Abstract
Ectopic activation and conduction may give rise to arrhythmias when a diseased myocardial substrate exists. Electrophysiological mapping studies that record electrical properties of the heart in sinus rhythm may fail to uncover pro-arrhythmic substrates that are triggered by ectopy. In this study we use simulation and experimental models of clinical, trackable, loop catheters to interrogate regions of myocardium by stimulating and recording with multiple activation patterns. Longitudinal and traverse conduction velocities of the tissue were acquired from the pacing protocol. Artifacts resulting from variable distance between the recording electrodes and pacing site were also detected and removed. This study demonstrates that the mapping of local tissue properties with variable activation patterns is feasible and can expose features of the electrophysiological substrate that can not be recovered during sinus conduction.
1. Introduction
The onset and entrenchment of atrial fibrillation (AF) is strongly associated with remodeling of the atrial myocardial substrate. As a results, as remodeling progresses, ablation strategies based solely on compartmentalization lose efficacy. Consequently, great clinical interest has centered on substrate mapping strategies that can identify proarrhythmic tissues for targeted therapy. In this scenario intracardiac electrogram (EGM) parameters such as voltage amplitude and presence and degree of fractionation are employed as markers of tissue with abnormal conduction properties [1,2]. However, traditional mapping strategies for AF, whether recorded in sinus rhythm or AF, have neglected the effect of variable activation patterns on EGM parameters of interest.
A key feature of an ectopically triggered beat in the heart is that the resulting activation does not follow the same conduction patterns as a normal sinus beat. Thus, it is even conceivable that sinus conduction may mask the remodeled and pro-arrhythmic substrates that can only be exposed by extrasystole. In this study, we demonstrate the feasibility of mapping electrophysiological substrate features using varied activation patterns by pacing and recording within the same region of heart using a clinical loop catheter.
To demonstrate the feasibility of substrate mapping with controlled activation patterns, we selected conduction velocity (CV) as our initial parameter of interest. Conduction velocity is an important factor in the initiation of re-entry and is affected by substrate remodeling (heterogeneity and slowing) [3]. We explored factors associated with measurement of CV in this manner, and evaluated the information such measurements provide about myocardial substrate.
2. Methods
This study incorporated computation modeling and direct recording of electrograms in large mammal experiments. In both cases, a pacing protocol involving stimulation from bipolar electrodes on a 10 pole loop catheter was performed to interrogate the conduction properties of the tissue. Specifically, a depolarization wave was activated by pacing from each pair of adjacent electrodes on the loop catheter, i.e., 1-2, 2-3,…,9-10, to stimulate the region contained by the loop with 9 different activation patterns. EGMs from the non-pacing electrodes were acquired for each activation pattern and from them, activation times were determined from unipolar EGMs by computing the maximum negative slope, or from bipolar EGMs by a nonlinear energy operator [4]. CV was calculated as the distance, from the pacing site to the recording electrode, over the time to activation. CVs and directions from the respective studies were visualized using SCIRun[5].
2.1. Simulation
We implemented a 3-dimensional bidomain simulation of the spread of activation from pacing sites on slab models of atrial tissue with the Cardiac Arrhythmia Research Package (CARP) software [6]. The models were a 26.0×26.0×3.0 mm slab composed of isotropic hexahedral elements with 0.1 mm edge length immersed in 1.0 mm of bath surrounding all slab surfaces. For each activation pattern, we computed complete activation of the slab using the Courtemanche-Ramirez-Nattel cell model of atrial myocyte kinetics [7]. Pseudo-EGMs were generated at each electrode (1 kHz sampling frequency) for the entire 50 ms of simulation. Model conductivities were varied to produce both isotropic and anisotropic models (Figure 1).
Figure 1.
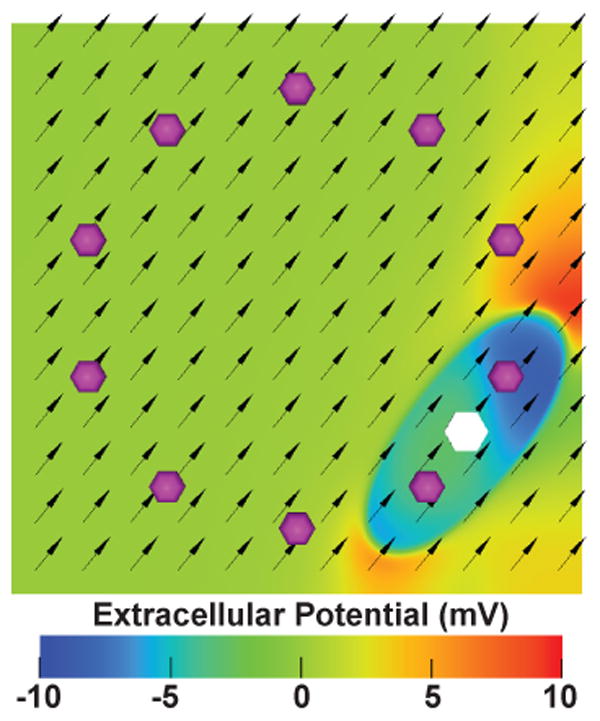
Anisotropic conductivity model of excitation propagation initiated by loop catheter pacing. Extracellular potential of this model is shown 10 ms after the start of pacing from the white hexagonal region. The purple spheres indicate the position of recording electrodes. The black arrows indicate the fiber orientation of the model.
2.2. Characterization of Conduction
The relationship between conduction velocity and the distance of the recording electrode from the pacing site was plotted and fit by linear regression. The slope of this regression was then used to normalize all CVs as though they had been recorded 20 mm from the pacing site.
CVs and their respective directions of conduction were compared to the fiber orientation of the computational models. Specifically, the angle between the fiber axis and directions of minimum and maximum conduction velocity were qualitatively assessed for correlation.
2.3. In Vivo Experimentation
The feasibility of pacing and recording from a single loop catheter for assessment of conduction velocities was tested during experimentation on a single canine. A 10 pole trackable circular mapping catheter (Lasso Nav, Biosense Webster, Diamond Bar, CA)was introduced into the right atrium via percutaneous access of the femoral vein. The electrode array was placed in the high right atrium on the lateral wall and positioned such that clear EGMs were visible on all channels. Pacing was achieved with 600 ms cycle length, 10 mA, and 2 ms pulse width stimulation. Myocardial capture was confirmed then location and electrogram recordings were acquired with a CARTO 3 electrophysiological recording system.
3. Results
3.1. Simulation
Activation times were found for all loop catheter electrodes in the computational model. CV and the direction of conduction were plotted as color-mapped vectors over the model (Figure 2, Left column), where both the color and magnitude correspond to the CV. In both the isotropic and anisotropic models CVs measured from electrodes in proximity to the pacing site showed slower CVs than observed at distant recording electrodes.
Figure 2.
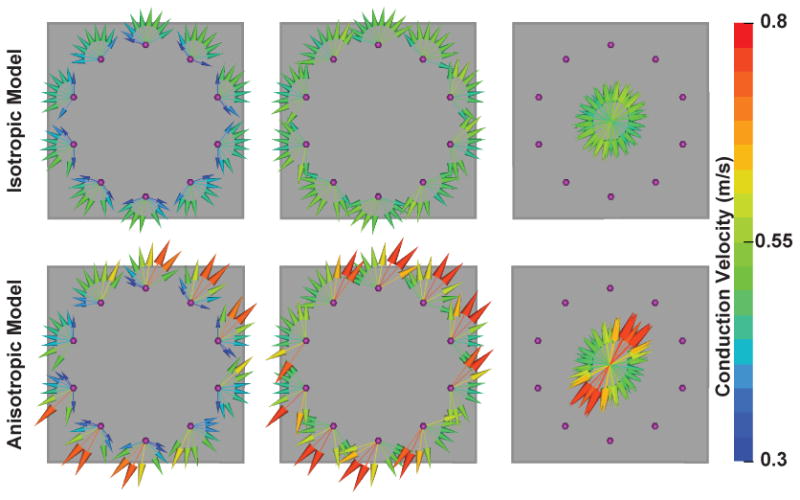
Characterization of local conduction properties with loop catheter. Left Column – Vectors at recording electrodes (purple spheres) point away from pacing site. Length and color correspond to conduction velocity (CV). Center Colum – CV vectors normalized to fixed distance from pacing site. Right Column – Compact representation of regional conduction.
3.2. Characterization of Conduction
Figure 3 shows a linear regression of conduction velocity as a function of conduction distance. In the center column of Figure 2, the CVs are normalized to a fixed distance (20 mm) by the slopes of the respective regressions. In the isotropic model (top row of Figure 2) the pre-normalization vectors (leftmost column) demonstrate anisotropy of CVs inconsistent with the spread of activation in the model. The normalized vectors (middle column), in contrast, indicate a uniform spread of activation that is consistent with the model conductivities. Anisotropy is apparent before normalization in the CVs of the anisotropic model, however, the CVs from electrode to electrode are inconsistent, even when oriented in the same direction, due to variable proximity of the recording electrodes to the paced sites. The normalization improves the consistency of CVs among vectors pointing in the same direction (visible in the center and right column of the second row of Figure 2). In the anisotropic model, the direction of highest CV lay along the fiber orientation (see Figure 1). As a compact representation of the conduction properties, the normalized vectors from each recording site were projected onto a point at the center of the loop catheter (Figure 2, right column).
Figure 3.
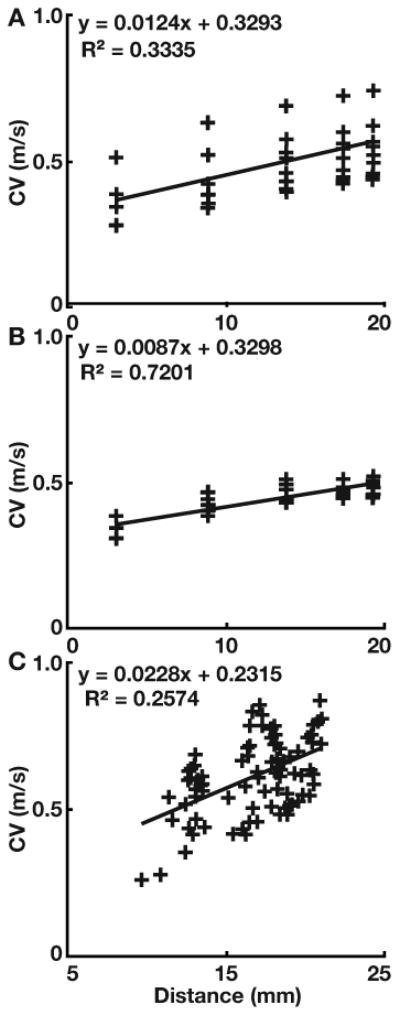
Linear regression of CV versus distance of the recording electrode from the pacing site. The slope for the anisotropic (top), isotropic (center) computational models, and in vivo model (bottom) was used to normalize all CV measurements to 20 mm from the pacing site.
3.3. In Vivo Experimentation
The pacing protocol to stimulate myocardial tissue with multiple activation patterns was successfully carried out at 2 locations in the canine model. Activation times were acquired on a median of 5 (min = 3, max = 6) bipolar channels for each pacing site. The number of interpretable EGMs was limited due to pacing artifact that saturated channels near the pacing site or by channels with no detectable activation signals. Similar to the simulation results, CV also increased with distance of the recording electrode from the pacing site. Figure 4 shows the result of normalizing and plotting CV vectors from measurements from two locations on the RA of the animal.
Figure 4.
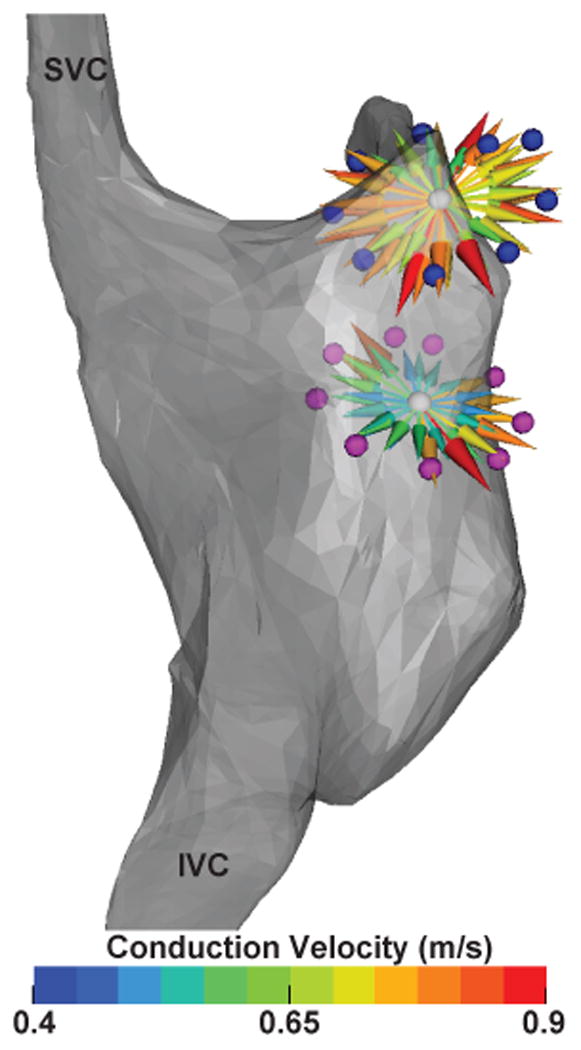
Compact representation of inter-loop conduction properties of right atrium at two sites. Both sites were interrogated with multiple activation patterns by bipolar pacing between electrodes around the loop. Normalized CV vectors indicate direction and velocity of conduction
4. Discussion
Our study based on simulation and animal experimentation shows that interrogation of regions of myocardium with multiple activation patterns can elucidate basic conduction properties of that tissue, e.g., CV and anisotropy, in a way that corrects for possible artifacts from the location of the pacing cite. The results of such evaluations of the myocardial substrate may be useful to establish patient specific ablation strategies for arrhythmias like AF.
The rate of conduction from a focal pacing site is not constant even in the immediate area around the pacing site, and increases as the depolarization wave propagates outward [3]. Consequently, electrodes in proximity to the pacing site will observe relatively slower conduction than distant electrodes. The normalization by distance we propose allows for inter-electrode comparison of CVs and improves the characterization of local conduction properties. The maximum conduction distance for these models was about 20 mm and the acceleration of conduction remained relatively constant over these distances.
Previous studies have reported using loop catheters to record CV from planar wavefronts during sinus conduction [8], however, their results have been indicative of conduction in the longitudinal direction only, i.e., parallel to the predominate fiber orientation. More generally, wavefronts initiated by local pacing have longitudinal and transverse aspects that permit characterization of the anisotropy of conduction. The findings of this study demonstrate how both longitudinal and transverse CVs may be recovered with this protocol.
The primary tenet of this controlled activation pattern mapping protocol is that sinus conduction is inherently stable and not prone to arrhythmogenesis. Thus, electroanatomical substrate mapping of tissue properties during sinus rhythm may fail to identify pro-arrhythmic substrates that are unmasked by ectopy and non-sinus conduction. However, this protocol is a preliminary effort and further considerations, e.g., CV restitution, may be required to robustly identify tissues with arrhythmogenic conduction properties. Robust characterization of the atrial conduction substrate may facilitate better understanding of the mechanisms behind AF initiation and provide better targets for therapeutic interventions.
References
- 1.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000 Mar;101(11):1288–96. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 2.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004 Jun;43(11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 3.Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiological Reviews. 2004 Apr;84(2):431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen MP, Schilling C, Dössel O. A new approach for automated location of active segments in intracardiac electrograms. World Congress on Medical Physics. 2010 [Google Scholar]
- 5.SCIRun: A Scientific Computing Problem Solving Environment, Scientific Computing and Imaging Institute (SCI) Download from: http://www.scirun.org.
- 6.Vigmond EJ, Hughes M, Plank G, Leon LJ. Computational tools for modeling electrical activity in cardiac tissue. J Elec-trocardiol. 2003;36(Suppl):69–74. doi: 10.1016/j.jelectrocard.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc Res. 1999 May;42(2):477–89. doi: 10.1016/s0008-6363(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 8.Weber FM, Schilling C, Seemann G, Luik A, Schmitt C, Lorenz C, Dössel O. Wave-direction and conduction-velocity analysis from intracardiac electrograms–a single-shot technique. IEEE Transactions on Biomedical Engineering. 2010 Oct;57(10):2394–2401. doi: 10.1109/TBME.2010.2055056. [DOI] [PubMed] [Google Scholar]


