Abstract
The size of in vitro engineered skeletal muscle tissue is limited due to the lack of a vascular network in vitro. In this article, we report tissue-engineered skeletal muscle consisting of human aligned myofibers with interspersed endothelial networks. We extend our bioartificial muscle (BAM) model by coculturing human muscle progenitor cells with human umbilical vein endothelial cells (HUVECs) in a fibrin extracellular matrix (ECM). First, the optimal medium conditions for coculturing myoblasts with HUVECs were determined in a fusion assay. Endothelial growth medium proved to be the best compromise for the coculture, without affecting the myoblast fusion index. Second, both cell types were cocultured in a BAM maintained under tension to stimulate myofiber alignment. We then tested different total cell numbers containing 50% HUVECs and found that BAMs with a total cell number of 2 × 106 resulted in well-aligned and densely packed myofibers while allowing for improved interspersed endothelial network formation. Third, we compared different myoblast-HUVEC ratios. Including higher numbers of myoblasts improved endothelial network formation at lower total cell density; however, improvement of network characteristics reached a plateau when 1 × 106 or more myoblasts were present. Finally, addition of Matrigel to the fibrin ECM did not enhance overall myofiber and endothelial network formation. Therefore, in our BAM model, we suggest the use of a fibrin extracellular matrix containing 2 × 106 cells of which 50–70% are muscle cells. Optimizing these coculture conditions allows for a physiologically more relevant muscle model and paves the way toward engineering of larger in vitro muscle constructs.
Introduction
Skeletal muscle tissue has a capacity for self-renewal upon muscle injury, but when this is impaired, nonfunctional scar tissue replaces the damaged or lost muscle. Current therapeutic interventions include autologous muscle transpositions, but these are associated with donor site morbidity and poor tissue survival and integration.1 As an alternative for autologous muscle transpositions, human skeletal muscle organoids can be created in vitro by tissue engineering. Next to potential applications in regenerative medicine, muscle organoids may also be used as an in vitro model for myogenesis or myopathology.
Culture and differentiation of myogenic progenitor cells on natural and synthetic biodegradable scaffolds2,3 result in differentiated myofibers, which are often unaligned. Parallel alignment of the myofibers can be induced when cells are subjected to a unidirectional force during differentiation and results in functional bioartificial muscles (BAMs), which can be electrically and mechanically stimulated.4–6
However, the size of in vitro created skeletal muscle tissue is limited due to the lack of a vascular network in vitro. Coculturing endothelial cells and myoblasts, muscle precursor cells, might overcome this problem. This way, the newly formed muscle can be prevascularized, meaning that an endothelial network is formed in vitro in the tissue-engineered muscle. The goal is to anastomose this network to the host vasculature after transplantation. Although prevascularization of tissue-engineered muscle has been described on scaffolds,7,8 myofibers do not align in one direction on these scaffolds, precluding muscle contractility. Other tissue engineering approaches such as the use of soft hydrogels, the combination of endothelial cell sheets with myoblast cell sheets, and microfabrication techniques such as 3D printing may be more suitable to obtain aligned myofibers.7,9–13 Engineering muscle precursor cells in a soft hydrogel such as fibrin allows the myofibers to align in one direction while fibrin behaves as a proangiogenic, making it a good scaffold for both the formation of myofibers and endothelial networks.
Prevascularization of aligned tissue-engineered muscle tissue has been described for murine muscle based on coculture of C2C12 murine myoblasts and embryonic heart endothelial cells.14 Another report describes the engineering of aligned, functional rat muscle with postimplantation vascular integration in mice.15 To our knowledge, however, prevascularization of human aligned myofibers has not been described. In this work, we aimed to determine suitable culture conditions allowing both aligned myofiber formation and endothelial network formation as a first step toward a human vascularized BAM. Therefore, we used a coculture of human muscle cells, containing both myoblasts and fibroblasts, and human umbilical vein endothelial cells (HUVECs) in a fibrin extracellular matrix (ECM).
Methods
Cell culture
HUVECs labeled with green fluorescent protein (GFP) (Angio-proteomie) were cultured in gelatin-coated culture flasks (0.1% gelatin, Millipore, preincubated for 1 h at 37°C) in endothelial growth medium (EGM-2 with bullet kit; Lonza). GFP-HUVECs were split at 90% confluence and used in the experiment at passage 7. Human muscle cells (gift from H. Vandenburgh, Brown University, USA) were isolated by needle biopsy on a 27-year-old male volunteer from a muscle biopsy (vastus lateralis) according to procedures approved by the Institutional Clinical Review Board of the Miriam Hospital (Providence, RI). They were cultured in skeletal muscle growth medium (SkGM; Lonza) supplemented with 15% fetal bovine serum (FBS; Lonza). Muscle cells were split at 60–70% confluence and used in experiments at 21 doublings.
3D tissue construct
Muscle cells and GFP-HUVECs were mixed with 500 μL thrombin (4 U/mL, Tissucol) and cast into 25-mm-long silicone rubber molds with end attachment sites. Then, 500 μL fibrinogen (2 mg/mL; Tissucol), with or without the addition of 20% Matrigel (BD Biosciences), was added and the cell–gel mix was mixed by quickly pipetting up and down to form a fibrin gel (1 mg/mL, with or without 10% Matrigel), including both cell types. Following 2 h of incubation at 37°C, EGM-2 or SkGM supplemented with fibrinolysis inhibitors, aprotinin (92.5 μg/mL; Carl Roth) and tranexamic acid (400 μM; Sigma), was added and replaced every two days. For BAMs containing only muscle cells, the medium was switched to differentiation medium two days after casting (SkFM; DMEM with 10 ng/mL hEGF, 10 μg/mL insulin, 50 μg/mL BSA, and 50 μg/mL gentamicin). BAMs were made with different cell type ratios and numbers. The medium was replaced every 2 days and BAMs were kept in culture for 7 days before thickness measurement and fixation.
Thickness measurements
Cross-sectional thickness of BAMs in culture was measured with a sterile micrometer at day 7 after casting.
Immunocytochemistry
Immunocytochemistry was performed to (i) determine the amount of myoblasts and fibroblasts in the cell population and (ii) to characterize the fusion index of the myoblasts. For desmin staining, muscle cells were cultured in SkGM in 12-well dishes for 2–3 days until 60–70% confluent. For determination of fusion index, the cells were cultured to 80% confluence in SkGM, and then switched to SkFM for 4 days to induce fusion into myofibers. Fixation was performed in a 1:1 methanol–acetone mix at −20°C for 10 min. Next, cells were permeabilized in blocking buffer containing 0.2% Triton X-100 (Sigma) and 1% bovine serum albumin (BSA; Sigma) in PBS (30 min, RT). Subsequently, cells were incubated for 2 h (RT) with a monoclonal mouse antibody against desmin (Sigma; D1033, 1:200 in blocking buffer) or tropomyosin (Sigma, T9283, 1:100 in blocking buffer) to determine the myoblast percentage and the fusion index, respectively. Cells were labeled with a polyclonal goat anti-mouse secondary antibody (Alexa Fluor 568, A11004; Invitrogen) for 30 min in the dark and subsequently incubated with DAPI (0.1 μg/mL in PBS; Life Technologies) for 1 h. The percentage of myoblasts in a muscle cell population was defined as the ratio of desmin-positive cells to the total amount of cells (identified by the DAPI-stained nuclei). Fusion index was defined as the ratio of tropomyosin-positive cells to the total amount of myoblasts in the population.
Whole-mount immunofluorescence staining and confocal microscopy
Constructs were washed (3 × 15 min in PBS), and then removed from the attachment sites. Then, they were pinned on styrofoam to preserve their original shape while fixing in 4% formaldehyde (Merck) for 1 h and stored at 4°C in PBS. Immediately before whole-mount staining, samples were fixed a second time in −20°C methanol for 10 min and permeabilized in blocking buffer for 1 h. Subsequently, BAMs were incubated overnight at 4°C with a monoclonal mouse antibody against tropomyosin (Sigma; T9283, 1:100 in blocking buffer). Next, BAMs were washed and incubated with a polyclonal goat anti-mouse secondary antibody (Alexa Fluor 568, A11004, Invitrogen, 1:200) for 30 min in the dark, followed by incubation with DAPI (Life Technologies; 0.1 μg/mL in PBS) for 1 h. BAMs were stored in PBS in the dark until visualized.
Confocal imaging and data analysis
BAMs were placed on coverslips and visualized by confocal microscopy (Zeiss LSM710) within 48 h. Per BAM, 5 z-stacks were acquired, each containing 20–40 images (depending on the intensity of the signal at various depths) every 5 μm toward the center of the BAM. For myofiber analysis, each 20-μm z-projection (image grouping performed by the ImageJ software16) was manually analyzed for different parameters of myofiber formation: myofiber alignment, length, and diameter, as well as number of myofibers per microscopic field. Myofiber alignment was determined by the standard deviation of the angles of the myofibers. A lower number thus indicates better alignment. For endothelial network analysis, each 60-μm z-projection was automatically analyzed by a customized version of the Angiogenesis Analyzer, an ImageJ plugin created by Gilles Carpentier.17 This tool quantifies endothelial networks in an objective manner by extracting characteristic information of the network. Parameters to define endothelial networks were junctions (group of joined nodes, pixels with at least three neighbors), branches (elements delimited by a junction and one extremity), isolated segments (binary lines, which are not branched), total length of endothelial network (interconnected segments, branches, and isolated segments),% branching length (length of interconnected segments and branches divided by total network length), and meshes (areas enclosed by segments) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
Statistics
Number of replicates refers to number of analyzed images for microscopic analyses or to number of BAMs for thickness analyses. D'Agostino and Pearson normality test and Bartlett's test were used to verify normality of the data and equality of variances, respectively. Normally distributed data with equal variances were analyzed by an unpaired Student t-test when two groups were compared. For comparing several normally distributed groups, a one-way ANOVA was used with a Bonferroni multiple comparison post-test. For groups that were not normally distributed and/or had unequal variances, a nonparametric Mann–Whitney test was used for comparison between two groups, while the Kruskal–Wallis test followed by Dunn's post-test was performed for multiple comparisons. All values are expressed as mean ±standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, NS=not significant.
Results
Aligned myofibers are formed in fibrin BAMs cultured in EGM-2
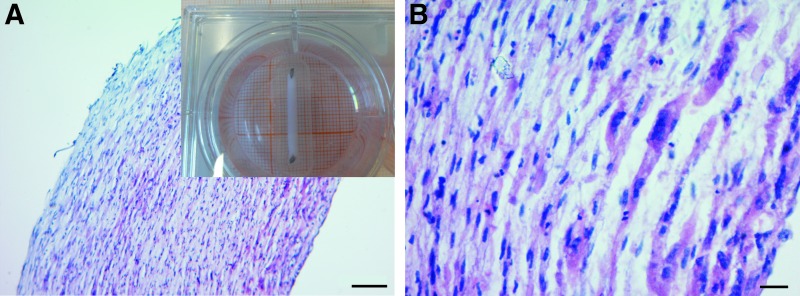
We first characterized the cells expanded from human muscle biopsy. The myogenic progenitor population (further referred to as muscle cells) contained 92.1% ± 4.0% (n = 15) myoblasts and 7.9% ± 4.0% fibroblasts as assessed by desmin staining. Myoblasts had a fusion index of 70.0% ± 5.7% (n = 20) as determined by tropomyosin staining. To make BAMs, 1 × 106 of these cells were mixed in a fibrin ECM (1 mg/mL) and cultured in growth medium (SkGM) for 2 days, followed by differentiation medium (SkFM) for 5 days. The cell-ECM mix contracted around 2 attachment sites and formed 2-cm-long muscle organoids (Fig. 1, inset) containing aligned myofibers (Figs. 1A, B, and 2A).
FIG. 1.
H&E staining of a bioartificial muscle (BAM) with 1 × 106 muscle cells in a fibrin extracellular matrix shown with a 10 × (A) and 40 × (B) objective. Inset shows a seven-day-old BAM in a six-well plate, fixed to two attachment sites, which are 2 cm apart. Myofibers are aligned and homogeneously distributed over the entire BAM. Scale bars represent 50 (A) and 10 μm (B). Color images available online at www.liebertpub.com/tea
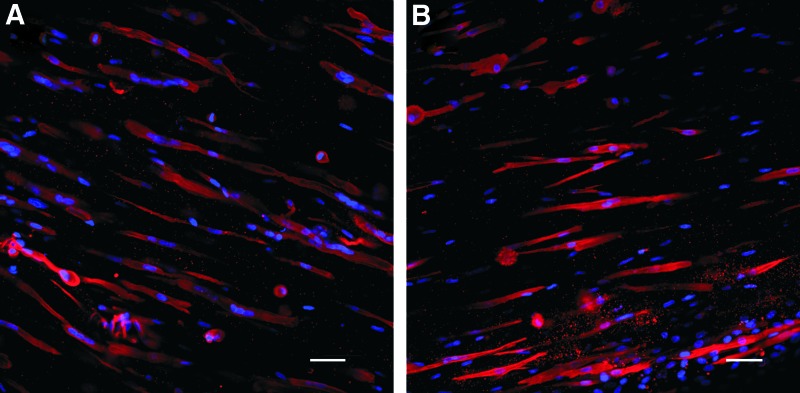
FIG. 2.
Multinucleated myofibers in BAMs with 1 × 106 muscle cells, cultured in (A) SkGM-SkFM and (B) EGM-2. Blue: nuclei stained with DAPI; red: myofibers stained with tropomyosin immunofluorescence. Color images available online at www.liebertpub.com/tea
Aiming at the formation of endothelial networks and aligned myofibers in BAMs, cocultures of HUVECs and muscle cells were mixed in a fibrin ECM in a 50:50 ratio. They are further referred to as coculture BAMs, in contrast to regular BAMs that do not contain endothelial cells. To investigate if endothelial networks can be formed in these organoids, we first applied the same tissue engineering procedure to cocultures of the muscle cells and endothelial cells. However, culturing 5 × 105 muscle cells and 5 × 105 GFP-HUVECs under these culture conditions resulted in poor survival of HUVECs as assessed by confocal fluorescence microscopy (data not shown). Since HUVECs are cultured in EGM-2, muscle cell survival and proliferation in this medium were examined in 2D. Muscle cells proliferated in EGM-2, although significantly slower (doubling time 39.71 ± 1.59 h, n = 9) compared with SkGM (30.34 ± 0.55 h, n = 9). Optimal differentiation conditions for 2D cultures are obtained by growing the muscle cells for 2–3 days in SkGM until 80% confluency, followed by 3–4 days in SkFM (SkGM-SkFM). Performing the differentiation in EGM-2 for 5 days resulted in myofibers with significantly less nuclei per fiber (2.49 ± 0.10 nuclei per fiber, n = 29) compared with SkGM-SkFM differentiation (7.06 ± 0.58 nuclei per fiber, n = 18). However, the fusion index does not differ significantly between EGM-2 (0.61 ± .012, n = 29) and SkGM-SkFM (0.67 ± 0.01, n = 18).
Next, we compared myofiber formation in the 3D organoid with 1 × 106 muscle cells cultured in EGM-2 versus SkGM-SkFM. Whole-mount tropomyosin staining was performed to visualize the formation of myofibers. Multinucleated myofibers formed in both EGM-2 and SkGM-SkFM. However, myofibers cultured in EGM-2 contained a significantly lower number of nuclei per myofiber (2.29 ± 0.10, n = 10) compared with those cultured in SkGM-SkFM (3.09 ± 0.15, n = 10) (Fig. 2). Culturing BAMs in EGM-2 also decreased the diameter of the formed myofibers. On the other hand, there was no significant difference between the use of the two media in the number and length of myofibers and the alignment factor (Table 1 columns A and D). In addition, the total thickness of the fibrin BAMs with 1 × 106 muscle cells was similar in SkGM-SkFM (2.48 ± 0.224 mm, n = 3) versus EGM-2 (2.76 ±0.159 mm, n = 3). Since EGM-2 allowed culturing of muscle cells and HUVECs with formation of myofibers while enabling an optimal survival of the HUVECs, we further optimized coculture BAMs in EGM-2.
Table 1.
Myofiber Formation in Fibrin BAMs with Only Muscle Cells (5 × 106 or 1 × 106 Cells) Or Coculture BAMs with 50% HUVECs and 1 × 106 Or 2 × 106 Cells in Total, Cultured in SkGM-SkFM (Column A), Or EGM-2 (Columns B–G) for 7 Days
| A (n = 12) | B (n = 8) | C (n = 11) | D (n = 10) | E (n = 34) | F (n = 11) | G (n = 75) | A–D | B–C | D–E | C–E | B–D | D–F | D–G | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # Myoblasts | 1 × 106 | 5 × 105 | 5 × 105 | 1 × 106 | 1 × 106 | 1 × 106 | 1 × 106 | |||||||
| # HUVECs | 0 | 0 | 5 × 105 | 0 | 1 × 106 | 0 | 0 | |||||||
| Medium | SkGM/SkFM | EGM-2 | EGM-2 | EGM-2 | EGM-2 | EGM-2 | EGM-2 | |||||||
| +10% Matrigel outer 20 μm | +10% Matrigel inner 140 μm | |||||||||||||
| Number/microscopic field | 42.20 ± 7.64 | 21.51 ± 2.34 | 25.63 ± 10.02 | 39.96 ± 5.75 | 40.48 ± 10.25 | 37.09 ± 17.14 | 35.21 ± 8.24 | NS | NS | NS | ** | * | NS | NS |
| Alignment | 10.34 ± 4.95 | 11.36 ± 4.00 | 16.24 ± 5.96 | 8.04 ± 1.89 | 5.09 ± 1.33 | 7.51 ± 3.76 | 14.07 ± 4.45 | NS | NS | NS | *** | NS | NS | *** |
| Length (μm) | 147.60 ± 14.11 | 118.7 ± 16.30 | 118.90 ± 6.93 | 126.30 ± 7.02 | 127.4 ± 37.56 | 176.30 ± 52.45 | 113.40 ± 21.21 | NS | NS | NS | NS | NS | *** | NS |
| Diameter (μm) | 15.34 ± 3.78 | 10.81 ± 4.26 | 12.79 ± 4.60 | 12.20 ± 3.00 | 13.26 ± 3.69 | 14.27 ± 3.22 | 13.75 ± 3.67 | * | NS | NS | NS | NS | NS | NS |
Columns A–E are discussed and compared in the Aligned myofibers are formed in fibrin BAMs cultured in EGM-2 section, columns F–G are discussed in the Matrigel impacts myofiber distribution, but does not improve myofiber and endothelial network formation section. In the last seven columns, statistical significances are shown for comparisons of indicated groups. Multiple comparisons were made by a Kruskal–Wallis test followed by Dunn's post-test, except for the parameter diameter where a parametric ANOVA followed by a Bonferroni post-test was used.
BAM, bioartificial muscle; EGM, endothelial growth medium; HUVEC, human umbilical vein endothelial cell.
Formation of aligned myofibers and endothelial networks in fibrin BAMs with a coculture of muscle cells and HUVECs
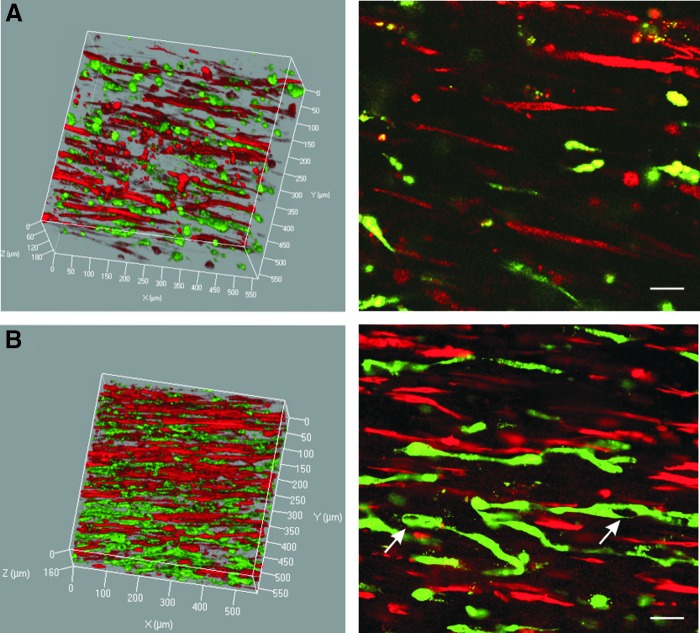
Coculture BAMs with 50% endothelial cells were further studied in EGM-2 with different total cell numbers (Fig. 3). The thickness of the BAM was inversely proportional with the total number of cells used both in BAMs with and without HUVECs (Table 2 column A).
FIG. 3.
Fluorescent confocal images of coculture BAM with 50% human umbilical vein endothelial cells (HUVECs) and 50% muscle cells and a total cell number of 1 × 106 cells (A) and 2 × 106 cells (B). Endothelial networks (green) between aligned myofibers (red) in 3D (left) and an xy-cross section in 2D (right). Scale bars represent 50 μm, meshes are indicated by arrows. Color images available online at www.liebertpub.com/tea
Table 2.
Thickness (in mm) of Different Fibrin BAMs Cultured for 7 Days in EGM-2
| A. Fibrin ECM | B. Fibrin + 10% Matrigel ECM | |
|---|---|---|
| 5 × 105 muscle cells | 3.20 ± 0.07 (n = 3) | 3.23 ± 0.35 (n = 3) |
| 1 × 106 muscle cells | 2.48 ± 0.39 (n = 3) | 3.32 ± 0.13 (n = 3) |
| 5 × 105 muscle cells + 5 × 105 GFP-HUVECs | 2.20 ± 0.36 (n = 3) | 2.20 ± 0.18 (n = 3) |
| 1 × 106 muscle cells + 1 × 106 GFP-HUVECs | 1.27 ± 0.22 (n = 12) | ND |
| 12 × 105 muscle cells + 8 × 105 GFP-HUVECs | 1.30 ± 0.03 (n = 4) | ND |
| 14 × 105 muscle cells + 6 × 105 GFP-HUVECs | 1.28 ± 0.21 (n = 4) | ND |
In the Formation of aligned myofibers and endothelial networks in fibrin BAMs with a coculture of muscle cells and HUVECs section, only column A is discussed. In the Matrigel impacts myofiber distribution, but does not improve myofiber and endothelial network formation section, a comparison is made between column A and B. ND, not determined.
BAMs and coculture BAMS, containing the same number of muscle cells (5 × 105 or 1 × 106), showed similar myofiber formation and alignment (Table 1 columns B-C and D-E, Supplementary Fig. S2). Myofibers were better aligned and more densely packed in coculture BAMs with a higher total cell number of 2 × 106 versus 1 × 106 (Fig. 3). Compared with coculture BAMs with a total number of 1 × 106, coculture BAMs with 2 × 106 cells showed a significant increase in myofiber density and alignment (Table 1 columns C and E, Supplementary Fig. S2 and Supplementary Video S1). We hypothesized that also endothelial network formation would be enhanced. Indeed, the endothelial networks were significantly improved in the coculture BAMs with 2 × 106 cells (Fig. 4, Table 3 columns A and D, Supplementary Fig. S3 and Supplementary Video S2). The presence of meshes was detected in coculture BAMs and confirms formation of extended endothelial networks (Figs. 3 and 4).
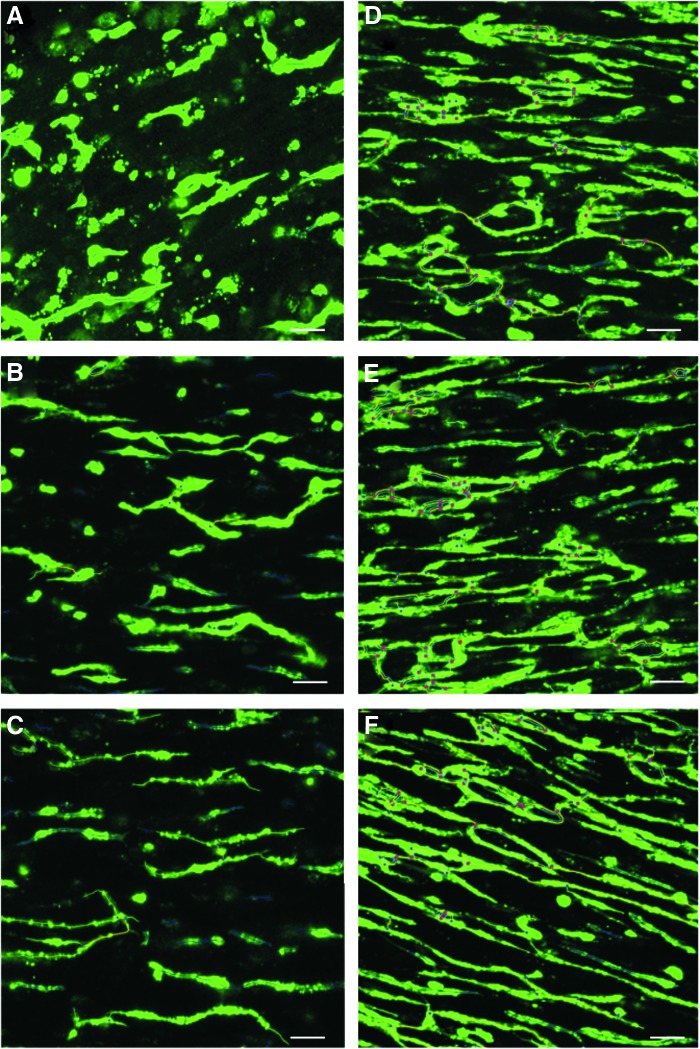
FIG. 4.
(A) Analysis by Angiogenesis Analyzer ImageJ's plugin, visualizing endothelial network formation of 60-μm z-projection images of fibrin BAMs with a total of 1 × 106 cells with 50% (A), 40% (B), and 30% (C) HUVECs or with a total of 2 × 106 cells with 50% (D), 40% (E), and 30% (F) HUVECs. Angiogenesis Analyzer indicates master junctions (pink dots), master segments (yellow), meshes (light blue), branches (green), and isolated segments (blue). Scale bar represents 50 μm. Color images available online at www.liebertpub.com/tea
Table 3.
Parameters Characterizing the Formation of Endothelial Networks in Fibrin Coculture BAMs with a Total of 1 × 106 Cells with 50% (A), 40% (B), and 30% (C) HUVECs or with a Total of 2 × 106 Cells with 50% (D), 40% (E), and 30% (F) HUVECs
| A (n = 37) | B (n = 8) | C (n = 5) | D (n = 219) | E (n = 65) | F (n = 50) | A–B | A–C | A–D | B–E | C–F | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total # cells in BAM | 1 × 106 | 1 × 106 | 1 × 106 | 2 × 106 | 2 × 106 | 2 × 106 | |||||
| # Muscle cells | 5 × 105 | 6 × 105 | 7 × 105 | 10 × 105 | 12 × 105 | 14 × 105 | |||||
| # HUVECs | 5 × 105 | 4 × 105 | 3 × 105 | 10 × 105 | 8 × 105 | 6 × 105 | |||||
| % branching length | 20.96 ± 13.28 | 39.05 ± 15.67 | 48.29 ± 8.19 | 76.36 ± 11.84 | 79.21 ± 8.01 | 75.92 ± 11.05 | ** | *** | *** | *** | *** |
| Number of isolated segments | 51.24 ± 14.29 | 36.00 ± 8.40 | 29.60 ± 2.70 | 18.72 ± 9.89 | 15.37 ± 4.78 | 16.44 ± 5.41 | ** | *** | *** | *** | *** |
| Number of junctions | 3.62 ± 2.64 | 7.63 ± 4.03 | 8.60 ± 2.61 | 47.43 ± 21.61 | 50.13 ± 13.54 | 49.64 ± 28.78 | ** | ** | *** | *** | *** |
| Number of branches | 7.78 ± 4.92 | 15.13 ± 6.47 | 15.40 ± 5.37 | 45.96 ± 13.84 | 48.33 ± 9.68 | 46.14 ± 17.55 | ** | ** | *** | *** | *** |
| Total length (μm) | 16422 μm ± 3591 | 20349 μm ± 5295 | 21488 ± 4574 | 38262 μm ± 7147 | 39920 μm ± 4942 | 40165 ± 8298 | * | * | *** | *** | *** |
| Number of meshes | 0.49 ± 0.8035 | 0.75 ± 0.71 | 1.00 ± 0.70 | 10.50 ± 7.14 | 12.35 ± 5.182 | 12.38 ± 8.82 | NS | NS | *** | *** | *** |
In the last five columns, statistical significances are shown for comparisons of indicated groups. Comparisons B–C, D–E, D–F, and E–F were also made, but did not result in statistically significant differences for any of the parameters. For multiple comparisons within groups [1 × 106 total cell count (A–C) or 2 × 106 total cell count (D–F)], a nonparametric Kruskal–Wallis test with Dunn's post-test was used. For pairwise comparisons between groups (A–D, B–E, and C–F), a Mann–Whitney test was performed.
30% HUVECs suffice to induce endothelial networks between aligned myofibers
Based on our previous findings and on research from other groups,7,9,18 we started experiments with coculture BAMs with a ratio of 50% muscle cells and 50% HUVECs to obtain myofiber and endothelial network formation. To optimize this ratio, BAMs with a total of 1 × 106 or 2 × 106 cells were made with different ratios, ranging from 50–70% muscle cells and 50–30% HUVECs.
In coculture BAMs with a total of 1 × 106 cells, a muscle cell:HUVEC ratio of 60:40 or 70:30 showed significantly better endothelial network formation compared with coculture BAMs with a 50:50 ratio (Table 3 columns A-C, Fig. 4 and Supplementary Fig. S3). Although there was a trend toward further improvement of the endothelial network formation in the 70:30 ratio compared with the 60:40 ratio, this was not significantly different. In the condition with a high percentage of HUVECs (50%), dense clusters of HUVECs occurred at certain places on the surface of the BAMs, which could not be analyzed by the Angiogenesis Analyzer. When a total number of 2 × 106 cells were used, all ratios performed equally well (Table 3 columns D–F). However, when compared with the same ratio in BAMs with 1 × 106 cells, there was a significant improvement in endothelial network formation for each ratio, indicating that increasing the total cell number to 2 × 106 is beneficial for all ratios studied (Supplementary Video S2). The dense clusters of HUVECs mentioned above were not observed in BAMs with a total cell count of 2 × 106 cells.
In coculture BAMs with a total of 1 × 106 cells, a ratio of 60:40 or 70:30 also showed significantly better myofiber formation compared with coculture BAMs with a 50:50 ratio: there was a significant increase in myofiber density and alignment (Table 4 columns A-C and Supplementary Fig. S4). When a total cell number of 2 × 106 was used, no major changes toward improvement of myofiber formation could be detected with increasing ratios of myoblasts (Table 4 columns D-F and Supplementary Fig. S4). When comparing the same ratios in BAMs with 1 × 106 versus 2 × 106 cells, there only was a consistent improvement in alignment of myofibers for the BAMs with 2 × 106 cells.
Table 4.
Parameters Characterizing the Formation of Myofibers in Fibrin Coculture BAMs with a Total of 1 × 106 Cells with 50% (A), 40% (B), and 30% (C) HUVECs or with a Total of 2 × 106 Cells with 50% (D), 40% (E), and 30% (F) HUVECs
| A (n = 11) | B (n = 24) | C (n = 19) | D (n = 34) | E (n = 20) | F (n = 15) | A–B | A–C | B–C | D–E | D–F | E–F | A–D | B–E | C–F | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total # cells in BAM | 1 × 106 | 1 × 106 | 1 × 106 | 2 × 106 | 2 × 106 | 2 × 106 | |||||||||
| # Muscle cells | 5 × 105 | 6 × 105 | 7 × 105 | 10 × 105 | 12 × 105 | 14 × 105 | |||||||||
| # HUVECs | 5 × 105 | 4 × 105 | 3 × 105 | 10 × 105 | 8 × 105 | 6 × 105 | |||||||||
| Number/microscopic field | 25.63 ± 10.02 | 47.08 ± 15.41 | 48.68 ± 12.56 | 40.48 ± 10.25 | 48.29 ± 13.62 | 49.16 ± 7.26 | ** | *** | NS | NS | ** | NS | ** | NS | NS |
| Alignment | 16.24 ± 5.96 | 6.25 ± 0.84 | 7.72 ± 4.30 | 5.09 ± 1.33 | 3.65 ± 0.79 | 4.912 ± 1.12 | *** | * | NS | *** | NS | ** | *** | *** | *** |
| Length (μm) | 118.90 ± 6.93 | 123.4 ± 13.9 | 119.7 ± 15.9 | 127.4 ± 37.56 | 121.3 ± 15.37 | 119.2 ± 13.18 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Diameter (μm) | 12.79 ± 4.60 | 16.88 ± 8.82 | 24.05 ± 7.18 | 13.26 ± 3.69 | 12.22 ± 2.88 | 11.96 ± 1.058 | NS | *** | ** | NS | NS | NS | NS | * | *** |
In the last nine columns, statistical significances are shown for comparisons of indicated groups. For multiple comparisons within groups [1 × 106 total cell count (A–C) or 2 × 106 total cell count (D–F)], a nonparametric Kruskal–Wallis test with Dunn's post-test was used. For pairwise comparisons between groups (A–D, B–E, and C–F), a Mann–Whitney test was performed.
Matrigel impacts myofiber distribution, but does not improve myofiber and endothelial network formation
In tissue engineering, Matrigel is often used as a (component of) hydrogel because it contains a complex mixture of ECM components and growth factors and has a beneficial impact on tissue growth in vitro.1,14 We hypothesized that addition of Matrigel to the fibrin ECM might enhance myofiber and endothelial network formation.
We assessed the addition of 10% Matrigel to the ECM of fibrin. This did not significantly change the thickness of the BAMs, neither in BAMs with 1 × 106 muscle cells nor in coculture BAMs with 5 × 105 muscle cells and 5 × 105 HUVECs (Table 2).
While in BAMs with 1 × 106 muscle cells in fibrin only, myofibers are evenly distributed throughout the BAM, the addition of 10% Matrigel to the ECM causes an uneven distribution and formation of myofibers. An outer region of 20–30 μm was observed, containing densely packed and highly aligned myofibers and a core with lower cell density containing significantly lower length and alignment of myofibers (Table 1, compare columns F-G with column D and Supplementary Fig. S5).
Compared with the homogeneously distributed fibrin BAMs, the outer densely packed region shows significantly longer myofibers, but there is no significant difference in alignment, fiber diameter, or number of myofibers (Table 1, column F versus D). The inner region contains myofibers that are less well aligned with no significant difference in the other parameters (Table 1, column G versus D). To conclude, the addition of 10% Matrigel does not result in a better myofiber formation throughout the entire BAM, but only in a small outer region of the BAM. In contrast, a decreased myofiber alignment is observed in the inner region. Overall, myofiber formation is heterogeneous throughout the BAM in the presence of Matrigel in the ECM.
Discussion
Obtaining thicker tissue-engineered constructs is one of the important goals in the field of tissue engineering. To succeed, vascularization is essential for obtaining viable constructs with increased survival time in vitro and improved engraftment upon transplantation.7,9,18 To do this, networks of endothelial cells, which form the innermost layer of blood vessels, must be established within the tissue-engineered muscle. The current BAM model consists of aligned multinucleated myofibers, without endothelial cells, starting from a population of human myoblasts and fibroblasts cast in ECM.19 We used the natural hydrogel fibrin as a scaffold for the cells rather than collagen or other alternatives. This choice was made because of its stiffness characteristics and angiogenic properties.20–24 The formation of 3D tissue-engineered human muscle in a fibrin ECM was already described in detail before; however, endothelial cells were not present in these 3D cultures.24
In our work, HUVECs were added to the muscle cell–fibrin mix to obtain endothelial networks between the myofibers. HUVECs are relatively easy to obtain from the umbilical cord, are robust in culture, and are capable of endothelial cell tube formation in vitro.25,26 Adding multiple cell types in an organoid presents a challenge regarding culture conditions.27 In addition, in our system, culture conditions needed to be modified to sustain growth and differentiation of both cell types. We observed that endothelial cells fail to survive and form networks in the optimal culture condition for fibrin BAMs in skeletal muscle growth and differentiation medium. Therefore, we analyzed myofiber formation in BAMs cultured in EGM-2, a growth medium optimized for endothelial cells. This resulted in thinner myofibers with fewer nuclei per fiber compared with the original medium. This may be explained both by a decreased proliferation of myoblasts during the first 2 days in EGM-2 versus SkGM, resulting in a lower cell density and a decreased myoblast fusion in EGM-2 as observed by a lower number of nuclei per myofiber. Nevertheless, aligned myofibers were still formed in BAMs cultured in EGM-2 while allowing the survival of HUVECs, making it the preferred medium for sustaining coculture BAMs.
Next, we investigated varying total cell numbers per BAM and different ratios of HUVECs versus muscle cells to determine the impact on endothelial network formation as well as myofiber formation. For BAMs with 1 × 106 total cells and 50% HUVECs, limited branching and mesh formation were observed. Network characteristics could be improved in cocultures with 40% or even 30% HUVECS. These endothelial networks have a higher number of branches and junctions and a lower number of isolated segments compared with the 50% HUVEC BAMs. The biggest improvement, however, was observed when a total cell number of 2 × 106 was used instead of 1 × 106 (Supplementary Video S2). In these BAMs, the total length of the endothelial network doubled, concurrent with a higher number of meshes, branches, and junctions and a lower number of isolated segments. For 2 × 106 cells, there was no further improvement when the muscle cell:HUVEC ratio was increased from 50:50 to 60:40 or 70:30. This suggests that for the given system and conditions tested, a maximal endothelial network formation was reached when 2 × 106 cells were used, independent of the muscle cell:HUVEC ratio. In addition, for myofiber formation, the 30% and 40% HUVEC BAMs with a total cell number of 1 × 106 contained a higher number of myofibers that were thicker and better aligned than in the 50% HUVEC BAMs. Moreover, in the 50% HUVEC BAMs, HUVECs were unequally distributed throughout the BAM with denser layers toward the surface. These dense layers of HUVECs were not observed in the conditions with 2 × 106 cells, pointing to a better distribution of the HUVECs within the BAM. Overall, parameters of myofiber formation performed equally well or better when using 2 × 106 cells versus 1 × 106 cells. Similar to endothelial network formation, changing the muscle cell:HUVEC ratio did not further influence myofiber formation for 2 × 106 total cells. These and the above findings suggest an optimal combination of myofiber and endothelial network formation in coculture BAMs with 2 × 106 cells and a minimum of 50% muscle cells (Supplementary Video S3). The presence of 1 × 106 muscle cells (both in BAMs with or without endothelial cells) has a double advantage over BAMs with only 5 × 105 muscle cells. First, as can be expected when using more muscle cells, myofiber density is higher. Second, a concurrent positive effect is observed in the alignment of myofibers. At the tissue level, BAMs with a higher total cell number have a significantly smaller thickness regardless of the type of cells. This may be explained by an augmented cell-mediated contraction of the ECM. We can conclude that the presence of HUVECs does not impair the formation of myofibers.
In addition to a better endothelial network formation when doubling total cell number per BAM, an increased amount of muscle cells has an important effect on endothelial network formation in the presence of similar amounts of HUVECs (compare Table 4 column A vs column F). It has been described that vascular endothelial growth factor (VEGF) is also produced by myoblasts and this expression is enhanced when myofibers are kept under uniaxial tension.14 In the experiments described in this article, we used EGM-2, which contains 0.5 ng/mL VEGF. Addition of VEGF in constructs with only endothelial cells leads to a more organized growth pattern of the endothelial cells.14 Although the amount of VEGF secreted by the myofibers in the cell culture medium (<0.3 ng/mL)14 was much lower than the amount of VEGF present in EGM-2, local VEGF concentrations in our extracellular fibrin matrix may be much higher because of VEGF binding to fibrin.28 This is consistent with the observation that even lower total amounts of HUVECs still result in better endothelial network formation when the total cell number is kept at 1 × 106 since the total amount of myofibers is higher.
In none of the myofibers we visualized, we could detect cross-striations. These have been described in vitro both in 2D29 as well as in 3D muscle cultures.1,30 We performed confocal microscopy at day 7 of cocultures, whereas cross-striations were reported only after 14 days of culture. Next to a prolonged culture time, supplementing EGM-2 with specific growth factors to stimulate myoblast fusion, such as MAGIC-F1 protein, IGF-1, and follistatin,31 can be explored in the future. In addition, subjecting BAMs to electrical and/or mechanical stimulation has been described to further improve several muscle characteristics, such as viscoelastic properties, protein synthesis, myofiber diameter, and density, thus improving the level of maturation of myofibers.32,33
Next to HUVECs, other endothelial cell populations may be worthwhile for further investigation. In particular, endothelial colony-forming cells (ECFCs), also termed blood outgrowth endothelial cells, are able to perform de novo tube formation and are proangiogenic in vitro and in vivo.34 Human peripheral blood and umbilical cord blood ECFCs form de novo functional blood vessels when seeded into a matrix and implanted in vivo.35 Microvessel density was similar for ECFCs and HUVECs, indicating a robust vasculogenic potential for both cell types.36 Adult peripheral blood ECFCs formed unstable vessels, while umbilical cord blood-derived ECFCs transiently formed vessels when implanted alone and were stable for four months when coimplanted with fibroblasts in an immunodeficient recipient.37 Our muscle cell population also contains fibroblasts. These have been described to provide support for endothelial cells as well as modulate their migration, viability, and network formation.38 Optimizing fibroblast ratios and/or using other cell populations such as pericytes to stabilize endothelial networks may also further improve the cocultures.39 It remains to be seen though if the type of endothelial cells, which are used in vitro, is a critical factor once the construct is implanted since the implanted endothelial network may be replaced by the host. Engineered human vascular networks, which were transplanted into third-degree burns, connected with the host vessels during the first week, but during a remodeling phase in the second week after transplantation, the transplanted vessels regressed40 and were replaced by host vessels. Transformation into mature blood vessels occurs through an inflammation-mediated process of vascular remodeling.41
As described by others,7,9,11,18 prevascularization of tissue-engineered muscle improves vascularization, blood perfusion, and survival of the muscle constructs upon transplantation. Specifically, Koffler et al.18 have shown that the degree of prevascularization in vitro, which highly depends on ECM, cell types, cell number, culture conditions, and duration of in vitro culture, determines the in vivo vascularization. The goal of our study was to optimize experimental conditions to obtain maximal endothelial network formation in coculture BAMs. These findings open perspectives toward culture of larger prevascularized BAMs with potential for human muscle transplantations as well as a physiologically relevant in vitro model for studying diseases and therapies.5,42,43 Host blood vessels are able to invade avascular muscle bundles15; however, this still limits the size of muscle bundles that can be kept in culture preimplantation. Ideally, anastomosis of the preimplantation network with host vessels occurs. Anastomosis between HUVEC-derived vessels and host (mouse) vessels has been detected8 and this occurs as early as 1 day after implantation,18 although it is not clear to what extent anastomosis occurs. Whether our prevascularized BAMs survive in vivo and allow for anastomosis will be assessed by future implantation experiments.
We also tested the addition of 10% Matrigel in coculture BAMs. Matrigel has been used in combination with collagen type I to form BAMs.19 It is a gelatinous protein mixture obtained from mouse sarcoma cells containing many ECM proteins and growth factors. Fibrin (1 mg/mL) without Matrigel supports a homogenous formation of myofibers. In contrast, in the BAMs with 10% Matrigel, the myofibers in the exterior 20 μm of the BAM are longer than in the condition without Matrigel. In the center of the BAM, the myofibers are less well aligned compared with the fibrin-only ECM. To conclude, we do not suggest using Matrigel as part of the ECM of BAMs with a fibrin-based ECM to obtain homogeneously distributed and formed myofibers. Moreover, the composition of Matrigel is not fully characterized.
In addition, different gel formulations and gelation conditions can strongly influence fibrin biochemistry and ultrastructure, which in turn has an important effect on its angiogenic properties and on cell behavior.44 Fibrin ultrastructure (and cell behavior) is also affected by fibrinogen and thrombin concentrations, calcium ion content, ionic strength, temperature, and pH during gelation. For both thrombin and fibrinogen, there is not only a difference between brands but also within one brand, depending on the source of the fibrin.44 Therefore, a direct comparison of these results with previous results in literature is difficult since different research groups not only use different types of fibrin but also different cell types, cell numbers, and volumes of ECM in tissue engineering of BAMs.5,7,9,12,18–20
To create muscle tissue similar to that in the adult body, still other hurdles need to be overcome. A major one is the formation of neuromuscular synapses. Therefore, myofibers should express functional nicotinic acetylcholine receptors (AchRs) capable of generating acetylcholine-induced action potentials. Acetylcholine receptor expression increases sharply just before myoblast fusion,45 but is distributed randomly along the surface. During later phases of myogenesis, these receptors cluster at the motor endplates. In the absence of neural cells, BAMs have been shown to form mature AchR clusters by the addition of agrin and laminin.46 In the absence of mature AchR clusters, BAMs can still be stimulated by electrical current, which results in increased protein synthesis, increased force production, and excitability.5,47 The introduction of neural cells to a monolayer of myotubes resulted in the formation of neuromuscular-like junctions with clustering of acetylcholine receptors.48 It is not known whether endothelial cells could affect the expression of muscle nicotinic receptors and/or induce clustering of AchRs. This could be further studied with our coculture system. Furthermore, BAMs should remain under tension in vivo to avoid atrophy. Therefore, another issue to be addressed is the integration of BAMs with (bioartificial) tendons. Our model system allows for extensive biochemical, physical, cellular, and electrical characterization of the effect of adding different cell types to muscle cells during myofiber formation. It thus bridges the gap between 2D culture systems and in vivo transplantation of exogenous muscle tissue.
To our knowledge, we have tissue engineered for the first time a human BAM containing advanced endothelial cell networks by coculturing HUVECs and human muscle cells in a fibrin ECM.
Supplementary Material
Acknowledgments
The authors thank Petra D'hooge for technical assistance with confocal microscopy and Sigrid Vanryckeghem for administrative support. This work was funded by the Research Fund KU Leuven (CREA/12/034). L.T. is a Postdoctoral Fellow of the Research Foundation-Flanders (FWO).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hinds S., Bian W., Dennis R.G., and Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 32, 3575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishman J.M., Tyraskis A., Maghsoudlou P., Urbani L., Totonelli G., Birchall M., et al. . Skeletal muscle tissue engineering: which cell to use? Tissue Eng Part B Rev 44, 1, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Klumpp D., Horch R.E., and Beier J.P. Tissue engineering of skeletal muscle. In: Eberli D., ed. Tissue Engineering and Organ Regeneration. Intechopen, 2011. DOI: 10.5772/1146, ISBN 978-953-307-688-1 [DOI] [Google Scholar]
- 4.Thorrez L., Shansky J., Wang L., Fast L., VandenDriessche T., Chuah M., et al. . Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials 29, 75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenburgh H., Shansky J., Benesch-Lee F., Barbata V., Reid J., Thorrez L., et al. . Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37, 438, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Shansky J., and Chromiak J. A simplified method for tissue engineering skeletal muscle organoids in vitro. In vitro cell. Dev Biol Anim 33, 659, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Levenberg S., Rouwkema J., Macdonald M., Garfein E.S., Kohane D.S., Darland D.C., et al. . Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23, 879, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Shandalov Y., Egozi D., Koffler J., Dado-Rosenfeld D., Ben-Shimol D., Freiman A., et al. . An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci U S A 111, 6010, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesman A., Koffler J., Atlas R., Blinder Y.J., Kam Z., and Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials 32, 7856, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Nagamori E., Ngo T.X., Takezawa Y., Saito A., Sawa Y., Shimizu T., et al. . Network formation through active migration of human vascular endothelial cells in a multilayered skeletal myoblast sheet. Biomaterials 34, 662, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Carosio S., Barberi L., Rizzuto E., Nicoletti C., Del Prete Z., and Musarò A. Generation of eX vivo-vascularized Muscle Engineered Tissue (X-MET). Sci Rep 3, 1420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criswell T.L., Corona B.T., Wang Z., Zhou Y., Niu G., Xu Y., et al. . The role of endothelial cells in myofiber differentiation and the vascularization and innervation of bioengineered muscle tissue in vivo. Biomaterials 34, 140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller J.S., Stevens K.R., Yang M.T., Baker B.M., Nguyen D.-H.T., Cohen D.M., et al. . Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11, 1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Schaft D.W.J., van Spreeuwel A.C.C., van Assen H.C., and Baaijens F.P.T. Mechanoregulation of vascularization in aligned tissue-engineered muscle: a role for vascular endothelial growth factor. Tissue Eng Part A 17, 2857, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Juhas M., Engelmayr G.C., Fontanella A.N., Palmer G.M., and Bursac N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc Natl Acad Sci U S A 111, 5508, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasband W. ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA: http://imagej.nih.gov/ij/ [Google Scholar]
- 17.Carpentier G. Gilles carpentier research web site: computer image analysis. Angiogenesis Analyzer for ImageJ, 2012 [Google Scholar]
- 18.Koffler J., Kaufman-Francis K., Yulia S., Dana E., Daria A.P., Landesberg A., et al. . Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci U S A 108, 14789, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorrez L., Vandenburgh H., Callewaert N., Mertens N., Shansky J., Wang L., et al. . Angiogenesis enhances factor IX delivery and persistence from retrievable human bioengineered muscle implants. Mol Ther 14, 442, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Rosso F., Marino G., Giordano A., Barbarisi M., Parmeggiani D., and Barbarisi A. Smart materials as scaffolds for tissue engineering. J Cell Physiol 203, 465, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Morin K.T., and Tranquillo R.T. Guided sprouting from endothelial spheroids in fibrin gels aligned by magnetic fields and cell-induced gel compaction. Biomaterials 32, 6111, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Morin K.T., Smith A.O., Davis G.E., and Tranquillo R.T. Aligned human microvessels formed in 3D fibrin gel by constraint of gel contraction. Microvasc Res 90, 12, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morin K.T., Dries-Devlin J.L., and Tranquillo R.T. Engineered microvessels with strong alignment and high lumen density via cell-induced fibrin gel compaction and interstitial flow. Tissue Eng Part A 20, 553, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiron S., Tomczak C., Duperray A., Lainé J., Bonne G., Eder A., et al. . Complex interactions between human myoblasts and the surrounding 3D fibrin-based matrix. PLoS One 7, e36173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakatsu M.N., Sainson R.C.A., Aoto J.N., Taylor K.L., Aitkenhead M., Pérez-del-Pulgar S., et al. . Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res 66, 102, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Arnaoutova I., George J., Kleinman H.K., and Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis 12, 267, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Baldwin J., Antille M., Bonda U., De-Juan-Pardo E.M., Khosrotehrani K., Ivanovski S., et al. . In vitro pre-vascularisation of tissue-engineered constructs A co-culture perspective. Vasc Cell 6, 13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahni A., and Francis C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 96, 3772, 2000 [PubMed] [Google Scholar]
- 29.Engler A.J., Griffin M.A., Sen S., Bönnemann C.G., Sweeney H.L., and Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166, 877, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin N.R.W., Passey S.L., Player D.J., Khodabukus A., Ferguson R.A., Sharples A.P., et al. . Factors affecting the structure and maturation of human tissue engineered skeletal muscle. Biomaterials 34, 5759, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Cassano M., Biressi S., Finan A., Benedetti L., Omes C., Boratto R., et al. . Magic-factor 1, a partial agonist of Met, induces muscle hypertrophy by protecting myogenic progenitors from apoptosis. PLoS One 3, e3223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell C.A., Smiley B.L., Mills J., and Vandenburgh H.H. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol 283, C1557, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Rangarajan S., Madden L., and Bursac N. Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles. Ann Biomed Eng 42, 1391, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Beem R.T., Verloop R.E., Kleijer M., Noort W.A., Loof N., Koolwijk P., et al. . Blood outgrowth endothelial cells from cord blood and peripheral blood: angiogenesis-related characteristics in vitro. J Thromb Haemost 7, 217, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Yoder M.C., Mead L.E., Prater D., Krier T.R., Mroueh K.N., Li F., et al. . Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109, 1801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melero-Martin J.M., Khan Z.A., Picard A., Wu X., Paruchuri S., and Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109, 4761, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Au P., Daheron L.M., Duda D.G., Cohen K.S., Tyrrell J.A., Lanning R.M., et al. . Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood 111, 1302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunz-Schughart L.A., Schroeder J.A., Wondrak M., van Rey F., Lehle K., Hofstaedter F., et al. . Potential of fibroblasts to regulate the formation of three-dimensional vessel-like structures from endothelial cells in vitro. Am J Physiol Cell Physiol 290, C1385, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Sampaolesi M., Blot S., D'Antona G., Granger N., Tonlorenzi R., Innocenzi A., et al. . Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444, 574, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Hanjaya-Putra D., Shen Y.-I., Wilson A., Fox-Talbot K., Khetan S., Burdick J.A., et al. . Integration and regression of implanted engineered human vascular networks during deep wound healing. Stem Cells Transl Med 2, 297, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh J.D., Sawh-Martinez R., Brennan M.P., Jay S.M., Devine L., Rao D.A., et al. . Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 107, 4669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandenburgh H., Shansky J., Benesch-Lee F., Skelly K., Spinazzola J.M., Saponjian Y., et al. . Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. FASEB J 23, 3325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandenburgh H. High-content drug screening with engineered musculoskeletal tissues. Tissue Eng Part B Rev 16, 55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morin K.T., and Tranquillo R.T. In vitro models of angiogenesis and vasculogenesis in fibrin gel. Exp Cell Res 319, 2409, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krause R.M., Hamann M., Bader C.R., Liu J.H., Baroffio a, and Bernheim L. Activation of nicotinic acetylcholine receptors increases the rate of fusion of cultured human myoblasts. J Physiol 489, 779, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Shansky J., and Vandenburgh H. Induced formation and maturation of acetylcholine receptor clusters in a defined 3D bio-artificial muscle. Mol Neurobiol 48, 397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donnelly K., Khodabukus A., Philp A., Deldicque L., Dennis R.G., and Baar K. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng Part C Methods 16, 711, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Larkin L.M., Van der Meulen J.H., Dennis R.G., and Kennedy J.B. Functional evaluation of nerve-skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim 42, 75, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.