INTRODUCTION
Clozapine is a dibenzodiazepine atypical antipsychotic indicated for the management of treatment-resistant schizophrenia.1 It is well known for its risk of agranulocytosis; it is also associated, though less commonly, with potentially fatal adverse cardiovascular effects, particularly myocarditis. This article reports a case of suspected clozapine-induced myocarditis.
CASE REPORT
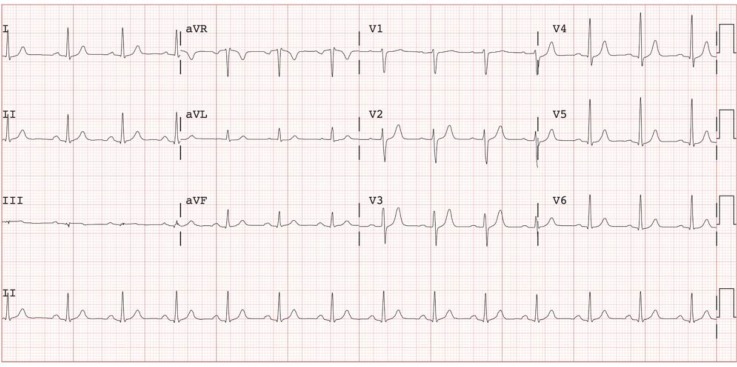
A non-obese patient (age mid-30s), with a history of schizophrenia diagnosed when the patient was a teenager, was admitted to the psychiatry service of an urban hospital.* One month after admission, the patient experienced 2 episodes of sudden-onset, centralized chest pressure. On both occasions, the pressure resolved spontaneously after 1–2 min and was not associated with heart failure or flu-like symptoms. The patient denied any history of cardiovascular disease or family history of premature cardiovascular disease, as well as any history of recent alcohol abuse, recreational drug use, or viral infection. Initial electrocardiography showed normal sinus rhythm with a heart rate of 82 beats/min and no acute ischemic changes (Figure 1). Initial bloodwork on the psychiatry unit showed that troponin I and creatine kinase were elevated. Medications for acute coronary syndrome were initiated, and the patient was transferred to the coronary intensive care unit (CICU) for further investigations and monitoring.
Figure 1.
Electrocardiogram on initial presentation (while on the psychiatry unit).
The remainder of the patient’s medical history was noncontributory. The only regularly scheduled medication before transfer to the CICU was clozapine 100 mg by mouth daily, which the patient had been receiving consistently for 1 month. It is not known whether the patient had received clozapine in the past. On the day before onset of the cardiac symptoms, this medication had been changed to paliperidone 150 mg via intramuscular injection every 4 weeks to improve adherence with therapy.
On admission to the CICU, the patient was afebrile, with blood pressure 139/88 mm Hg, heart rate 75 beats/min, respiratory rate 18 breaths/min, and oxygen saturation 96% on room air. The results of a cardiac examination were unremarkable. On admission to the CICU several hours later, levels of troponin I and creatine kinase remained elevated (Table 1).
Table 1.
Results of Laboratory Testing for a Patient Admitted to the Coronary Intensive Care Unit
| Variable | Patient’s Value | Normal Range |
|---|---|---|
| Troponin I (µg/L) | 4.22 | < 0.15 |
| Creatine kinase (U/L) | 420 | < 250 |
| B-type natriuretic peptide (pg/mL) | 28 | < 100 |
| Leukocyte count (× 109/L) | 7.3 | 4.0–11.0 |
| Eosinophil count (× 109/L) | 0.4 | 0.0–0.7 |
| Serum creatinine (µmol/L) | 74 | 50–115 |
| Hemoglobin (g/L) | 144 | 135–175 |
| Thyroid-stimulating hormone (mU/L) | 1.63 | 0.20–4.00 |
| Low-density lipoprotein cholesterol (mmol/L) | 1.42 | NA |
Repeat electrocardiography showed no changes, and subsequent coronary angiography demonstrated no coronary artery disease. In light of these results, cardiac magnetic resonance (CMR) imaging was performed, which revealed mild left ventricular systolic dysfunction, with an ejection fraction of 52%. Mild hypokinesis was noted in the inferior and inferolateral walls, with associated edematous changes. Delayed gadolinium-enhanced imaging revealed patchy subepicardial enhancement in the inferolateral, lateral, and inferior walls, consistent with a diagnosis of myocarditis.
On the basis of these results, the medications for acute coronary syndrome were discontinued. The patient was treated supportively with analgesics and was discharged from hospital, with approval, after 48 h in the CICU. The patient’s condition was stable from a psychiatric perspective, as the first dose of paliperidone had been given 3 days before hospital discharge. Repeat CMR imaging performed 3 months later showed partial resolution of enhancement on delayed gadolinium imaging.
An assessment of causality using the Naranjo algorithm2 indicated a possible adverse drug reaction (score 4/13). Points were assigned for previous conclusive reports of this adverse reaction, appearance of the adverse event after administration of the medication and improvement after discontinuation, and confirmation by objective evidence (CMR imaging). One point was deducted for alternative etiology, as an idiopathic cause could not be ruled out. Rechallenge with clozapine and measurement of serum levels of the drug were not performed.
DISCUSSION
Multiple reports of clozapine-associated myocarditis, pericarditis, and cardiomyopathy have appeared in the published literature.3–10 According to the manufacturer’s Canadian monograph, the estimated incidence of myocarditis is 1 per 14 000 patient-years, with a mortality rate of 23%.1 Other studies have estimated the incidence at between 0.015% and 0.188% (up to about 1 in 500), with one case series estimating the incidence to be as high as 0.7%–1.2% (up to about 1 in 100).4,5 The postulated mechanism is immunoglobulin E–mediated hypersensitivity, as up to about two-thirds of patients present with hypereosinophilia.3–5 The risk of cardiovascular toxicity is highest in, but not limited to, the first month of therapy; however, the published literature includes cases of myocarditis occurring several years after initiation of clozapine.6 A review of 10 fatal cases of clozapine-associated myocarditis found that, compared with nonfatal cases, there was a higher incidence of obesity and longer duration of clozapine therapy (21 days versus 17 days) in fatal cases.7 A baseline cardiovascular risk assessment is recommended for all patients before clozapine is started.3 The product monograph indicates that clozapine is contraindicated for patients with “severe” cardiac disease.1
The symptoms of clozapine-associated myocarditis (or any other form of myocarditis) are often nonspecific and may include fever, lethargy, dyspnea, chest discomfort, and palpitations.3–5 The diagnosis of clozapine-associated myocarditis can be challenging, as the symptoms may resemble adverse effects associated with dose titration, such as fever, tachycardia, and lethargy. Signs may include elevation of jugular venous pressure, pericardial friction rub, crackles on respiratory auscultation, and pulmonary edema evident on chest radiography. Electrocardiography may show sinus tachycardia, diffuse nonspecific ST-segment and T-wave abnormalities, and intraventricular conduction deficits. Left ventricular dysfunction may or may not be present on echocardiography.
In a recent case series, Ronaldson and others3 compared 75 cases of suspected clozapine-associated myocarditis and 94 controls. The mortality rate among the cases was 12%, and most cases occurred between 2 and 3 weeks after clozapine initiation. Although about 90% of the patients presented with positive troponin I or T, only 50% of the patients had chest pain. Other common symptoms included tachycardia, fever, and tachypnea. Over 50% of the patients had eosinophilia, and two-thirds had left ventricular dysfunction on echocardiography. On the basis of this case series, the authors3 proposed a monitoring protocol for the first 4 weeks of clozapine therapy to detect cases of myocarditis. This protocol involves baseline measurement of troponin and C-reactive protein, echocardiography and vital signs every 2 days for 4 weeks, and serial measurement of troponin and C-reactive protein weekly for 4 weeks. For any patient who exhibits signs or symptoms of myocarditis, the authors recommended either increasing the frequency of monitoring or discontinuing clozapine and consulting the cardiology service. They do not advocate monitoring for eosinophilia, as this condition does not occur in all cases. Similarly, Annamraju and others8 recommended a simple 6-item myocarditis symptom questionnaire (fatigue, dyspnea, chest discomfort, palpitations, fever or flu-like illness, peripheral edema) to be performed weekly for the first 8 weeks of clozapine therapy to improve early detection.
It is recommended that clozapine be immediately and permanently discontinued for all patients with suspected clozapine-associated myocarditis.1 However, this may present a therapeutic challenge, as clozapine is indicated for patients with refractory schizophrenia. A small number of case reports in the published literature have described successful rechallenge with clozapine in patients with suspected clozapine-associated myocarditis.9,10 If rechallenge is undertaken, clozapine should be started at a low dose and should progress with slow dose titration and frequent monitoring of both troponin and cardiac function.
All clinicians who care for patients receiving clozapine should be aware of this rare but potentially fatal cardiac adverse effect. Monitoring protocols or symptom questionnaires may help in the early detection and treatment of clozapine-associated myocarditis, particularly in the first 4 weeks of therapy. Any patient who exhibits signs or symptoms of myocarditis while receiving clozapine should undergo an immediate cardiac assessment.
This case represents a typical, though subtle, case of clozapine-associated myocarditis, as the patient presented with nonspecific chest discomfort within the first month of therapy, but no other signs or symptoms. Indeed, early diagnosis may have been aided by the use of CMR imaging. Coincidentally, clozapine had already been discontinued in favour of a 4-week intramuscular injection to aid patient adherence with therapy; therefore, management was appropriate despite clozapine being discontinued for another indication. This case had a favourable outcome, but it highlights the importance of rapid cardiac assessment when a patient presents with any cardiac symptoms.
Footnotes
It was not feasible to obtain the patient’s informed consent for publication of this case report. Approval from the University of Alberta Health Research Ethics Board was not obtained, as such approval is not required for individual case reports. Potentially identifying details not pertinent to understanding of the case have been omitted.
Competing interests: None declared.
Funding: None received.
References
- 1.Clozaril (clozapine tablets) [product monograph] Dorval (QC): Novartis Pharmaceuticals Canada Inc; 2014. Aug 19, [Google Scholar]
- 2.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 3.Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458–65. doi: 10.3109/00048674.2011.572852. [DOI] [PubMed] [Google Scholar]
- 4.Haas SJ, Hill R, Krum H, Liew D, Tonkin A, Demos L, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993–2003. Drug Saf. 2007;30(1):47–57. doi: 10.2165/00002018-200730010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Merrill DB, Ahmari SE, Bradford JME, Lieberman JA. Myocarditis during clozapine treatment. Am J Psychiatry. 2006;163(2):204–8. doi: 10.1176/appi.ajp.163.2.204. Erratum in: Am J Psychiatry. 2006;163(3):556. [DOI] [PubMed] [Google Scholar]
- 6.Lang UE, Willbring M, von Golitschek R, Schmeisser A, Matschke K, Malte Tugtekin S. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576–80. doi: 10.1177/0269881107082136. [DOI] [PubMed] [Google Scholar]
- 7.Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ. Clinical course and analysis of ten fatal cases of clozapine-induced myocarditis and comparison with 66 surviving cases. Schizophr Res. 2011;128(1–3):161–5. doi: 10.1016/j.schres.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Annamraju S, Sheitman B, Saik S, Stephenson A. Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol. 2007;27(5):479–83. doi: 10.1097/jcp.0b013e31814e5e68. [DOI] [PubMed] [Google Scholar]
- 9.Bray A, Reid R. Successful clozapine rechallenge after acute myocarditis. Aust N Z J Psychiatry. 2011;45(1):90. doi: 10.3109/00048674.2010.533365. [DOI] [PubMed] [Google Scholar]
- 10.Granja-Ingram NM, James J, Clark N, Stovall J, Heckers S. Successful re-exposure to clozapine after eosinophilia and clinically suspected myocarditis. Rev Bras Psiquiatr. 2013;35(1):95–6. doi: 10.1016/j.rbp.2012.10.005. [DOI] [PubMed] [Google Scholar]