device is secured in a neonatal rat adaptor (Stoelting, Wood Dale, IL) and the pup’s skull is leveled. Stereotaxic coordinates are then used to identify the exact locations for drilling. At this time, cannulae can be stereotaxically inserted for subsequent delivery of drugs or, alternatively, chemical or electrical lesions can be produced. If the experiment involves imaging, a craniotomy window is produced, with special care being taken not to damage the dura (e.g., Golshani et al., 2009; McVea et al., 2012; Tiriac et al., 2012). This part of the procedure typically requires 10 min to complete.
When the pup is ready for transfer, the second layer of gauze is removed and the pup is placed atop a metal bar and supporting platform (Fig. 2). The pup is secured to the bar using a piece of tape wrapped around the pup and platform. The rod that is attached to the platform inserts into the nose-piece holder of the stereotaxic apparatus, allowing for height and angle adjustments to the pup’s head and body. Each ear bar is attached to the bolts at the end of the head-fix device, and the ear bars are secured to the stereotaxic apparatus. At this point, the height and angle of the pup’s head is adjusted in relation to the torso to ensure maximal comfort. The pup’s lower jaw should rest on the platform. When this process is complete, the pup should appear comfortable with its legs dangling on either side of the platform.
A Plexiglas three-walled compartment with an open top is designed and built to fit inside the arms of the stereotaxic apparatus to surround the pup (Fig. 2). This compartment helps to isolate the pup from cool room air and directs heat from the heated glass chamber toward the pup. A small glass jar filled with water helps to prevent the air from becoming too dry within the compartment.
To measure brain temperature, we use a chromel-constantan thermocouple (Type E; #TT-E-40, Omega Engineering, Stamford, CT) and a thermocouple meter (450-AET). The thermocouple is inserted into a hole in the skull distant from the brain area of interest for the experiment. The thermocouple can remain in the brain for the duration of the experiment, but the meter should be turned off when data are recorded. For neurophysiological recordings, we maintain brain temperature at 36–37°C. To do this, and depending on the pup’s age and distance from the heat source, we set the water bath temperature at 40–65°C. Occasionally, and only for brief durations, a heat lamp located outside the Faraday cage can be used to help elevate brain temperature; however, the lamp should never be aimed directly at the pup.
Experimental Testing
Once positioned in the stereotaxic apparatus, we wait at least 1 hr for the pup to recover from anesthesia, habituate to the head-fix condition, and achieve a stable temperature. A fully recovered and well-acclimated pup exhibits the following characteristics: First, the nuchal EMG will exhibit clearly alternating periods of atonia and high muscle tone (see Fig. 3); long periods of intermediate muscle tone indicate a pup that is still recovering from anesthesia. Second, myoclonic twitches of the limbs and tail occur and are produced exclusively against a background of muscle atonia; conversely, high-amplitude wake movements are coincident with periods of high muscle tone. Third, the pup’s breathing is regular during periods of behavioral quiescence. If the pup exhibits full-body movements with each inspiration and a burst of activity is observed in the nuchal EMG, this can be a sign of breathing difficulties. It is also important to note that audible vocalizations can occasionally occur during periods of wakefulness, but they are very rare; long and persistent bouts of vocalization signify a pup that is physically uncomfortable. Under such conditions, the experimenter should check to ensure that the pup is not overheated, that the air is not too dry, and that the head is comfortably situated in relation to the rest of the body.
FIGURE 3.
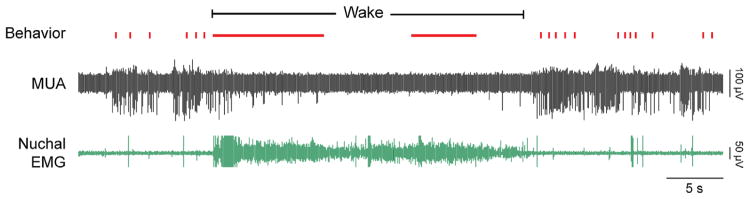
Representative behavioral and electrophysiological records in a head-fixed week-old rat. The figure depicts nuchal EMG (green), multiunit activity (MUA, black), and behavior (red; vertical ticks: myoclonic twitches; horizontal lines: wake-related movements). Note the increased nuchal muscle tone during wake and the preceding and following periods of muscle atonia; the EMG spikes that occur against a background of atonia are twitches. (Adapted from Tiriac et al. (2014). Self-generated movements with “unexpected” sensory consequences. Current Biology, 24, 2136–2141, with permission.)
At this stage the experiment can begin. In a typical experiment, we again level the skull. Then, we start the process of inserting an electrode through a pre-drilled hole in the skull to a desired location. For many of the electrodes that we use, we first coat the electrode with fluorescent DiI (Life Technologies, Grand Island, NY) using a fine brush or by dipping the electrode in the dye; this method permits histological verification of electrode location within the brain. We use a pneumatic drum drive (FHC, Inc., Bowdoin, ME) to lower the electrode slowly and with micrometer precision. We monitor neural activity throughout the process of lowering the electrode. Once we have arrived at the desired location—as determined stereotaxically or based on the pattern of neural activity observed—we allow the electrode to stabilize within the brain tissue for at least 10 min before commencing with data acquisition. During data acquisition, and in addition to EMG and neurophysiological data, we code behavior and/or record digital video in synchrony with the electrographic data. A brief artifact-free recording of multinunit activity, nuchal EMG, and behavior in a week-old head-fixed rat is shown Figure 3.
COMMON PROBLEMS AND TROUBLESHOOTING
Electrical Noise
Electrical noise is a perennial problem in neurophysiology. In our set-up, most electrical noise is removed by using a grounded Faraday cage. The only devices that should be placed inside the Faraday cage are the stereotaxic apparatus, a battery-powered thermocouple meter and pre-amplifier, the double-walled glass chamber, and the Plexiglas box. The thermocouple meter can be a source of noise, but only when turned on. All other electrical appliances (e.g., heat lamps, computers, monitors, phones) should be positioned outside the Faraday cage or removed from the room altogether. Inadequate grounding of the animal can also cause problems with noise. Finally, dry room air increases static electricity that, in turn, increases the occurrence of electrical noise, especially when recording local field potentials; increasing humidity in the room to 20–40% helps to reduce this problem.
Mechanical Vibration and Movement Artifact
Mechanical vibrations in the recording apparatus can lead to artifacts in the electrophysiological data. One main source of mechanical vibration is the water circulating through the double-walled glass chamber. All air bubbles should be removed from the closed system and appropriately sized tubing should link the water circulator to the water heater to minimize turbulent flow. Mechanical vibration can also arise from unsecured EMG electrode wires connected to the pup that move as the pup breathes or twitches. Securing loose wires with tape to a stable platform usually resolves this problem.
Strict adherence to the procedures described here typically result in artifact-free recordings from the forebrain and midbrain. However, hindbrain recordings are more prone to movement artifact, especially when the nuchal muscle, which attaches to the occipital bone, is active. To resolve this, we perform an additional surgical step to detach the nuchal muscle from its anchor point to the skull. This can be done during surgery via blunt dissection using a pair of small forceps or with a cautery. There are two anchor points, one on each side of the skull, and both should be severed to minimize movements of the occipital bone. If movement artifacts continue to occur, a drop of Vetbond can be applied to the occipital bone to harden it.
The Head-Fix Device Does Not Adhere to the Skull
If the skull is not adequately cleaned and dried before the head-fix device is attached, problems with adherence may occur. When performing the surgery, all connective tissue should be removed and the skull thoroughly cleaned with bleach or hydrogen peroxide. This aspect of the procedure is crucial, as inadequate cleaning and drying of the skull will result in a weak bond with the device and possible failure of attachment during data acquisition.
Distress
Any experimental procedure involving restraint requires special attention to ensure that pain and distress are minimized. Such attention is necessary as an ethical obligation by the experimenters toward their animal subjects, as well as to ensure that reliable data are produced. For head-restraint procedures in adult rats and mice, repeated habituation is used to minimize distress. Such habituation procedures are not necessary in infant subjects, and it is worth detailing how it is that we know that they are not necessary. First, under our testing conditions, newborn rats sleep as much as or more than unrestrained pups; based on EMG activity and associated behavior, the sleep states of head-fixed pups are organized similarly to freely moving pups (although bout lengths may be altered), with twitches occurring exclusively against a background of muscle atonia and wake movements occurring exclusively against a background of high muscle tone. Second, restrained pups rarely exhibit signs of distress, such as struggling limb movements or audible vocalizations.
Nonetheless, a head-fixed pup may occasionally exhibit signs of distress and its behavior provides vital clues to the experimenter for resolving them. When a pup exhibits prolonged struggling limb movements when awake or vocalizes audibly for extended periods of time, temperature and humidity should immediately be checked to ensure that the pup is neither overheated nor dry. Also, the experimenter should ensure that the head and neck are comfortably positioned in relation to the body.
Breathing complications can occur, especially in pups younger than 10 days of age. The causes of abnormal breathing patterns include uncomfortable positioning of the body in relation to the head and gauze wrapped too tightly around the torso. Also, because pups can only urinate reflexively upon mechanical stimulation of the anogenital region, a full bladder can occur over the course of an extended experiment, resulting in significant behavioral activation. The experimenter can resolve this issue by gently stimulating the anogenital region with a cotton-tipped applicator. Importantly, when signs of distress cannot be resolved, the experiment should be terminated and the pup euthanized.
LIMITATIONS OF THE HEAD-RESTRAINT METHOD
We have discussed here the numerous advantages of head restraint for investigating neural circuitry and brain–behavior interactions in newborn rats and mice. Nonetheless, the limitations of this experimental approach should also be acknowledged. Perhaps most obviously, pups in these experiments are separated for prolonged periods from their natural habitat, which comprises their mother and littermates and the tactile, olfactory, and thermal features of that habitat (Alberts, Blass, & Cramer, 1988). Head-restrained pups cannot produce over-ground locomotion or regulate their temperature behaviorally through huddling. However, we can envision future modifications to the head-fix procedure in which various features of the home-cage environment are reintroduced. For example, as has been done with adult head-fixed mice, locomotion can be studied in a head-fixed subject by situating it atop an air-supported Styrofoam ball (Dombeck et al., 2007). Similarly, just as investigators can investigate the neural correlates of licking in head-restrained adult mice (Bryant et al., 2009), it should be possible to study the neural correlates of suckling and associated behaviors in infants (Westneat & Hall, 1992). Nonetheless, the very nature of head fixation necessary limits the degree to which concerns about ecological and ethological validity can be fully alleviated. Ultimately, as with any experimental method, investigators must weigh the relative costs and benefits of the head-fix method in relation to the scientific questions they wish to answer.
CONCLUDING THOUGHTS AND FUTURE DIRECTIONS
Until now, rats have been the predominant species of choice, but it is likely that mice will become increasingly popular in the coming years, for several reasons. First, genetically modified mice can be used to test specific hypotheses in ways that do not require surgical or pharmacological manipulation, an advantage that is particularly useful when performing experiments on newborns. For example, to test the hypothesis that proprioceptors provide sensory feedback from twitching limbs to the sleeping brain, we recorded neural activity in cerebellar cortex of infant ErbB2 knockout mice, which fail to develop muscle spindles (Uitermarkt, Sokoloff, Weiner, Fritzsch, & Blumberg, 2013).
Second, genetically modified mice are proving very useful for imaging neural activity, primarily in superficial layers of cerebral cortex. Initially, voltage-sensitive or calcium dyes were applied topically to the surface of the brain, loaded into cells, or virally transduced. These methods are technically challenging and time consuming; perhaps most critically for developmental studies, viral transduction is too slow to make it useful for investigating newborns. In contrast, a growing array of genetically modified mice allow direct fluorescent imaging of neural activity without any surgical preparation; genetically encoded calcium indicators are but one example (Zariwala et al., 2012). With these mice, investigators can investigate neural activity locally or globally using 2-photon or wide-field imaging, respectively.
Finally, optogenetic manipulation of neurons provides specificity and temporal precision that is lacking using more conventional methods. Viral transduction of opsin genes has been the standard method used in adults, but again the time required for viral expression and transfer to target sites places severe limitations on the use of optogenetics in newborns. It is now possible to circumvent these limitations using mice engineered to express opsins in a Cre-dependent manner in specific brain regions (Madisen et al., 2012).
Thus far, the head-fix preparation has been used primarily to investigate spontaneous neural activity across sleep and wakefulness, activity driven by or evoked by stimulation of the sensory periphery (e.g., limb, whisker, retina), or intrinsic network activity. But so much more can be done. With some modifications and additions to the basic head-fix method, it should be possible to explore brain-behavior relations in other experimental contexts to address issues that have formed the traditional core of developmental psychobiology, including suckling and feeding (Hall & Williams, 1983; Teicher & Blass, 1977), learning and memory (Flory, Langley, Pfister, & Alberts, 1997; Freeman & Nicholson, 2000; Johanson & Hall, 1979; Moran, Lew, & Blass, 1981), vocalization (Blumberg, Efimova, & Alberts, 1992; Oswalt & Meier, 1975), behavioral and physiological thermoregulation (Alberts, 1978; Blumberg, 2001), olfaction and olfactory learning (Brunjes & Alberts, 1979; Sullivan, Wilson, & Leon, 1989), and the effects of isolation (Hofer & Shair, 1991; Levine, Huchton, Wiener, & Rosenfeld, 1992) and maternal licking and grooming (Francis, Diorio, Liu, & Meaney, 1999; Moore, 1995) on development. Moreover, the head-fix method can be used to study pathophysiological processes, including neonatal seizures and spasms (Baram, 1993; Jensen, 2009; Mohns, Karlsson, & Blumberg, 2007; Schuchmann et al., 2006). By applying state-of-the-art neurophysiological methods to these and other contexts, we can expand and potentially reshape our understanding of neural circuit function, formation, and plasticity in typically and atypically developing animals.
Acknowledgments
Contract grant sponsor: NIH
Contract grant numbers: HD81168, HD63071
NOTES
Preparation of this paper was made possible by NIH grants (R37-HD81168, R01-HD63071) to M.S.B. The Fulbright Foreign Student Program supported C.D.R.-B.
References
- Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts JR. Huddling by rat pups: Group behavioral mechanisms of temperature regulation and energy conservation. Journal of Comparative and Physiological Psychology. 1978;92:231–245. doi: 10.1037/h0077459. [DOI] [PubMed] [Google Scholar]
- Alberts JR, Blass E, Cramer C. Ecology and experience, sources of means and meaning of developmental change. In: Blass EM, editor. Handbook of behavioral neurobiology. Vol. 8. New York: Handbook of Behavioral Neurobiology; 1988. pp. 1–39. [Google Scholar]
- An S, Kilb W, Luhmann HJ. Sensory-evoked and spontaneous gamma and spindle bursts in neonatal rat motor cortex. Journal of Neuroscience. 2014;34:10870–10883. doi: 10.1523/JNEUROSCI.4539-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Yang JW, Sun H, Kilb W, Luhmann HJ. Long-term potentiation in the neonatal rat barrel cortex in vivo. Journal of Neuroscience. 2012;32:9511–9516. doi: 10.1523/JNEUROSCI.1212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Reid RC. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Frontiers in Cellular Neuroscience. 2010;4:1–16. doi: 10.3389/fncel.2010.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram T. Pathophysiology of massive infantile spasms: Perspective on the putative role of the brain adrenal axis. Annals of Neurology. 1993;33:231–236. doi: 10.1002/ana.410330302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes TL, Langston RF, Kruge IU, Moser EI, Moser MB. Coherence among head direction cells before eye opening in rat pups. Current Biology. 2014;25:1–9. doi: 10.1016/j.cub.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS. The developmental context of thermal homeostasis. In: Blass EM, editor. Handbook of developmental neurobiologyy. Vol. 13. New York: Plevin Press; 2001. pp. 199–228. [Google Scholar]
- Blumberg MS. Beyond dreams: Do sleep-related movements contribute to brain development? Frontiers in Neurology. 2010;1:140. doi: 10.3389/fneur.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Efimova I, Alberts JR. Ultrasonic vocalizations by rats pups: The primary importance of ambient temperature and the thermal significance of contact comfort. Developmental Psychobiology. 1992;25:229–250. doi: 10.1002/dev.420250402. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Freeman JH, Robinson SR, editors. Oxford handbook of developmental behavioral neuroscience. New York: Oxford University Press; 2010. [Google Scholar]
- Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Current Biology. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. European Journal of Neuroscience. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Brockmann MD, Poschel B, Cichon N, Hanganu-Opatz IL. Coupled oscillations mediate directed interactions between prefrontal cortex and hippocampus of the neonatal rat. Neuron. 2011;71:332–347. doi: 10.1016/j.neuron.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Brunjes P, Alberts JR. Olfactory stimulation induces filial preferences for huddling in rat pups. Journal of Comparative and Physiological Psychology. 1979;93:548–555. doi: 10.1037/h0077571. [DOI] [PubMed] [Google Scholar]
- Bryant JL, Roy S, Heck DH. A technique for stereotaxic recordings of neuronal activity in awake, head-restrained mice. Journal of Neuroscience Methods. 2009;178:75–79. doi: 10.1016/j.jneumeth.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT. Rapid developmental emergence of stable depolarization during wakefulness by inhibitory balancing of cortical network excitability. Journal of Neuroscience. 2014;34:5477–5485. doi: 10.1523/JNEUROSCI.3659-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, Khazipov R. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67:480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Khazipov R. “Slow activity transients” in infant rat visual cortex: A spreading synchronous oscillation patterned by retinal waves. Journal of Neuroscience. 2010;30:4325–4337. doi: 10.1523/JNEUROSCI.4995-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner M, Bour H. Postnatal development of spontaneous neuronal discharges in the pontine reticular formation of free-moving rats during sleep and wakeful-ness. Experimental Brain Research. 1984;54:66–72. doi: 10.1007/BF00235819. [DOI] [PubMed] [Google Scholar]
- Corner M, Kwee P. Cyclic EEG and motility patterns during sleep in restrained infant rats. Electroencephalography and Clinical Neurophysiology. 1976;41:64–72. doi: 10.1016/0013-4694(76)90215-7. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron. 2007;56:43–57. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory G, Langley C, Pfister J, Alberts JR. Instrumental learning for a thermal reinforcer in 1-day-old rats. Developmental Psychobiology. 1997;30:41–47. doi: 10.1002/(sici)1098-2302(199701)30:1<41::aid-dev4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney M. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Frank M, Heller H. Development of REM and slow wave sleep in the rat. American Journal of Physiology. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Nicholson DA. Developmental changes in eye-blink conditioning and neuronal activity in the cerebellar interpositus nucleus. Journal of Neuroscience. 2000;20:813–819. doi: 10.1523/JNEUROSCI.20-02-00813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshani P, Goncalves JT, Khoshkhoo S, Mostany R, Smirnakis S, Portera-Cailliau C. Internally mediated developmental desynchronization of neocortical network activity. Journal of Neuroscience. 2009;29:10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen A. The development of the EEG in the rat. Developmental Psychobiology. 1976;9:501–515. doi: 10.1002/dev.420090604. [DOI] [PubMed] [Google Scholar]
- Hall WG, Williams CL. Suckling isn’t feeding, or is it? A search for developmental continuities. Advances in the Study of Behavior. 1983;13:219–254. [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. Journal of Neuroscience. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu-Opatz IL. Between molecules and experience: Role of early patterns of coordinated activity for the development of cortical maps and sensory abilities. Brain Research Reviews. 2010;64:160–176. doi: 10.1016/j.brainresrev.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Hofer M, Shair H. Trigeminal and olfactory pathways mediating isolation distress and companion comfort responses in rat pups. Behavioral Neuroscience. 1991;105:699–706. doi: 10.1037//0735-7044.105.5.699. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Neonatal seizures: An update on mechanisms and management. Clinics in Perinatology. 2009;36:881–900. doi: 10.1016/j.clp.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson I, Hall W. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Developmental Psychobiology. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130:275–283. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KA, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biology. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KA, Mohns EJ, Vianna di Prisco G, Blumberg MS. On the co-occurrence of startles and hippocampal sharp waves in newborn rats. Hippocampus. 2006;16:959–965. doi: 10.1002/hipo.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Buzsáki G. Early patterns of electrical activity in the developing cortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford handbook of developmental behavioral neuroscience. New York: Oxford University Press; 2010. pp. 161–177. [Google Scholar]
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends in Neurosciences. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O’Connor DH, Zhang YX, Huber D, Hooks BM, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser MB. Development of the spatial representation system in the rat. Science. 2010;328:1576–1580. doi: 10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsáki G. Correlated bursts of activity in neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- Levine S, Huchton D, Wiener S, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Developmental Psychobiology. 1992;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature Neuroscience. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcano-Reik AJ, Blumberg MS. The corpus callosum modulates spindle-burst activity within homotopic regions of somatosensory cortex in newborn rats. European Journal of Neuroscience. 2008;28:1457–1466. doi: 10.1111/j.1460-9568.2008.06461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcano-Reik AJ, Prasad T, Weiner JA, Blumberg MS. An abrupt developmental shift in callosal modulation of sleep-related spindle bursts coincides with the emergence of excitatory-inhibitory balance and a reduction of somatosensory cortical plasticity. Behavioral Neuroscience. 2010;124:600–611. doi: 10.1037/a0020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVea DA, Mohajerani MH, Murphy TH. Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat. Journal of Neuroscience. 2012;32:10982–10994. doi: 10.1523/JNEUROSCI.1322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel GF, Moore C. Developmental psychobiology: An interdisciplinary science. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. Journal of Neurophysiology. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. NMDA receptors pattern early activity in the developing barrel cortex in vivo. Cerebral Cortex. 2009;19:688–696. doi: 10.1093/cercor/bhn115. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R. Early gamma oscillations synchronize developing thalamus and cortex. Science. 2011;334:226–229. doi: 10.1126/science.1210574. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Corner M. Neuronal discharge patterns in the occipital cortex of developing rats during active and quiet sleep. Brain Research. 1982;255:37–48. doi: 10.1016/0165-3806(82)90074-8. [DOI] [PubMed] [Google Scholar]
- Mitrukhina O, Suchkov D, Khazipov R, Minlebaev M. Imprecise whisker map in the neonatal rat barrel cortex. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu169. [DOI] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms. Journal of Neuroscience. 2008;28:10134–10144. doi: 10.1523/JNEUROSCI.1967-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. Journal of Neuroscience. 2010;30:3438–3449. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, Karlsson KA, Blumberg MS. The preoptic hypothalamus and basal forebrain play opposing roles in the descending modulation of sleep and wakeful-ness in infant rats. European Journal of Neuroscience. 2006;23:1301–1310. doi: 10.1111/j.1460-9568.2006.04652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohns EJ, Karlsson KA, Blumberg MS. Developmental emergence of transient and persistent hippocampal events and oscillations and their association with infant seizure susceptibility. European Journal of Neuroscience. 2007;26:2719–2730. doi: 10.1111/j.1460-9568.2007.05928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. Maternal contributions to mammalian reproductive development and the divergence of males and females. Advances in the Study of Behavior. 1995;24:47–118. [Google Scholar]
- Moran T, Lew M, Blass E. Intracranial self-stimulation in 3-day-old rat pups. Science. 1981;214:1366–1368. doi: 10.1126/science.6975999. [DOI] [PubMed] [Google Scholar]
- Oswalt G, Meier G. Olfactory, thermal, and tactual influences on infantile ultrasonic vocalization in rats. Developmental Psychobiology. 1975;8:129–135. doi: 10.1002/dev.420080205. [DOI] [PubMed] [Google Scholar]
- Sarro EC, Wilson DA, Sullivan RM. Maternal regulation of infant brain state. Current Biology. 2014;24:1664–1669. doi: 10.1016/j.cub.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchmann S, Schmitz D, Rivera C, Vanhatalo S, Salmen B, Mackie K, Kaila K. Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nature Medicine. 2006;12:817–823. doi: 10.1038/nm1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. Thermal and nutritional modulation of sleep in infant rats. Behavioral Neuroscience. 2005;19:603–611. doi: 10.1037/0735-7044.119.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke AMH, Blumberg MS. Developmental appearance and disappearance of cortical events and oscillations in infant rats. Brain Research. 2010;1324:34–42. doi: 10.1016/j.brainres.2010.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff G, Uitermarkt BD, Blumberg MS. REM sleep twitches rouse nascent cerebellar circuits: Implications for sensorimotor development. Developmental Neurobiology. 2014 doi: 10.1002/dneu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Wilson D, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. Journal of Neuroscience. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamásy VV, Korányi LL. Multiunits in the mesencephalic reticular formation: Ontogenetic development of wakefulness and sleep cycle in the rat. Neuroscience Letters. 1980;17:143–147. [PubMed] [Google Scholar]
- Tamásy V, Korányi L, Lissák K. Multiunit activity in the mesencephalic reticular-formation and septal area of freely moving newborn rat. Brain Research Bulletin. 1979;4:715–719. doi: 10.1016/0361-9230(79)90002-9. [DOI] [PubMed] [Google Scholar]
- Tamásy V, Korányi L, Lissák K. Early postnatal development of wakefulness-sleep cycle and neuronal responsiveness: A multiunit activity study on freely moving newborn rat. Electroencephalography and Clinical Neurophysiology. 1980;49:102–111. doi: 10.1016/0013-4694(80)90356-9. [DOI] [PubMed] [Google Scholar]
- Teicher M, Blass E. First suckling response of the newborn albino rat: The roles of olfaction and amniotic fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-generated movements with “unexpected” sensory consequences. Current Biology. 2014;24:2136–2141. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Current Biology. 2012;22:2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. Journal of Neuroscience. 2012;32:692–702. doi: 10.1523/JNEUROSCI.1538-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitermarkt BD, Sokoloff G, Weiner JA, Fritzsch B, Blumberg MS. Newborn mice lacking muscle spindles exhibit reduced twitch-related Purkinje cell activity during active sleep. San Diego, CA: Society for Neuroscience; 2013. [Google Scholar]
- Westneat MW, Hall WG. Ontogeny of feeding motor patterns in infant rats: An electromyographic analysis of suckling and chewing. Behavioral Neuroscience. 1992;106:539–554. doi: 10.1037//0735-7044.106.3.539. [DOI] [PubMed] [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, O’Keefe J. Development of the hippocampal cognitive map in pre-weanling rats. Science. 2010;328:1573–1576. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, An S, Sun JJ, Reyes-Puerta V, Kindler J, Berger T, Luhmann HJ. Thalamic network oscillations synchronize ontogenetic columns in the newborn rat barrel cortex. Cerebral Cortex. 2012;23:1299–1316. doi: 10.1093/cercor/bhs103. [DOI] [PubMed] [Google Scholar]
- Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. Journal of Neuroscience. 2009;29:9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagha E, Casale AE, Sachdev RNS, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–578. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. Journal of Neuroscience. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]