Abstract
Aims
To measure the prevalence and correlates of nicotine replacement therapy (NRT) use for reasons other than quitting smoking among smokers in four countries.
Design and setting
Population-based, cross-sectional telephone survey with nationally representative samples of adult smokers in Canada, the United States, the United Kingdom and Australia, conducted in 2005.
Participants
A total of 6532 adult daily smokers in Canada (n = 1660), the United States (n = 1664), the United Kingdom (n = 1617) and Australia (n = 1591).
Measurements
Survey questions included demographics, smoking behaviour, use of NRT and reasons for NRT use, as well as access and availability of NRT.
Findings
Approximately 17% of smokers surveyed had used NRT in the past year. Among NRT users, approximately one-third used NRT for a reason other than quitting smoking, including temporary abstinence or reducing the number of cigarettes smoked. The prevalence of non-standard NRT use was remarkably consistent across countries. Using NRT for reasons other than quitting was associated with higher education level, heavier smoking, having no quit intentions, having no past-year quit attempts, the type of NRT product used and accessing NRT without a prescription.
Conclusions
The use of NRT for purposes other than quitting smoking is fairly common and may help to explain the difficulty in detecting significant quitting benefits associated with NRT use in population studies. Tobacco control policies, including the accessibility of NRT, may have important implications for patterns of NRT use.
Keywords: Nicotine replacement therapy, smoking cessation, stop smoking medications, tobacco use
INTRODUCTION
Nicotine plays a central role in both the development and treatment of tobacco dependence. Evidence from more than 100 randomized controlled trials indicates that nicotine replacement therapy (NRT) is an effective method for reducing the symptoms of tobacco withdrawal and promoting smoking cessation [1,2]. As a result, various policies have been introduced to promote greater use of NRT, including changes in regulatory status from prescription to over-the-counter (OTC) and subsidization policies to minimize the cost of NRT. These efforts appear to have succeeded in increasing NRT use [3]: in the United States, NRT use increased more than 150% in the year after it became available OTC [4], and NRT use more than tripled in California between 1992 and 1999 [5,6].
Several studies suggest that the increase in population rates of NRT use in the United States may be associated with a decline in its overall efficacy. Population-based studies conducted in California and Massachusetts reported lower cessation rates for NRT users following the switch to OTC status [5,7,8]. Although more recent studies suggest that OTC and prescription NRT have equivalent quit rates [3,9–11], the ‘negative’ findings from California and Massachusetts remain largely unexplained. Unfortunately, relatively few data are available from other countries to examine similar trends.
One potential explanation for the US findings lies in how nicotine replacement therapy is being used by smokers. Several studies have reported that, following the switch to OTC status, many smokers did not use NRT for the recommended duration or in conjunction with counselling [5,7,12]. Smokers may also be more likely to use OTC NRT for reasons other than quitting, such as for reducing, rather than stopping smoking and for temporary abstinence. For example, one study of cessation aids noted that approximately one-fifth of smokers who reported NRT use in the past 6 months did not report a quit attempt for the same period [13]. These types of ‘non-standard’ NRT use might be expected to increase in response to comprehensive work-place smoking restrictions, which have been shown to reduce cigarette consumption [14–16]. To date, however, there is little research examining NRT use for reasons other than quitting, including the effect of OTC status and smoke-free policies.
The current study sought to examine patterns of non-standard NRT use among smokers from four countries—the United States, Canada, the United Kingdom and Australia—using data from the International Tobacco Control (ITC) Four Country Survey, a cohort survey of adult smokers. In particular, the study examined: (i) the proportion of non-standard NRT use; (ii) individual and country-level differences in non-standard use; and (iii) the association between reason for NRT use, access to NRT (prescription versus OTC, payment) and smoking restrictions.
METHODS
The ITC Four Country Survey is a cohort survey conducted every 12 months with adult smokers from Canada, the United States, the United Kingdom and Australia. The ITC survey is designed to evaluate the impact of key national-level tobacco control policies upon behavioural and psychosocial predictors of tobacco use, including tobacco denormalization.
Sample
The current analysis includes adult daily smokers (18 years or older, smoked more than 100 cigarettes in their life, and smoked at least one cigarette per day) from wave 4 of the ITC Four Country Survey conducted in 2005. Participants were 6532 daily smokers across the four countries: Canada (n = 1660), the United States (n = 1664), the United Kingdom (n =1617) and Australia (n = 1591). A complete breakdown of the ITC Four Country sample and characteristics has been published elsewhere [17]. Table 1 shows the characteristics of the sample used in this analysis.
Table 1.
Sample characteristics (n = 6532).
| Canada (n = 1660)
|
United States (n = 1664)
|
United Kingdom (n = 1617)
|
Australia (n = 1591)
|
|||||
|---|---|---|---|---|---|---|---|---|
| % | (n) | % | (n) | % | (n) | % | (n) | |
| Sex | ||||||||
| Male | 42.8 | (710) | 41.0 | (682) | 43.5 | (704) | 44.7 | (711) |
| Female | 57.2 | (950) | 59.0 | (982) | 56.5 | (913) | 55.3 | (880) |
| Age group (years) | ||||||||
| 18–24 | 7.2 | (120) | 6.6 | (110) | 4.6 | (75) | 8.9 | (141) |
| 25–39 | 25.7 | (426) | 22.3 | (371) | 25.7 | (416) | 31.4 | (500) |
| 40–54 | 41.9 | (696) | 38.4 | (639) | 38.1 | (616) | 38.5 | (613) |
| 55+ | 25.2 | (418) | 32.7 | (544) | 31.5 | (510) | 21.2 | (337) |
| Education | ||||||||
| Low | 46.0 | (764) | 42.9 | (714) | 61.3 | (991) | 64.5 | (1026) |
| Moderate | 38.7 | (643) | 39.1 | (651) | 25.1 | (406) | 21.1 | (335) |
| High | 15.2 | (253) | 18.0 | (299) | 13.6 | (220) | 14.5 | (230) |
| Income | ||||||||
| Not stated | 6.6 | (110) | 5.2 | (86) | 7.9 | (128) | 5.3 | (84) |
| Low | 27.5 | (457) | 36.9 | (614) | 31.3 | (506) | 29.9 | (476) |
| Moderate | 35.4 | (588) | 35.3 | (588) | 33.6 | (544) | 32.9 | (523) |
| High | 30.4 | (505) | 22.6 | (376) | 27.1 | (439) | 31.9 | (508) |
| Ethnicity (minority status) | ||||||||
| Identified minority | 8.5 | (141) | 15.4 | (257) | 3.9 | (63) | 11.1 | (177) |
| Other | 91.5 | (1519) | 84.6 | (1407) | 96.1 | (1554) | 88.9 | (1414) |
| Past-year quit attempts | ||||||||
| None | 63.5 | (1054) | 62.7 | (1044) | 61.1 | (988) | 60.8 | (967) |
| At least one | 36.5 | (606) | 37.3 | (620) | 38.9 | (629) | 39.2 | (624) |
| Intentions to quit | ||||||||
| None | 76.3 | (1266) | 68.8 | (1144) | 63.8 | (1031) | 73.8 | (1175) |
| Any | 23.7 | (394) | 31.2 | (520) | 36.2 | (586) | 26.2 | (416) |
| Cigarettes per day | ||||||||
| Mean | 17.1 | (1660) | 18.7 | (1664) | 17.2 | (1617) | 18.0 | (1591) |
| SD | 9.4 | 11.5 | 9.2 | 10.1 | ||||
| HSI | ||||||||
| Mean | 2.8 | (1660) | 2.8 | (1664) | 2.6 | (1617) | 2.7 | (1591) |
| SD | 1.5 | 1.5 | 1.4 | 1.6 | ||||
HSI: Heaviness of Smoking Index; SD: standard deviation.
Procedure
The ITC cohort was constructed from probability sampling methods with telephone numbers selected at random from the population of each country, within strata defined by geographic region and community size. Eligible households were identified by asking a household informant the number of adult smokers. The ‘next birthday method’ [18] was used to select the respondent in households with more than one eligible adult smoker. Respondents lost to attrition at each survey wave were ‘replenished’ using the same sampling methods as at wave 1 recruitment.
The surveys were conducted using computer-assisted telephone interviewing (CATI) software. In order to increase recruitment rates [19], participants were mailed compensation equivalent to USr10 prior to completing the main survey.
The study protocol was cleared for ethics by the institutional review boards or research ethics boards of the University of Waterloo (Canada), Roswell Park Cancer Institute (US), University of Illinois at Chicago (US), University of Strathclyde (UK) and the Cancer Council Victoria (Australia). A full description of the ITC methodology and survey rates is available at http://www.itcproject.org.
Measures
The ITC survey was standardized across the four countries: respondents in each country were asked the same questions, with only minor variations for colloquial language.
Demographics
Age was grouped into four categories: 18–24, 25–39, 40–54 and 55+ years. Level of education consisted of three categories: high school diploma or lower; technical, trade school, community college or some university; and university degree. Annual income was categorized into ‘under $30 000’, ‘$30 000–59 999’ and ‘$60 000 and over’ for the US, Canadian and Australian samples. For the UK sample, we used the following categories: ‘£15 000 or under’, ‘£15 001–30 000’ and ‘£30 001 and over’. Ethnicity was measured using the relevant Census question for each country and then analysed as a dichotomous variable (minority versus not) to allow for comparisons across countries. In Canada, the United States and the United Kingdom, race/ethnicity was classified as ‘white’ versus ‘non-white and mixed race’. In Australia, language was used as a proxy for Australian ethnicity (‘English-speaking’ = white, ‘non-English speaking’ = non-white), as is consistent with the Australian census.
Smoking behaviour
The survey included validated measures of smoking behaviour and quit history. A heaviness-of-smoking index (HSI; range: 0–6) comprised the sum of the scores from two categorical variables: time to first cigarette and cigarettes per day. Time to first cigarette was assigned a value of 0 for >60 minutes, 1 for 31–60 minutes, 2 for 6–30 minutes, or 3 for 5 or fewer minutes, and cigarettes per day was assigned a value of 0 for 0–10, 1 for 11–20, 2 for 21–30 or 3 for >30. Quit attempts were assessed by asking participants whether they had made any attempt to quit smoking since the last survey date, approximately 12 months earlier. Participants were then asked about the duration of the quit attempt and only quit attempts that lasted longer than 24 hours were coded as a quit attempt. Previous quit attempts were coded as a dichotomous variable: 0 = no attempts, 1 = one or more attempts. Intention to quit was assessed by asking: ‘Are you planning to quit in the next month, 6 months, beyond 6 months, or not at all?’, and coded to reflect no plans to quit (0) or plans to quit (1).
NRT use
Respondents were asked to list what, if any, stop-smoking medications they had used in the past year. ‘Any’ NRT use was defined as reporting the use of at least one of the following: nicotine gum, patch, lozenge, tablet, inhaler or spray. Respondents who reported using NRT were asked: ‘What was the main reason you used [NRT type]: to stop smoking completely, to reduce the amount you smoke, to cope with times when you could not or were not allowed to smoke, or some other reason?’. Non-standard NRT use was defined as use for any reason other than to stop smoking completely. NRT users were also asked whether they had obtained the most recent product they had used by prescription, OTC/off the shelf or from a friend, as well as whether they had paid full price for the product. At the time of the survey, nicotine gum and the patch were available OTC in all four countries; nicotine inhalers were OTC in Canada, Australia and the UK, while nicotine lozenges were available OTC in the United States, Australia and the UK.
Smoke-free policies
Respondents were asked to report the rules about smoking indoors in their home, work-place, restaurants and bars. For the current analysis, responses for each setting were coded as ‘completely restricted’ (1) or not (0; including ‘no restrictions’, ‘some restrictions’ and ‘did not work outside the home’).
Analyses
All analyses were conducted using the survey procedures available in SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) to account for the stratified design of the ITC survey. Analyses were also weighted to be representative of age and sex prevalence estimates within geographic strata and to account for survey non-response. χ2 tests were used for between-country comparisons and other categorical variables. Logistic regression was used to model reasons for NRT use (standard versus non-standard use). All odds ratios presented in the Results are adjusted for country, gender, age, ethnicity, income, education, Heaviness of Smoking Index (HSI), quit intentions and past-year quit attempts. Variables such as type of NRT product, OTC versus prescription attainment, paying full price for NRT and the presence of smoking restrictions were added to the regression model in subsequent steps.
RESULTS
Patterns of NRT use
Across all four countries, 17.1% (n =1149) of smokers reported using NRT in the past 12 months. There was a significant overall difference in the prevalence of NRT use by country (χ2 =44.04, P <0.001). Smokers in the United Kingdom were more likely to report using NRT than those in Canada (22.3% versus 16.5%; χ2 =18.7, P <0.001), the United States (13.2%; χ2 =41.1, P <0.001) and Australia (16.3%; χ2 =15.7, P <0.001). Figure 1 shows patterns of NRT use by type of product. The nicotine patch was the most widely used product, reported by 72% of all NRT users in the past year, whereas 31% reported using nicotine gum. Use of the nicotine lozenge or tablet and nicotine inhalers was significantly lower, while use of nicotine spray was less than 1% of NRT users in all countries (data not shown). The use of multiple NRT products in the past year was reported by 13% of respondents across countries. Several between-country differences were observed. NRT users in the United Kingdom were more likely to report using gum than Australia (χ2 = 5.02, P = 0.025), and lozenge use was greater in Australia compared to both Canada (χ2 = 20.32, P < 0.001) and the United Kingdom (χ2 = 5.78, P = 0.016). Lozenge use was also significantly lower in Canada than in the United States (χ2 = 14.82, P < 0.001) and the United Kingdom (χ2 = 11.74, P = 0.001). No significant between-country differences were observed in the use of the nicotine patch or inhaler.
Figure 1.
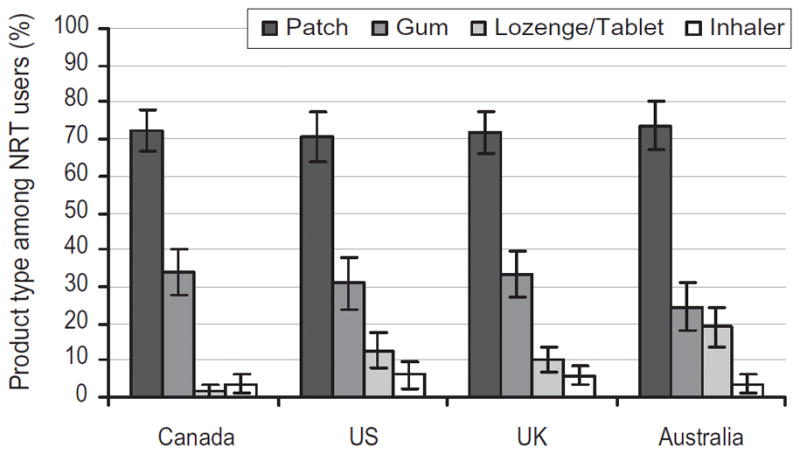
Types of nicotine replacement therapy (NRT) products used in the past 12 months among NRT users (n = 1149)
Non-standard NRT use
Overall, approximately one-third of smokers in all four countries who used NRT in the past 12 months did so for a reason other than quitting (Table 2). The prevalence of nonstandard use did not vary significantly by country.
Table 2.
Reasons for NRT use (n = 1149).
| Country | Reason | % | (n) | (95% CI) |
|---|---|---|---|---|
| Canada | Quit completely | 67.6 | (195) | (61.7–73.6) |
| Non-standard use | 32.4 | (88) | (26.4–38.3) | |
| Reduce amount smoked | 9.8 | (27) | (6.0–13.6) | |
| To cope when cannot smoke | 9.4 | (26) | (5.5–13.2) | |
| Other/not stated | 13.2 | (35) | (8.7–17.8) | |
| United States | Quit completely | 63.0 | (154) | (55.5–70.5) |
| Non-standard use | 37.0 | (90) | (29.5–44.5) | |
| Reduce amount smoked | 5.7 | (17) | (2.6–8.9) | |
| To cope when cannot smoke | 10.9 | (30) | (6.7–15.1) | |
| Other/not stated | 20.4 | (43) | (13.7–27.0) | |
| United Kingdom | Quit completely | 64.5 | (235) | (58.4–70.5) |
| Non-standard use | 35.5 | (123) | (29.5–41.6) | |
| Reduce amount smoked | 8.2 | (24) | (4.3–12.1) | |
| To cope when cannot smoke | 7.4 | (26) | (3.7–11.1) | |
| Other/not stated | 19.9 | (73) | (15.1–24.7) | |
| Australia | Quit completely | 65.3 | (171) | (58.8–71.8) |
| Non-standard use | 34.7 | (93) | (28.2–41.2) | |
| Reduce amount smoked | 9.0 | (22) | (4.9–13.1) | |
| To cope when cannot smoke | 6.5 | (19) | (3.3–9.7) | |
| Other/not stated | 19.2 | (52) | (13.9–24.4) | |
| Overall | Quit completely | 65.2 | (755) | (61.9–68.4) |
| Non-standard use | 34.8 | (394) | (31.6–38.1) | |
| Reduce amount smoked | 8.3 | (90) | (6.3–10.2) | |
| To cope when cannot smoke | 8.4 | (101) | (6.5–10.2) | |
| Other/not stated | 18.2 | (203) | (15.6–20.8) |
CI: confidence interval.
Predictors of non-standard NRT use
A logistic regression analysis was conducted among NRT users (n = 1149) to examine the socio-demographic variables associated with non-standard NRT use. NRT users with high education level had greater odds of non-standard NRT use than smokers with low [odds ratio (OR) = 1.82, 95% confidence interval (CI) = 1.11, 2.99] or moderate (OR = 2.24, 95% CI = 1.29, 3.86) education level. Heavier smokers (higher HSI scores) also had higher odds of non-standard NRT use (OR = 1.12, 95% CI = 1.00, 1.26). Smokers who did not intend to quit (OR = 2.20, 95% CI = 1.32, 3.68) had higher odds of nonstandard NRT use than those with any quit intentions. In addition, smokers who had not made a quit attempt in the last year (OR = 2.94, 95% CI = 2.01, 4.28) had increased odds of non-standard NRT use compared to those who had made a quit attempt. Non-standard NRT use was not related significantly to country, gender, age, ethnicity or income.
Non-standard NRT use varied by product type: NRT was used for a reason other than quitting by 20.7% of patch users, 55.7% of gum users and 87.9% of those who used both the patch and gum (χ2 = 144.7, P < 0.001). After adjusting for country, gender, age, ethnicity, income, education, HSI, quit intentions, past-year quit attempts, paying full price and receiving by prescription, smokers who used nicotine gum alone were 3.6 (95% CI = 2.38, 5.56) times more likely to report non-standard use than those using the patch alone. Those who used both the nicotine patch and gum had far greater odds of non-standard NRT use than those who used the patch alone (OR = 41.6, 95% CI = 21.73, 79.75). No significant differences were observed by country in non-standard use by product type.
Policy-relevant variables and non-standard NRT use
As Fig. 2 indicates, the proportion of NRT users that obtained NRT by prescription in the last year varied significantly by country (χ2 = 133.4, P <0.001). Those who obtained NRT by prescription had much lower odds of non-standard NRT use (OR = 0.44, 95% CI = 0.28, 0.67) compared to those who obtained NRT through other sources, including OTC, after adjusting for the set of covariates described above as well as type of NRT products used. Between-country differences were also observed in the proportion of NRT users who paid full price for their NRT product (χ2 = 114.3, P <0.001). As Fig. 2 illustrates, paying full price followed a pattern inverse to that of prescription attainment. Respondents who paid full price for their NRT were significantly less likely to use NRT for a purpose other than quitting after adjusting for NRT type, receiving NRT by prescription and other covariates (OR = 0.46 95% CI = 0.30, 0.71).
Figure 2.
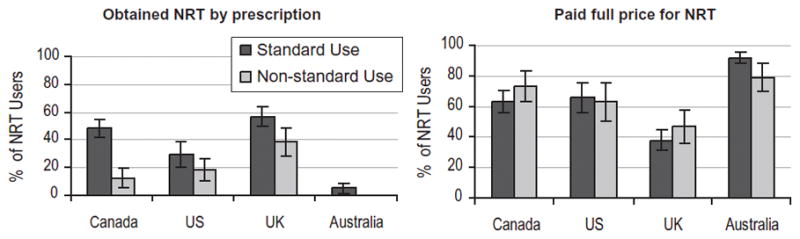
Proportion of nicotine replacement therapy (NRT) users obtaining any NRT product by prescription and paying full price in the past 12 months, by country, 2005 (n = 1149)
The association between reasons for using NRT and the presence of smoking restrictions was also examined. Complete smoking restrictions varied widely across countries, as reported previously [20,21]. For example, by 2005 complete smoking restrictions in bars were reported by 52.2% of Canadian respondents, 30.7% in the United States, 17.3% in Australia and 5.1% in the United Kingdom (χ2 = 861.2, P <0.001). Complete smoking restrictions in homes, restaurants and bars were not associated with non-standard NRT use after adjusting for the covariates listed above. However, United Kingdom smokers reporting restrictions in the work-place were significantly less likely to use NRT for non-standard use (OR = 0.27, 95% CI = 0.13, 0.54). No other significant effects were detected.
DISCUSSION
The findings indicate that roughly one-sixth of smokers reported using NRT in the past year. UK smokers reported the highest prevalence of NRT use, due possibly to a comprehensive national Stop Smoking Service, which includes subsidized NRT [22,23]. In terms of the types of NRT products, use of the nicotine patch was reported twice as often as nicotine gum, consistent with previous studies [23–25]. More recent NRT products, including the nicotine lozenge and inhaler, were significantly less likely to be used.
The findings reveal that a substantial proportion of NRT use in Canada, the United States, the United Kingdom and Australia is for a reason other than quitting smoking. Approximately one-third of all NRT use was for the purpose of reducing smoking, temporary abstinence or another reason other than quitting smoking. These estimates are considerably higher than previous estimates of non-standard NRT use. A population-based survey conducted in 2000–01 in Massachusetts found that approximately 19% of smokers reported ‘ever’ using NRT for a reason other than quitting [26]. Data from a 2002 survey conducted in California also found that 14% of smokers reported a reason other than quitting when asked about their history of NRT use [27]. The higher estimates in the current survey may be due to differences in the populations studied or they may reflect increases in the prevalence of non-standard NRT use in the 3–4 years since these earlier surveys were conducted.
The prevalence of non-standard NRT use was remarkably consistent across countries: estimates differed by less than 5% across the four countries. In contrast, striking between-country differences were observed in the source and access of NRT products. In the United Kingdom, approximately half of NRT products were obtained by prescription, compared to less than 5% in Australia. Subsidization followed a similar pattern: UK smokers were the least likely to pay full price for their NRT, while Australians were the most likely. The differences in prescription rates and subsidization suggest greatly different cessation services and strategies across countries, at least as they relate to the provision of NRT. The source of NRT was also associated with non-standard use: smokers who received their NRT through prescription were significantly less likely to use NRT for the purpose of reduction, abstinence and other reasons.
Non-standard NRT use was also associated with the type of NRT product used: smokers were considerably more likely to report non-standard use of nicotine gum than the patch. This may reflect differences in the nature of the products: whereas the nicotine patch is designed to provide steady doses of nicotine over relatively long periods, nicotine gum can be used to provide nicotine doses over shorter periods and may be more suitable for temporary abstinence. Non-standard use was most common among smokers who reported using multiple NRT products in the past 12 months. Judging by these data, it would appear that using multiple NRT products for the purpose of quitting within a 12-month period is relatively rare.
Heavier smokers, and those with no recent attempts to quit and no intentions to quit smoking, were significantly more likely to report non-standard NRT use. This might be expected, given the more frequent need to smoke among these respondents. Smokers with higher education levels were also more likely to use NRT for reasons other than quitting; however, there was no association with age, income, ethnicity or gender, nor was there any association between non-standard use and smoking restrictions, with one exception: UK smokers reporting work-place smoking bans were less likely to use NRT for a reason other than quitting. Given that only a few ‘white-collar’ work-places in the United Kingdom were covered by comprehensive smoking restrictions at the time these data were collected, this finding may be attributable to the type of smoker in these work-places, rather than the effect of the smoking restrictions themselves. Longitudinal analyses using subsequent waves of the ITC Four Country survey have the potential to examine the relationship between work-place smoking restrictions and non-standard NRT use in greater depth.
Limitations
One potential limitation of this study concerns how ‘non-standard’ NRT use was assessed. Non-standard use was defined as any reason other than ‘to stop smoking completely’, which included ‘temporary abstinence’ and ‘to reduce consumption’, as well as an ‘other reason’ option, which was endorsed by a significant proportion of nonstandard users. It remains unclear what types of reasons might be included in this ‘other’ category. In addition, the current study examined only cross-sectional associations. Future research should examine the implications of non-standard NRT use for subsequent quitting behaviour, including whether using NRT for reasons other than quitting changes the likelihood that smokers will use NRT for subsequent quit attempts.
CONCLUSIONS AND IMPLICATIONS
The public health implications of these findings are some-what unclear. NRT products have a low addiction potential and the health risks of sustained use are low. As a result, the prevalence of non-standard NRT use is not necessarily cause for concern [28–31]. Although the direct health benefits of smoking reduction remain controversial [32], there is some evidence to suggest that using NRT to cut back consumption may, in fact, increase the likelihood of subsequent quitting [33–35]. Studies also indicate that smokers have an interest in gradual smoking reduction, and in using NRT to assist this process [36,37]. At present, NRT is approved for the purposes of smoking reduction in both Canada and the United Kingdom [38,39].
The findings from this study may help to explain the difficulty in demonstrating a clear cessation benefit for NRT in some population-based studies. The failure of such studies to take non-standard use of NRT into account would bias the results to show a lesser impact of NRT on cessation rates than would be the case if only those using NRT to make a quit attempt were considered. In this study, we found that non-standard use of NRT was significantly more common for non-prescription products, which may explain why the efficacy of NRT has appeared to have declined in parallel with OTC sales in some studies [5,7,8]. The switch to OTC may have spawned new uses of NRT more so than it decreased the effectiveness of NRT when used for the purposes of quitting. This is broadly consistent with previous reports that the demographic profile of NRT users may have shifted and that many smokers fail to use NRT for the recommended duration or as directed [5,7,8].
Overall, the findings from this study highlight the importance of monitoring consumer use of medications beyond the setting of clinical trials. Shifts in regulatory policy, including OTC status, may have important implications for who is using medication, how it is used, and the population-level benefit of the product.
Acknowledgments
This research was funded by grants from the National Cancer Institute of the United States (through R01 CA 100362 and through the Roswell Park Trans disciplinary Tobacco Use Research Center, P50 CA111236), the Robert Wood Johnson Foundation (045734), the Canadian Institutes of Health Research (57897), the National Health and Medical Research Council of Australia (265903), Cancer Research UK (C312/A3726) and the Canadian Tobacco Control Research Initiative (014578), with additional support from the Centre for Behavioural Research and Program Evaluation, National Cancer Institute of Canada/Canadian Cancer Society.
Footnotes
Declarations of interest
None.
References
- 1.Etter JE, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control. 2006;15:280–5. doi: 10.1136/tc.2005.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Shiffman S, Sweeney CT. Ten years after the Rx-to-OTC switch of nicotine replacement therapy: what have we learned about the benefits and risks of non-prescription availability? Health Policy. 2008;86:17–26. doi: 10.1016/j.healthpol.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Shiffman S, Gitchell J, Pinney JM, Burton SL, Kemper KE, Lara EA. Public health benefit of over-the counter nicotine medications. Tob Control. 1997;6:306–10. doi: 10.1136/tc.6.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierce JP, Gilpin EA. The impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA. 2002;28:1260–4. doi: 10.1001/jama.288.10.1260. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Use of FDA-approved pharmacologic treatments for tobacco dependence—United States, 1984–1998. MMWR. 2000;49:665–8. [PubMed] [Google Scholar]
- 7.Thorndike AN, Biener L, Rigotti NA. Effect on smoking cessation of switching nicotine replacement therapy to over-the-counter status. Am J Public Health. 2002;92:437–42. doi: 10.2105/ajph.92.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyland A, Rezaishiraz H, Giovino G, Bauer JE, Cummings KM. Over-the-counter availability of nicotine replacement therapy and smoking cessation. Nicotine Tob Res. 2005;7:547–55. doi: 10.1080/14622200500185975. [DOI] [PubMed] [Google Scholar]
- 9.Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control. 2003;12:21–7. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiffman S, Rolf CN, Hellebusch SJ, Gorsline J, Gorodetzky CW, Chiang YK, et al. Real-world efficacy of prescription and over-the-counter nicotine replacement therapy. Addiction. 2002;97:505–16. doi: 10.1046/j.1360-0443.2002.00141.x. [DOI] [PubMed] [Google Scholar]
- 11.Reed MB, Anderson CM, Vaughn JW, Burns DM. The effect of over-the-counter sales of the nicotine patch and nicotine gum on smoking cessation in California. Cancer Epidemiol Biomarkers Prev. 2005;14:2131–6. doi: 10.1158/1055-9965.EPI-04-0919. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman S, Hughes JR, Pillitteri JL, Burton SL. Persistent use of nicotine replacement therapy: an analysis of actual purchase patterns in a population based sample. Tob Control. 2003;12:310–16. doi: 10.1136/tc.12.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solberg LI, Boyle RG, Davidson G, Magnan S, Carlson CL, Alesci NL. Aids to quitting tobacco use: how important are they outside controlled trials? Prev Med. 2001;33:53–8. doi: 10.1006/pmed.2001.0853. [DOI] [PubMed] [Google Scholar]
- 14.Moher M, Hey K, Lancaster T. Workplace interventions for smoking cessation. Cochrane Database Syst Rev. 2005:CD003440. doi: 10.1002/14651858.CD003440.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Bauer JE, Hyland A, Li Q, Cummings KM. A longitudinal assessment of the impact of smoke-free worksite policies on tobacco use. Am J Public Health. 2005;95:1024–9. doi: 10.2105/AJPH.2004.048678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. BMJ. 2002;325:188. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond D, Fong GT, Borland R, Cummings KM, McNeill A, Driezen P. Text and graphic warnings on cigarette packages: findings from the ITC Four Country Survey. Am J Prev Med. 2007;32:202–9. doi: 10.1016/j.amepre.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binson D, Canchola JA, Catania JA. Random selection in a national telephone survey: a comparison of the Kish, next-birthday, and last-birthday methods. J Off Stat. 2000;16:53–60. [Google Scholar]
- 19.Singer E, van Hoewyk J, Maher MP. Experiments with incentives in telephone surveys. Public Opin Q. 2000;64:171–88. doi: 10.1086/317761. [DOI] [PubMed] [Google Scholar]
- 20.Borland R, Yong HH, Siahpush M, Hyland A, Campbell S, Hastings G, et al. Support for and reported compliance with smoke-free restaurants and bars by smokers in four countries: findings from the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15:iii34–41. doi: 10.1136/tc.2004.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borland R, Yong HH, Cummings KM, Hyland A, Anderson S, Fong GT. Determinants and consequences of smoke-free homes: findings from the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15:iii42–50. doi: 10.1136/tc.2005.012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UK Department of Health. [accessed 31 May 2007];NHS stop smoking services and nicotine replacement therapy. Available at: http://www.dh.gov.uk/en/Policyandguidance/Healthandsocialcaretopics/Tobacco/Tobaccogeneralinformation/DH_4002192.
- 23.West R, DiMarino ME, Gitchell J, McNeill A. Impact of UK policy initiatives on use of medicines to aid smoking cessation. Tob Control. 2005;14:166–71. doi: 10.1136/tc.2004.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Health Canada. [accessed 12 March 2007];Canadian Tobacco Use Monitoring Survey 2005 (CTUMS)—Summary of Annual Results for 2005. Available at: http://www.hc-sc.gc.ca/hl-vs/tobac-tabac/research-recherche/stat/ctums-esutc/2005/ann_summary-sommaire_e.html.
- 25.US Centers for Disease Control and Prevention. Assistance used to quit smoking by adults aged >18 years during the preceding 2 years, by type—National Health Interview Survey, United States, 2005. MMWR. 2007;56:507. [Google Scholar]
- 26.Levy DE, Thorndike AN, Biener L, Rigotti NA. Use of nicotine replacement therapy to reduce or delay smoking but not to quit: prevalence and association with subsequent cessation efforts. Tob Control. 2007;16:384–9. doi: 10.1136/tc.2007.021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Delaimy W, Gilpin E, Pierce J. When California smokers use nicotine replacement therapy, most are trying to quit smoking. Tob Control. 2005;14:359–60. doi: 10.1136/tc.2005.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatsukami D, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacol Biochem Behav. 2007;86:132–9. doi: 10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiffman S, Hughes JR, Di Marino ME, Sweeney CT. Patterns of over-the-counter nicotine gum use: persistent use and concurrent smoking. Addiction. 2003;98:1747– 53. doi: 10.1111/j.1360-0443.2003.00575.x. [DOI] [PubMed] [Google Scholar]
- 30.Hughes JR, Adams EH, Franzon MA, Maguire MK, Guary J. A prospective study of off-label use of, abuse of, and dependence on nicotine inhaler. Tob Control. 2005;14:49–54. doi: 10.1136/tc.2004.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiffman S, Hughes JR, Di Marino ME, Sweeney CT. Patterns of over-the-counter nicotine gum use: persistent use and concurrent smoking. Addiction. 2003;98:1747– 53. doi: 10.1111/j.1360-0443.2003.00575.x. [DOI] [PubMed] [Google Scholar]
- 32.Hatsukami DK, Henningfield JE, Kotlyar M. Harm reduction approaches to reducing tobacco-related mortality. Annu Rev Public Health. 2004;25:1–19. doi: 10.1146/annurev.publhealth.25.102802.124406. [DOI] [PubMed] [Google Scholar]
- 33.Bolliger CT, Zellweger JP, Danielsson T, van Biljon X, Robidou A, Westin A, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. BMJ. 2000;321:329–33. doi: 10.1136/bmj.321.7257.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batra A, Klingler K, Landfeldt B, Friederich HM, Westin A, Danielsson T. Smoking reduction treatment with 4-mg nicotine gum: a double-blind, randomized, placebo-controlled study. Clin Pharmacol Ther. 2005;78:689–96. doi: 10.1016/j.clpt.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Wennike P, Danielsson T, Landfeldt B, Westin A, Tonnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98:1395–402. doi: 10.1046/j.1360-0443.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- 36.Shiffman S, Hughes JR, Ferguson SG, Pillitteri JL, Gitchell JG, Burton SL. Smokers’ interest in using nicotine replacement to aid smoking reduction. Nicotine Tob Res. 2007;9:1177–82. doi: 10.1080/14622200701648441. [DOI] [PubMed] [Google Scholar]
- 37.Hughes JR, Callas PW, Peters EN. Interest in gradual cessation. Nicotine Tob Res. 2007;9:671–5. doi: 10.1080/14622200701365293. [DOI] [PubMed] [Google Scholar]
- 38.MHRA. Report of the Committee on Safety of Medicines Working Group on Nicotine Replacement Therapy. London: Committee on Safety of Medicines, Medicine and Healthcare Products Regulatory Agency; 2005. [Google Scholar]
- 39.National Institute for Health and Clinical Excellence (NICE) Cut Down to Quit with Nicotine Replacement Therapies (NRT) in Smoking Cessation: Systematic Review of Effectiveness and Economic Analysis. London: National Health Service, NICE; 2007. [Google Scholar]


