Abstract
Dopaminergic systems regulate the release of several hormones including growth hormone (GH), thyroid hormones, insulin, glucocorticoids and prolactin (PRL) that play significant roles in the regulation of various Cytochrome P450 (CYP) enzymes. The present study investigated the role of dopamine D2-receptor-linked pathways in the regulation of CYP1A1, CYP1A2 and CYP1B1 that belong to a battery of genes controlled by the Aryl Hydrocarbon Receptor (AhR) and play a crucial role in the metabolism and toxicity of numerous environmental toxicants. Inhibition of dopamine D2-receptors with sulpiride (SULP) significantly repressed the constitutive and benzo[a]pyrene (B[a]P)-induced CYP1A1, CYP1A2 and CYP1B expression in the rat liver. The expression of AhR, heat shock protein 90 (HSP90) and AhR nuclear translocator (ARNT) was suppressed by SULP in B[a]P-treated livers, whereas the AhRR expression was increased by the drug suggesting that the SULP-mediated repression of the CYP1 inducibility is due to inactivation of the AhR regulatory system. At signal transduction level, the D2-mediated down-regulation of constitutive CYP1A1/2 and CYP1B1 expression appears to be mediated by activation of the insulin/PI3K/AKT pathway. PRL-linked pathways exerting a negative control on various CYPs, and inactivation of the glucocorticoid-linked pathways that positively control the AhR-regulated CYP1 genes, may also participate in the SULP-mediated repression of both, the constitutive and induced CYP1 expression. The present findings indicate that drugs acting as D2-dopamine receptor antagonists can modify several hormone systems that regulate the expression of CYP1A1, CYP1A2 and CYP1B1, and may affect the toxicity and carcinogenicity outcome of numerous toxicants and pre-carcinogenic substances. Therefore, these drugs could be considered as a part of the strategy to reduce the risk of exposure to environmental pollutants and pre-carcinogens.
Introduction
CYP1A1, CYP1A2 and CYP1B1 belong to a battery of genes that are transcriptionally activated by the aromatic hydrocarbon receptor [1]. More than 90% of known chemical carcinogens, including aromatic amines and polycyclic aromatic hydrocarbons (PAH)s, are substrates of these cytochromes [2–8], and their metabolism often results in the formation of active carcinogenic metabolites [9,10]. Benzo[a]pyrene (B[a]P) is the major PAH component in cigarette smoke and environmental mixtures, such as coal tar and diesel exhaust condensate and is found in the heavily polluted air of urban and industrial areas, in water and heavily cooked food [11]. B[a]P is partly metabolized by CYP1A isozymes to an electrophilic reactive intermediate that covalently binds to DNA and initiates carcinogenesis [3,5]. In addition, B[a]P, acts as a ligand of the AhR and as an inducer of the CYP1 enzymes. The dual role of B[a]P as an inducer of CYP1A1/2 and CYP1B1 and as a pre-carcinogenic substrate for these cytochromes, indicates that B[a]P and related compounds constitute a particularly important group of toxicants able to enhance their own metabolic activation and carcinogenicity [12].
Previous studies have shown that psychological stress and adrenergic receptor (AR)-linked pathways can regulate the expression of cytochrome P450 enzymes [13–18]. Specifically, restraint stress up-regulated CYP1A2 in the murine and rat liver [13,19,20], and AR-agonists or antagonists, and drugs modifying central and peripheral catecholaminergic activity, have a strong impact on the expression of constitutive and B[a]P-induced CYP1A1/2 expression [13]. These results suggest a strong regulatory role of stress and related adrenergic signalling pathways in the regulation of both constitutive and B[a]P induced CYP1A1/2 expression [13,21].
Dopaminergic systems play also significant roles in the regulation of several CYP isozymes catalyzing the metabolism of the majority of prescribed drugs [21–23]. In particular, inhibition of dopamine D2-receptors markedly repressed hepatic CYP2C11, CYP2D1/2, CYP2E1 and CYP3A1/2 expression in rats [22,23]. In this regulatory loop the role of insulin/PI3K/AKT signalling pathway is critical [24].
The D2-dopaminergic receptor-mediated CYP regulation is potentially highly significant as a wide array of drugs, prescribed for a variety of diseases, such as psychosis, depression, bipolar disorder and Parkinson's disease, exert their effects mainly via D2-dopaminergic receptor-linked pathways [25]. These drugs acting as either D2-receptor-agonists or antagonists can modify the activity of several hormonal pathways including the insulin/PI3K/AKT signalling pathway thus influencing the expression of various drug metabolizing cytochromes. This effect may lead to significant drug-drug interactions and may influence the outcome of pharmacotherapy and drug toxicity [18,26,27].
The aim of this study was to investigate the role of D2-dopaminergic receptor- related pathways in the regulation of cytochrome CYP1A1, CYP1A2 and CYP1B1 in the liver. For this purpose, rats were treated with selective D2-antagonists and exposed to either B[a]P or the vehicle alone [22]. The findings indicated the critical role of dopamine D2-receptors in the regulation of the constitutive and B[a]P-induced expression of these cytochromes, and suggest that drugs binding to dopamine D2-receptors may modify the toxicity of environmental pollutants and pre-carcinogens interfering with their metabolism.
Materials and Methods
Animals
Adult male inbred Wistar rats (Kuo/Ioa/rr) 3 months old (weighing 250–300g) were used for this study. All animals were housed in groups of 5 and maintained in plastic cages (Makrolon) with wood chip bedding, under a constant temperature (20°C) and a 12h light/dark cycle. Food (the standard rodent chow) and tap water was available ad libitum. All in vivo animal experiments and in vitro experiments employing primary hepatocytes isolated from rats were reviewed and approved by the Institutional Animal Care and Use Committee of the Medical School of the University of Ioannina, and the study has been carried out in strict accordance with the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23), revised 1996 and with the Guiding Principles in the Use of Animals in Toxicology, the adopted by the Society of Toxicology in 1989. Efforts were made in order to minimize the number of animals used and reduce their suffering.
Drugs
The following drugs have been used in this study: Sulpiride (Sigma, USA.); benzo[a]pyrene (Sigma- Aldrich, USA) and L-741,626 (SID 50104688 in PubChem; Sigma-Aldrich, USA).
Experimental procedure
Groups of five animals each received the selective dopamine D2-receptor antagonist, sulpiride (12mg/kg b.w., s.c.; SULP) or the vehicle (normal saline). Alternatively, the highly selective dopamine D2-receptor antagonist, L-741,626 (1.5mg/kg b.w., i.p.) was also administered in a group of rats. The drugs were administered twice daily and for four consecutive days (totally 7 injections in each treatment group). In parallel to SULP treatment, the animals received either olive oil or benzo[a]pyrene (10mg/kg b.w., i.p.) once daily for three consecutive days. The last dose of B[a]P was administered 24 hours before sacrifice.
At the end of the experiment, two hours after the last drug treatment, the animals were sacrificed by decapitation and trunk blood was collected for hormonal determinations. The samples were kept at -20°C until assayed. Simultaneously, the brains were rapidly removed and the dissected hypothalamus was immediately frozen in liquid nitrogen and stored at -80°C until assayed. Parts of the livers were also taken for microsome isolation, total RNA, nuclear and cytosol extraction and were kept at -80°C until analyzed.
Neurochemical analysis
Dopaminergic activity in the hypothalamus was assessed by measuring DA, DOPAC and HVA concentrations. Hypothalamic DA turnover ratio was determined by calculating the rates of DOPAC over DA levels in this brain region. The DOPAC/DA turnover ratio was used as an index of the DA turnover rate, which reflects the dopaminergic activity including the release and/or metabolism of DA, because evidence suggests that dopaminergic activity is better evaluated with DOPAC/DA than with tissue DA, DOPAC and HVA levels [28,29]. For the determination of DA and its metabolites concentration in the hypothalamus, the high performance liquid chromatography with electrochemical detection (HPLC-EC) was used, as previously described [30–32], with some minor modifications. Briefly, the samples were weighed and homogenized for 20 s with a sonicator in ice cold 0.2 N perchloric acid (HClO4). The homogenate was centrifuged at 13,000 rpm at 4°C for 15 min and the supernatant was divided into two portions. The measured compounds were DOPAC and HVA. An aliquot of 200 μl was transferred to an eppendorf tube containing 20 mg activated alumina and was used to extract dopamine (DA) and DOPAC from the homogenate prior to HPLC detection. The following operating conditions were used: column type: Apex-C-18 ODS 5μ (Jones) reverse phase column; mobile phase: 0.1 M sodium acetate (CH3-COONa), 0.1 M citric acid (C6H8O7.H2O), 2.7 x 10−4 M octyl sulphate (C8H17O4; Sna), with 25% methanol (v/v); flowrate: 0.6 ml/min; detector: electrochemical detector (Shimadzu, Japan) maintained at 0.75 V. Data were acquired on a PC-compatible computer using BAS-5 interface. Standard curves were constructed using 8 points between 0.625 and 80 pg/10 μl for dopamine, 23.4–3000 pg/10 μl for DOPAC and 15.62–2000 pg/10 μl for HVA. Correlation coefficients (r) of >0.98 were obtained for all curves. The working standard solutions were kept at -80°C and 10 μl of the standard solution was injected in the beginning of the analysis and between biological samples.
Isolation of microsomes
Microsomal fractions were prepared by homogenization of rat liver samples in ice-cold homogenization buffer (0.15 M KCl, 10 mM K2EDTA, 1mM Dithiothreitol, pH 7.4). The homogenates were centrifuged at 9,000 g (4°C) for 20 min. The upper phase was transferred carefully into new vials and was centrifuged for 70 min at 96,552 g (4°C). After removal of the liquid phase, followed washing of the microsomal pellet: re-suspension in ice-cold homogenization buffer, homogenization and centrifugation for 60 min at 96,552 g (4°C). The washed microsomal pellet was re-suspended in ice-cold storage buffer (K2HPO4/KH2PO4 pH 7.4, 1 mM K2EDTA, 0.1 mM dithiothreitol, 20% glycerol) and stored at -80°C until assayed [33].
Assessment of hepatic EROD and MROD activities
In the microsomes of rat livers the CYP1A1/2-dependent activities were determined. Microsomal protein content was determined by the method of Lowry et al. [34].
Ethoxyresorufin 7-deethylase activity (EROD) was measured fluorometrically in rat liver microsomes, using 7-ethoxyresorufin as substrate in order to assess cytochrome CYP1A1-dependent activity [35].
Methoxyresorufin 7-demethylase activity (MROD), which is mainly catalyzed by cytochrome CYP1A2, was determined fluorometrically in the rat liver microsomes according to Burke and Mayer [35].
Total P450 content was determined from CO-differential spectra of dithionite-reduced samples following the method, which was described by Omura and Sato [36]
Primary hepatocyte cultures
Primary hepatocytes were isolated from rats and used in cultures according to the method of Klaunig et al. [37], [22]. In brief, as previously described by Daskalopoulos et al. [22], primary hepatocytes were isolated from rats weighing 250–300 g using a two-step collagenase perfusion method. They were suspended in William's Medium E (Gibco) containing 1% L-glutamine (PAA) and 1% penicillin/streptomycin. The cells were counted in a Neubauer cell chamber and plated at a density of 1 x 105 cells per well, in 3.8 square centimeter diameter collagen type I coated dish (BIOCOAT, Cell Environment, Becton Dickinson Labware, UK). The viability of the isolated hepatocytes was checked with trypan blue dye 0.4% exclusion and only cells with viability higher than 85% just before plating were used. Hepatocytes were cultured at 37°C for 24 hours under an atmosphere of humidified 5% CO2, in order to allow them to adhere to the wells. Time and dose response experiments started 24 hours later. Primary hepatocyte cultures were treated either with SULP (10 or 25μM) or insulin (1μM) [8], in combination with wortmannin (1μM), an inhibitor of the PI3K/AKT signalling pathway. Wortmannin was added 30 min prior to insulin. Primary hepatocytes were also cultured in the presence of B[a]P at doses raging between 1 and 10μM. Either SULP or insulin were also added in the cell cultures 30 min prior to B[a]P. Time response experiments were conducted with SULP treatment of primary hepatocytes ranging between 1 and 24 hours. These in vitro experiments employing primary hepatocytes were approved by the Institutional Animal Care and Use Committee of the Medical School of the University of Ioannina.
Western blot analysis
Immunoblot analysis of the cytochrome CYPs, STAT5b and FOXO1 apoprotein levels was carried out using microsomes (CYPs) and nuclear extracts or cytosol of liver samples, respectively. For the preparation of the nuclear extracts and cytosol the NE-PER nuclear extraction kit (Pierce, Rockford, IL) was used. The content of the phosphorylated AKT and p70S6K was determined by western blot in total cellular proteins, extracted from the liver using RIPA buffer supplemented with protease inhibitors, PMSF (10μM), BGP (50μM) and NaF (50μM). Protein concentrations were determined by the BCA protein assay (Pierce, Rockford, IL). Proteins were subjected to SDS-PAGE gel electrophoresis and immunoblotting using the following antibodies: rat polyclonal CYP1A1 and CYP1A2 IgGs (they were kindly donated by Dr Ronald Wolf and Colin J. Henderson, London, UK), rat monoclonal total STAT5a/b IgG (Santa Cruz Biotechnology) and rabbit monoclonal p-STAT5b IgG (Tyr 694, Cell Signalling Technology), rabbit polyclonal p-p70S6K IgG (Thr 389), rabbit polyclonal total p70S6K IgG (Cell Signalling Technology), rabbit polyclonal p-FOXO1 (Ser 256) and total FOXO1 IgGs (Santa Cruz Biotechnology), as well as rabbit polyclonal p-AKT (Ser 473), total AKT IgGs (Santa Cruz Biotechnology) and rabbit monoclonal p-mTOR IgG (Ser2448, Cell Signalling) were also used. Secondary antibodies, conjugated with horseradish peroxidase (Santa Cruz Biotechnology) were used and the proteins were detected using a chemiluminescence detection kit (ECL, Amersham, GE Healtcare). Immunoblotting with GAPDH (Santa Cruz Biotechnology) and anti-mouse IgG horseradish peroxidase conjugated secondary antibody, were used as loading control. The membranes were developed by chemiluminescence using the Phototope-HRP Detection Kit for Western blotting (Biolabs INC, New England) and exposed to film.
Quantitative real-time PCR
The TRIzol reagent (Invitrogen) following the manufacturer’s protocol was used for the isolation of total RNA from liver tissue and primary hepatocytes. A spectrophotometric method was used for the determination of total RNA concentration in each sample. Quantitative real-time reverse transcriptase PCR (qPCR) was performed with cDNA generated from 1 μg total RNA with a SuperScript II reverse transcriptase kit (Invitrogen). The sequences of the forward and reverse gene-specific primers, which were used are shown in S1 Table. For the real-time PCR reactions the SYBR Green PCR master mix was used (Applied Biosystems, Warrington, UK). These reactions were carried out using the Thermal Cycler Real-Time Detection System C1000 (BioRad, Italy). Relative mRNA expression levels were normalized to β-actin (QuantiTect Primer Assay, Qiagen) and values were quantified using the comparative threshold cycle method.
Hormonal determinations
The GH serum levels were assessed using the rat growth hormone RIA kit (Millipore, MA, USA). The detection limit was 0.5 ng/ml and the intra-assay coefficient of variation was 10%. Prolactin (PRL) serum concentration was determined using the rat prolactin RIA kit (MP Biomedicals Europe, France) and the detection limit was 0.5 ng/ml. Serum thyroid hormone concentrations were determined using the Dynatest T3, Dynatest T4 and Dynatest TSH kits (Brahms, Germany). The normal ranges were 80–200 ng/dl (Dynatest T3), 4.5–12 μg/dl (Dynatest T4) and 0.4–4 mg/ml (Dynatest TSH), respectively. The insulin levels were measured using an EIA kit (Mercodia Rat ELISA kit for insulin, Uppsala, Sweden). The detection limit was 3.3ng/ml and the intra-assay coefficient of variation was 3.1%. The blood glucose levels were measured with a commercially available kit (Merck, Germany) using the technique of glucose oxidase (Trinder, 1969).
Statistical analysis
The data were expressed as means±SE and were analysed using one-way analysis of variance (ANOVA) followed by multiple comparisons with Bonferoni’s and Tuckey’s list honest significant difference methods. The significance level for all analyses was set at probability of less than or equal to 0.05.
Results
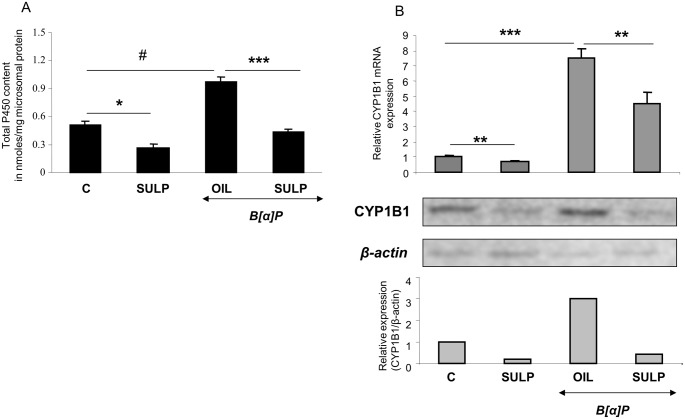
SULP, a dopamine D2- receptor antagonist, reduced total P450 content in the rat liver and prevented the B[a]P-induced increase in it (Fig 1A). These effects suggest that one or more hepatic Cytochrome P450 isoforms are down-regulated following blockade of the dopamine D2-receptor-linked signalling pathways.
Fig 1. D2-dopaminergic receptor-mediated effect on hepatic total P450 content and CYP1B1.
(A) Assessment of sulpiride effect on total P450 content in the liver of rats treated with either normal saline or benzo[a]pyrene. (B) Assessment of sulpiride effect on hepatic CYP1B1 mRNA expression following treatment with either normal saline or benzo[a]pyrene. Bonferroni’s correction and Tukey post-hoc tests took place in the comparisons of the data presented here. (C vs SULP, C vs B[a]P and B[a]P vs (B[a]P+SULP)). C: controls treated with normal saline; SULP: sulpiride (dopamine D2-antagonist); B[a]P: benzo[a]pyrene; OIL: olive oil; *P<0.05, **P<0.01, #P<0.005, ***P<0.001.
Blockade of D2-dopaminergic receptors down-regulates CYP1A1/2 and CYP1B1
Treatment of rats with the selective D2-antagonist SULP, markedly repressed constitutive CYP1A1 and CYP1A2 expression in the liver (Figs 2 and 3). This effect was evident at mRNA, apoprotein and enzyme activity (EROD and MROD) levels and was almost identical for both cytochromes. Furthermore, SULP markedly restricted the hepatic B[a]P-induced EROD and MROD activities (Figs 2 and 3). D2-receptor blockade also repressed the constitutive and B[a]P-induced CYP1B1 mRNA and protein expression in the rat liver (Fig 1B). These findings suggest that the D2-receptor-related signalling pathways potentially interfere with the mechanism of AhR activation.
Fig 2. D2-dopaminergic receptor-mediated regulation of hepatic CYP1A1.
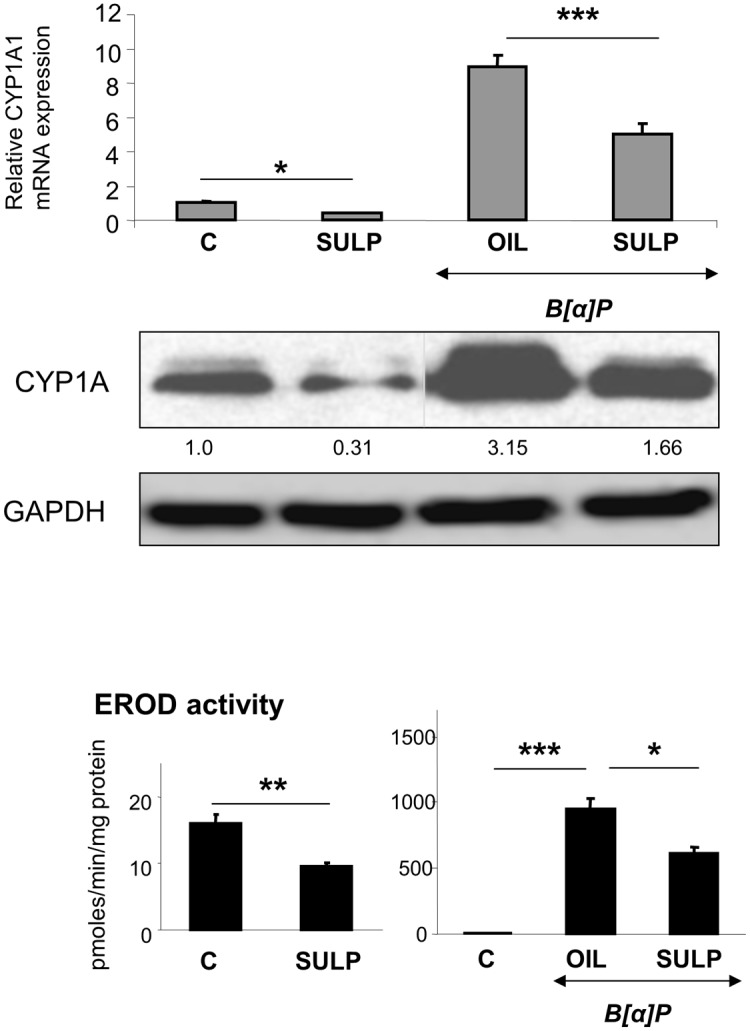
Assessments of the effects induced by the selective dopamine D2-antagonist sulpiride (SULP) on CYP1A1 relative mRNA expression by using quantitative PCR assays, on CYP1A1 apoprotein levels by using Western blotting, and on CYP1A1-catalyzed EROD activity by using a fluorometric assay. Bonferroni’s correction and Tukey post-hoc tests took place in the comparisons of the data presented here (C vs SULP, C vs B[a]P and B[a]P vs (B[a]P+SULP)). Numbers in the western blot captures, correspond to the lower lanes and indicate the relative CYP1A apoprotein expression following treatment compared to the control expression level that was set at 1. The antibody used against CYP1A1 is not highly specific and recognizes CYP1A2 as well. C: controls treated with normal saline; SULP: sulpiride (dopamine D2-antagonist); B[a]P: benzo[a]pyrene; OIL: olive oil; *P<0.05, **P<0.01, ***P<0.001.
Fig 3. D2-dopaminergic receptor-mediated regulation of hepatic CYP1A2.
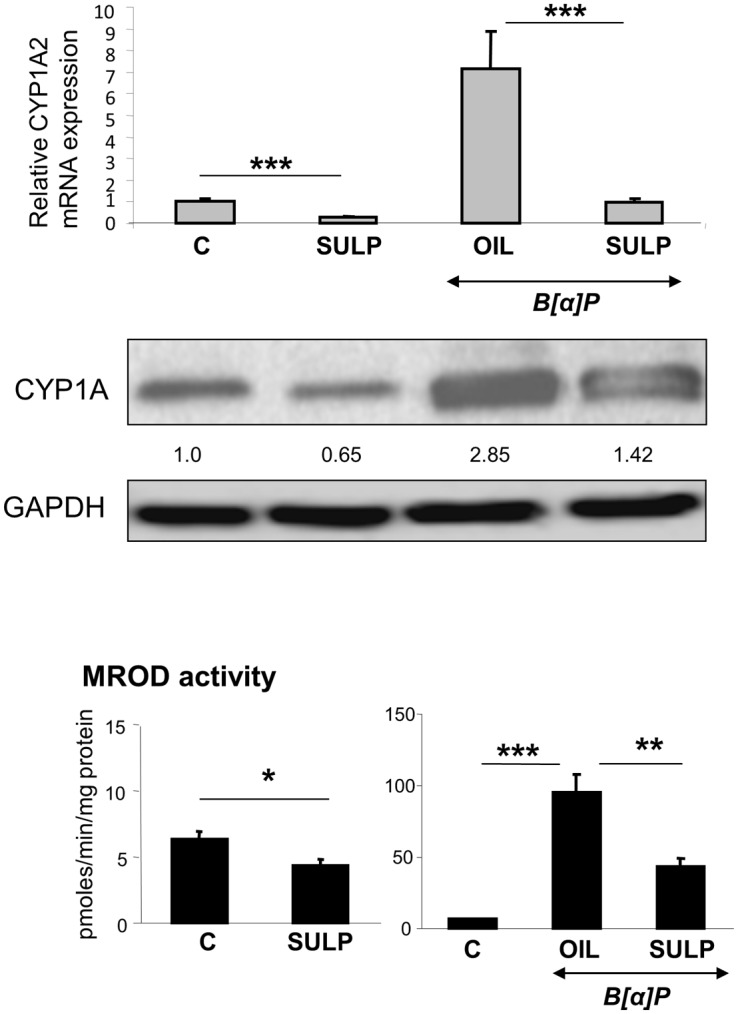
Assessments of the effects induced by the selective dopamine D2-antagonist sulpiride (SULP) on CYP1A2 relative mRNA expression by using quantitative PCR assays, on CYP1A2 apoprotein levels by using Western blotting, and on CYP1A2-catalyzed MROD activity by using a fluorometric assay. Bonferroni’s correction and Tukey post-hoc tests took place in the comparisons of the data presented here (C vs SULP, C vs B[a]P and B[a]P vs (B[a]P+SULP)). Numbers in the western blot captures indicate the relative CYP1A apoprotein expression following treatment compared to the control expression level that was set at 1. C: controls treated with normal saline; SULP: sulpiride (dopamine D2-antagonist); B[a]P: benzo[a]pyrene; OIL: olive oil; *P<0.05, **P<0.01, ***P<0.001.
In order to ensure that the effect of SULP on CYP1A1, CYP1A2 and CYP1B1 regulation is mediated by D2-dopaminergic receptors, animals were treated with the highly selective dopamine D2-antagonist, L-741,626. The data confirmed that blockade of D2-dopaminergic receptors results in significant repression of CYP1A1, CYP1A2 and CYP1B1 mRNA expression (P<0.001, Fig 4), indicating that the down-regulating effect of SULP is, indeed, mediated by inhibition of dopamine D2-receptors.
Fig 4. D2-dopaminergic receptor-mediated regulation of hepatic CYP1A1/2 and CYP1B1.
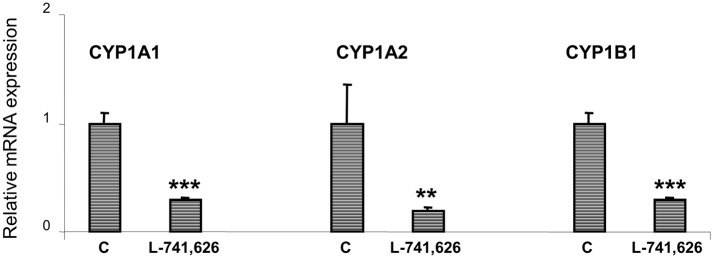
Assessments of the effects induced by the selective dopamine D2-antagonist L-741,626 on CYP1A1, CYP1A2 and CYP1B1 relative mRNA expression by using quantitative PCR assays. Bonferroni’s test took place in the comparisons of the data presented here (C vs L-741,626). C: controls treated with normal saline; L-741,626: highly selective dopamine D2-antagonist; ***P<0.001.
In vivo inhibition of dopamine D2-receptors down-regulates the hepatic AhR-dependent CYP regulation system
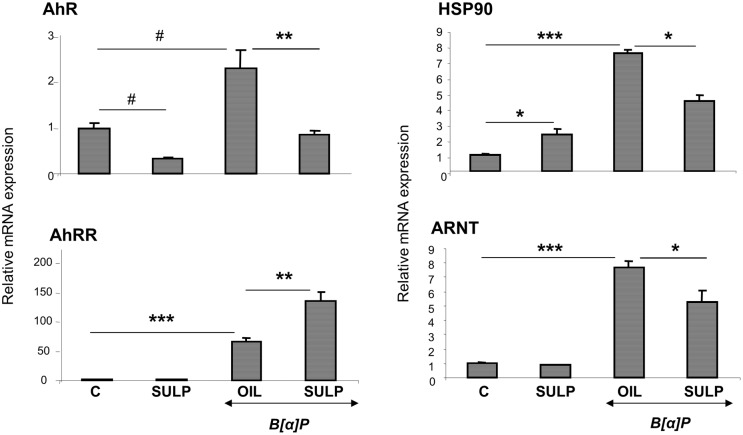
D2-receptor blockade with SULP resulted in repression of AhR, heat shock protein 90 (HSP90) and aryl hydrocarbon receptor nuclear translocator (ARNT) in the liver of rats exposed to B[a]P (Fig 5). In contrast, SULP further increased the B[a]P-induced AhR repressor (AhRR) mRNA expression (Fig 5). Interestingly, SULP did not affect ARNT and AhRR constitutive mRNA expression, whereas up-regulated HSP90 basal expression (Fig 5).
Fig 5. D2-dopaminergic receptor-mediated regulation of critical hepatocyte factors that regulate CYP1A1, CYP1A2 and CYP1B1.
Assessments of the effects induced by the selective dopamine D2-antagonist sulpiride on aryl hydrocarbon receptor [1], aryl hydrocarbon receptor repressor (AhRR), aryl hydrocarbon receptor nuclear translocator (ARNT) and heat shock protein 90 (HSP90) relative mRNA expression by using quantitative PCR assays. Bonferroni’s correction and Tukey post-hoc tests took place in the comparisons of the data presented here. In particular, comparisons of data (Relative ARNT and AhRR mRNA levels) between the group of controls (C) and SULP took place using the Bonferroni’s and Tukey tests and no difference was found. (C vs SULP, C vs B[a]P and B[a]P vs (B[a]P+SULP)). C: controls treated with normal saline; SULP: sulpiride (dopamine D2-antagonist); B[a]P: benzo[a]pyrene; OIL: olive oil; *P<0.05, **P<0.01, #P<0.005, ***P<0.001.
Role of the insulin/PI3K/AKT/FOXO1 pathway, in the D2-receptor-mediated down- regulation of CYP1A and CYP1B
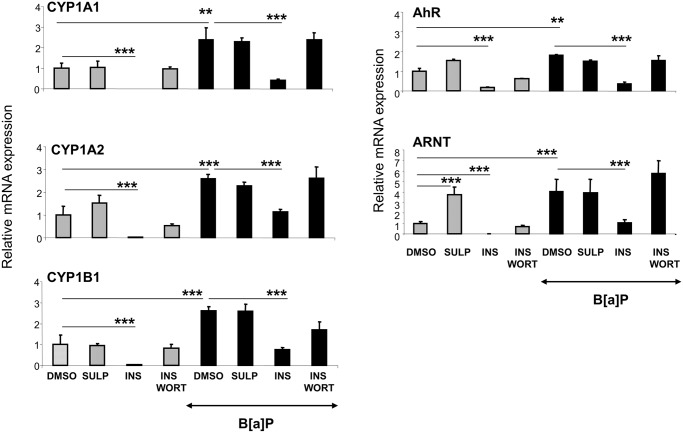
In order to elucidate the mechanism underlying the down-regulation of CYP1 enzymes following inhibition of D2-receptors, in vitro experiments were employed using primary hepatocyte cultures. Time- and dose-response experiments were conducted for the determination of the optimal conditions for the treatment of primary hepatocytes with SULP (S1 and S2 Figs). It was found that treatment of hepatocytes with SULP did not alter the constitutive and B[a]P-induced expression of CYP1A1/2 and CYP1B1 (Fig 6). The findings of this in vitro study, where SULP affected directly the hepatocytes, indicated that the down-regulation of the CYP1 genes observed following the in vivo SULP administration appears to involve primarily extrahepatic D2-receptor linked pathways.
Fig 6. In vitro assessment of the role of dopamine D2-receptor-mediated regulation of hepatic CYP1A1, CYP1A2 and CYP1B1.
Assessments of SULP effects on CYP1A1, CYP1A2 and CYP1B1 relative mRNA expression in primary hepatocytes by using quantitative PCR assays. The role of insulin in the regulation of the above mentioned CYPs was also assessed in primary hepatocyte cultures treated either with insulin (1μM, 24hr) alone or in combination with the inhibitor of the PI3K signalling pathway, wortmannin (1μM, 24hr) [76]. Controls were treated with dimethylsulfoxide (DMSO); SULP: sulpiride (selective dopamine D2-antagonist); INS: insulin; WORT: wortmannin; B[a]P: benzo[a]pyrene; ***P<0.001. Comparisons of the relative CYP1A1, CYP1A2, CYP1B1, AhR and ARNT mRNA expression between DMSO- and INS-, as well as between DMSO- and (INS+WORT)-treated hepatocytes were done using the Bonferroni’s test and were performed in both, constitutive and B[a]P-induced states. No differences were found between DMSO- and (INS+WORT)-treated hepatocytes, indicating that wortmannin has completely blocked the repressive effect of insulin on both, constitutive and B[a]P-induced mRNA expression of the above mentioned genes.
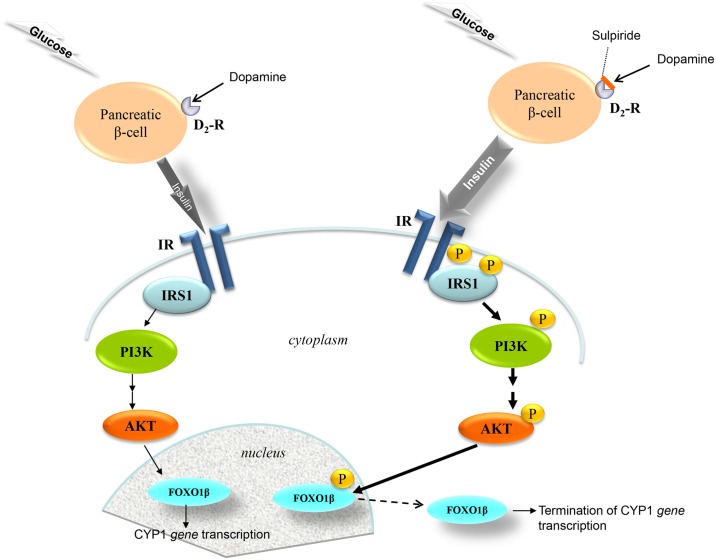
The findings coming from the in vivo study indicate that the pancreatic β-cell D2-receptor/insulin-related pathway potentially has a central role in the SULP-mediated repressive effect on the constitutive and B[a]P-induced CYP1A1, CYP1A2 and CYP1B1 expression. It is well documented that SULP by blockade of pancreatic islet β-cell D2-receptors increases insulin release (Fig 7), an effect that was confirmed by the present study (Table 1; [22]). It was also found that treatment of rats with SULP activated the insulin/PI3K/AKT/FOXO1 signalling pathway in the liver (Fig 7). Specifically, SULP increased AKT phosphorylation that subsequently activated FOXO1β in the nucleus, thus leading to its translocation into the cytoplasm, and termination of CYP1 gene transcription at a constitutive level (Fig 8). This hypothesis is also supported by the finding that treatment of primary hepatocytes with insulin strongly repressed CYP1A1, CYP1A2, CYP1B1, AhR and ARNT mRNA expression, effects that were completely prevented by wortmannin, a PI3K inhibitor, able to block the insulin/PI3K/AKT/FOXO1 signalling pathway (Fig 6), [38]. SULP also activated AKT and in turn, increased FOXO1β phosphorylation in the nucleus of hepatocytes of B[a]P-exposed rats, indicating an activation of the PI3K/AKT signalling pathway (Fig 8). In vitro treatment of primary hepatocytes with insulin (it is released in vivo following SULP treatment, [22]), resulted in a strong restriction of CYP1 inducibility by B[a]P, an effect that was prevented by wortmannin, a PI3K inhibitor (Fig 6).
Fig 7. The dopamine D2-receptor-mediated control of the insulin/PI3K/AKT/FOXO1 signalling pathway activation.
Dopamine stimulates D2-ARs on pancreatic β-cells and restricts the release of insulin in response to increased plasma glucose levels [61]. In contrast, blockade of D2-dopaminergic receptors by sulpiride, increases insulin release [22], which in turn, stimulates insulin receptors (IR) in hepatocyte plasma membranes, an effect resulting in the phosphorylation of the Insulin Receptor Substrate (IRS) at different docking sites, where the phosphatidylinositol 3-kinase (PI3K) binds. Activated PI3K converts phosphatidylinositol biphosphate to phosphatidylinositol triphosphate, which subsequently activates protein kinase B (AKT). Upon activation AKT phosphorylates the transcription factor forkhead box O1 (FOXO1), which then translocates into the cytoplasm thus terminating CYP1A1, CYP1A2 and CYP1B1 gene transcription [22,75].
Table 1. Sulpiride-induced effect on rat hormonal state.
| Treatment | T3 | T4 | TSH | PRL | GH | CORT | Insulin |
|---|---|---|---|---|---|---|---|
| Control | 130.7±6.8 | 2.9±0.1 | 2.1±0.1 | 38.4±6.4 | 129.8±3.5 | 193.1±14.4 | 0.44±0.02 |
| SULP | 78.54±5.6*** | 1.6±0.2*** | 1.9±0.1 | 194.9±13.0*** | 41.1±12.3*** | 85.1±11.0** | 1.4±0.2*** |
| B[a]P | 93.9±5.5*** | 2.5±0.1* | 2.1±0.01 | 28.9±4.4 | 110.8±9.1 | 196.8±19.4 | 0.35±0.03 |
| B[a]P + SULP | 65.0±3.5** | 1.3±0.1*** | 2.1±0.01 | 247.7±12.8*** | 59.7±0.3* | 102.3±2.0* | 1.5±0.2*** |
C; controls treated with normal saline; SULP: sulpiride (dopamine D2-antagonist); B[a]P: benzo[a]pyrene; OIL: olive oil; T3: triiodothyronin expressed in ng/dl; T4: thyroxin expressed in μg/dl; TSH: thyroid-stimulating hormone expressed in ng/ml; GH: growth hormone expressed in ng/ml; PRL: prolactin expressed in ng/ml; Corticosterone expressed in mg/ml; Insulin expressed in pg/ml. Values are expressed as mean ± SE (n = 10). The asterisks indicate the significance of the differences between SULP-treated rats and controls, and between B[a]P-exposed rats with and without concomitant treatment with SULP
*P<0.05
**P<0.005
***P<0.001
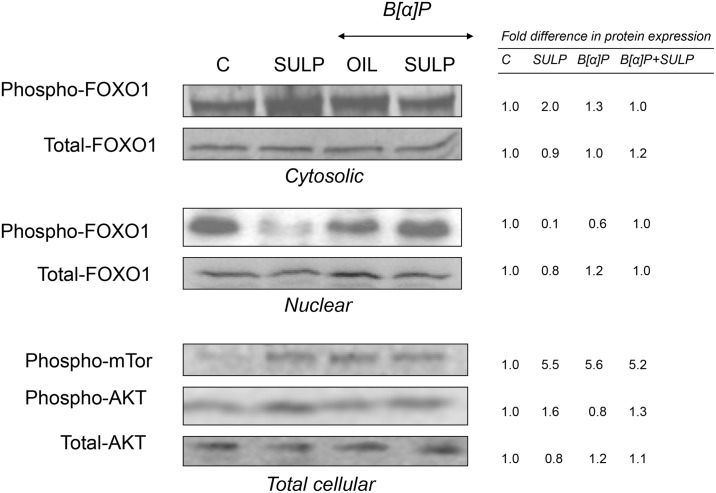
Fig 8. In vivo assessment of the effect of D2-receptor blockade on the insulin/PI3K/AKT/FOXO1 and mTOR signalling pathway.
Western blotting showing the SULP-mediated AKT phosphorylation that activated FOXO1 in the nucleus, which in turn translocated into the cytoplasm (reduced FOXO1β phosphorylation in the nucleus and increased FOXO1β phosphorylation in the cytoplasm). Numbers in the western blot captures indicate the relative FOXO1β, AKT and mTOR phosphorylation level following treatment compared to the control level that was set at 1. C: controls treated with normal saline; SULP: sulpiride (selective dopamine D2-antagonist); B[a]P: benzo[a]pyrene; OIL: olive oil.
Taken together these data indicate that the mechanism of SULP-mediated down-regulation of CYP1A and CYP1B constitutive expression potentially involves activation of the hepatic insulin/PI3K/AKT/FOXO1β signalling pathway. This activation, however, may not apply for the SULP-mediated restriction of the CYP1 inducibility by B[a]P. It is possible that factors, other than FOXO1β, downstream to the PI3K/AKT signalling pathway are involved and hold predominant roles in the SULP-mediated repression of CYP1 inducibility.
Based on the fact that there is a cross-talk between mTOR/HIF1a and the AhR-linked signalling pathway [39–41], the effect of SULP on mTOR phosphorylation and HIF1a expression was investigated. As mentioned above, D2-receptor blockade with SULP activated the PI3K/AKT signalling pathway in non exposed to B[a]P livers (Fig 8), an activation also seen at the level of mTOR (a down-stream element in this pathway). It should be noted though, that SULP did not alter the level of mTOR phosphorylation in the B[a]P-exposed livers (Fig 8). Furthermore, this D2-receptor antagonist did not alter both, constitutive and B[a]P-induced HIF1a mRNA expression in the rat liver (S3 Fig). Apparently, this cross-talk is not critically involved in the SULP-mediated down-regulation of CYP1 inducibility by B[a]P.
Dopamine D2-receptor blockade inactivates the GH/STAT5b signalling pathway
It is well established that dopamine stimulates the secretion of growth hormone (GH) from the anterior-pituitary lobe [42], which has a down- regulating effect on hepatic CYP1A expression via the Jak2/STAT5b pathway [43]. In the present experimental setting, SULP reduced serum GH levels (Table 1) and consequently, STA5b phosphorylation both, in B[a]P-exposed and non-exposed rats (Fig 9). Based on these findings a CYP1A and CYP1B up-regulation should be expected. However, the opposite is true indicating that the pancreatic D2-receptor-insulin-PI3K/AKT/FOXO1 pathway plays a dominant role overriding that of the GH/STAT5b pathway.
Fig 9. In vivo assessment of the effect of D2-receptor blockade on the activation of GH/STAT5b signalling pathway.
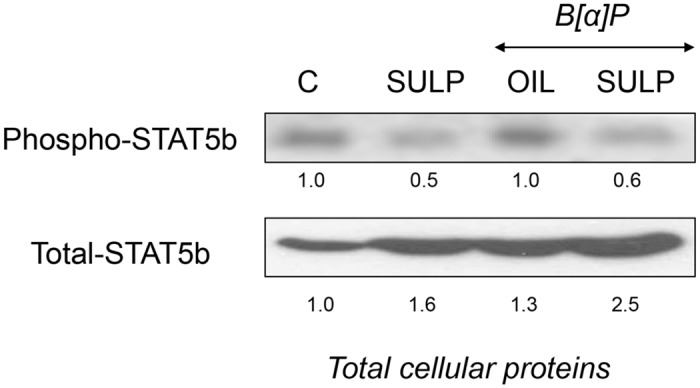
Western blotting showing the SULP-mediated suppression of STAT5b phosphorylation. Numbers in the western blot captures indicate the STAT5b phoshorylation level following treatment compared to the control level that was set at 1. Lanes C: control; SULP: sulpiride (selective dopamine D2-antagonist); B[a]P: benzo[a]pyrene; OIL: olive oil.
Effect of dopamine D2-receptor blockade on neurochemical and hormone levels
The role of hormones, such as GH, thyroid hormones, PRL and glucocorticoids in the regulation of CYP1A and CYP1B isozymes is well documented [26,44–48]. The secretion of these hormones is under a central noradrenergic and dopaminergic control [42]. Therefore, the levels of norepinephrine (NE) and dopamine (DA) were determined in the hypothalamus, the brain site where the corresponding hormone releasing factors are released from [42].
SULP reduced NE content in the hypothalamus of both, B[a]P-exposed and non-exposed rats (P<0.05 and P<0.01, respectively, Table 2). Hypothalamic DA levels were also reduced by SULP only in the non-exposed to B[a]P rats (P<0.05, Table 2), whereas DA turnover ratio was increased in this group of treatment (P<0.01, Table 2), indicating an increased dopaminergic activity in the hypothalamus.
Table 2. Sulpiride-induced effect on hypothalamic catecholamines, norepinephrine (NE) and dopamine (DA).
| Control | SULP | B[a]P | B[a]P+ SULP | |
|---|---|---|---|---|
| NA | 182.0±19.4 | 76.8±3.3** | 281.8±16.6 | 200.0±11.1* |
| DA | 18.9±2.3 | 8.1±1.5* | 19.4±0.7 | 16.1±1.1 |
| DOPAC | 13.3±1.0 | 17.4±0.1 | 11.1±0.7 | 13.8±1.1 |
| HVA | 3.3±0.5 | 3.1±0.2 | 2.0±0.1 | 3.5±0.4 |
| Turnover DA | 0.9±0.13 | 2.6±0.4** | 0.7±0.1 | 1.1±0.1 |
The evaluation of the effect of sulpiride (SULP), a dopamine D2-antagonist, on hypothalamic dopaminergic activity was based on the alterations observed at DA turnover ratio (HVA+DOPAC)/DA. DA, NE, DOPAC and HVA levels were expressed in nmoles/g tissue. HVA: homovanilic acid; DOPAC: dihydroxyphenylacetic acid. Values are expressed as mean ± SE (n = 10). The asterisks indicate the significance of the differences between SULP-treated rats and controls, and between benzo[a]pyrene (B[a]P)-exposed rats with and without concomitant treatment with SULP;
*P< 0.05
**P< 0.01
***P< 0.001
SULP reduced serum T3, T4, GH and corticosterone levels both, in B[a]P-exposed and non-exposed rats, but the drug increased PRL and insulin concentration in these rats (Table 1). Interestingly, B[a]P suppressed serum T3 and T4 levels (Table 1).
Discussion
Previous studies have shown that central and peripheral catecholaminergic systems play a critical role in the regulation of several CYPs that are involved in the metabolism of the majority of prescribed drugs and toxicants. In particular, noradrenergic systems hold prevalent roles in CYP regulation [13,18,20–22,26,49]. Accumulating evidence indicates that dopaminergic systems and mainly those related to dopamine D2-receptors, are also involved [22,23]. Specifically, inhibition of D2-dopaminergic receptors with either sulpiride or L,741,626 led to a robust down-regulation of CYP2C, CYP2D, CYP2E1, and CYP3A in the rat liver [22,23]. The present study has focused on the role of dopamine D2-receptor-linked pathways in the regulation of constitutive and B[a]P-induced CYP1A1/2 and CYP1B1 expression. It should be noted that dopamine D2-receptors are targets of drugs used in the treatment of various neurodegenerative and psychopathological disorders, such as the Parkinson’s disease, depression and psychosis [50–52]. The drugs prescribed in these diseases are either D2-agonists or antagonists that can influence the functional efficiency of the dopaminergic system by mimicking, blocking, or modifying the sensitivity of D2-receptors to dopamine.
The present findings indicated that blockade of dopamine D2-receptors markedly repressed both, the constitutive and B[a]P-induced expression of CYP1A1/2 and CYP1B1. It is well documented that AhR, a member of the basic helix-loop-helix/PER-ARNT-SIM family of DNA-binding proteins, is a determinant transcription factor that regulates both, constitutive and B[a]P-induced CYP1A1/2 and CYP1B1 expression[53]. Transcriptional activation of these CYP isozymes requires firstly binding of the ligand, such as B[a]P, to AhR, which is associated with HSP90 in the cytoplasm. Upon ligand binding the complex translocates into the nucleus where HSP90 dissociates. The ligand-AhR complex then forms a heterodimer with the ARNT [54] and interacts with the xenobiotic responsive elements at the promoters of the CYP1A1/2 and CYP1B1 genes, thus inducing their transcription [54,55]. Therefore, the findings of this study indicate that the reduction of CYP1A1/2 and CYP1B1 inducibility by B[a]P that was detected following D2-receptor blockade with SULP, and that of AhR, HSP90 and ARNT, indicates a mechanism that profoundly includes impaired transcription of the AhR responsive genes [56]. In support of this hypothesis is the fact that SULP further increased the B[a]P-induced AhRR expression. It is well established that contrary to what applies for the induced CYP1 gene expression, the induced levels of AhRR inhibit the AhR function by competing with it in forming a heterodimer with ARNT thus compromising its XRE binding activity [57].
The fact that SULP further enhanced the B[a]P-induced AhRR expression, instead of suppressing it, as in the case of other AhR target genes, confirms previous observations that the outcome of AhR-AhRR interactions is more complex than suggested by a simple AhR-induced, AhRR-mediated feedback model, which is based on the ability of AhR to transactivate the AhRR gene, and the ability of AhRR to repress AhR activity, at least as defined by CYP1 induction. In particular, it has been reported that the ligand-activated AhR may not transactivate AhRR in all tissues and under some circumstances, may actually repress AhRR transcription, thereby maximizing AhR activity [58].
It should be noted that AhRR, HSP90 and ARNT do not hold essential roles in the SULP-mediated repression of CYP1A and CYP1B constitutive expression, because the drug did not affect basal ARNT and AhRR expressions, whereas increased that of HSP90. Profoundly, SULP along with AhR, repressed some of the numerous ancillary factors that are involved in the activation of the AhR transcriptional complex that regulates constitutive CYP1 expression [59]. Finally, it appears that while AhR regulates both, the constitutive and the induced CYP1 expression, the mechanism mediating the SULP repressive effect on each expression state is not identical. There may be differences at the level of AhR interaction with other transcription factors, or potentially at some upstream signalling pathways, or at the AhR affinity level in binding with the respective gene promoters.
The role of dopamine D2-receptor-linked pathways in the down-regulation of hepatic CYP1A1/2 and CYP1B1 was confirmed with another, highly selective D2-antagonist, the L-741,626, which also repressed their expression. This D2-receptor mediated effect is probably indirect, because treatment of primary hepatocyte cultures with SULP had no effect on the expression of CYP1A1/2 and CYP1B1. It is therefore, hypothesized that the repressive effect on CYP1A1/2 and CYP1B1, which is observed when SULP is administered in vivo, is the outcome of the drug’s effects on central and peripheral dopaminergic and hormonal systems that have an impact on hepatic signalling pathways regulating these CYP genes [22].
Our results suggest a role for the hepatic insulin/PI3K/AKT/FOXO1 signalling pathway in the SULP-mediated down-regulation of constitutive CYP1A1/2 and CYP1B1 expression. It has been previously shown that inhibition of pancreatic D2-dopaminergic receptors with SULP triggers insulin secretion from pancreatic islet β-cells. This effect leads to activation of the insulin/PI3K/AKT signalling pathway in the hepatocytes [22] and ultimately, to phosphorylation of the nuclear factor FOXO1β and its subsequent translocation into the cytoplasm and termination of CYP1A1, CYP1A2 and CYP1B1 transcription. Upstream to the insulin/PI3K/AKT/FOXO1 pathway, dopamine is a significant regulatory factor that restricts the release of insulin in response to increased plasma glucose levels (Fig 7). Dopamine, exerts its negative control in insulin release via stimulation of pancreatic D2-receptors expressed in islet β-cell membranes [60]. In contrast, blockade of these receptors with SULP increases insulin release (Fig 7), [23], which in turn, activates the hepatic insulin/PI3K/AKT/FOXO1 signalling pathway, thus exerting a negative regulatory control on several P450s [22,60–64]. This hypothesis is also supported by the findings of an in vitro study. Treatment of primary hepatocyte cultures with insulin resulted in a strong down-regulation of CYP1A1, CYP1A2 and CYP1B1 via activation of the PI3K/AKT signalling pathway, because pre-treatment of these cells with wortmannin, a PI3K inhibitor, prevented the repressive effect of insulin on these cytochromes. The PI3K/AKT signalling pathway may also participate at least in part, in the SULP-mediated reduction of CYP1A1/2 and CYP1B1 inducibility by B[a]P. This hypothesis is based on the fact that SULP increased AKT and FOXO1β phosphorylation in the liver of B[a]P-exposed rats, indicating an activation of the insulin/PI3K/AKT signalling pathway. Furthermore, insulin represses CYP1 inducibility by B[a]P in primary heatocytes and wortmannin completely blocked this effect. The in vivo and in vitro findings indicate that profoundly, some distinct downstream elements in the insulin/PI3K/AKT signalling pathway participate in the SULP-mediated repression of CYP1A1/2 and CYP1B1 inducibility by B[a]P than those regulating the repression of CYP1 constitutive expression. However, further investigation involving potentially Chip assays is needed in order to completely clarify the involvement of PI3K/AKT signalling pathway in CYP1A and CYP1B regulation by SULP.
Previous studies reported a cross-talk between the AhR and HIF1a signalling pathways [39]. It is well established that activation of the PI3K/AKT/mTOR-related pathway up-regulates HIF1a [39,41], which in turn, inactivates AhR, thus repressing CYP1A inducibility [40]. It should be taken also into account the fact that the impact of this cross-talk depends on ARNT availability, which is an essential element in both, AhR and HIF1a signalling pathways [39] and SULP repressed the B[a]P-induced ARNT expression. The present study also indicated that SULP activated mTOR in the liver of non exposed to B[a]P rats, but had no effect on the B[a]P-exposed livers. In addition, SULP did not affect HIF1a inducibility by B[a]P. Combined these data indicate that the HIF1a/mTOR and AhR cross-talk did not have any critical role in the SULP-mediated suppression of the CYP1A1/2 and CYP1B1 inducibility by B[a]P.
In the D2-receptor related regulatory system, the cross-talk between the AhR- and insulin/PI3K/AKT-linked signalling pathways apparently, holds significant roles in CYP1 regulation [65–67]. In light of this interaction, it is suggested that the SULP-induced repression of CYP1A1/2 and CYP1B1, could be attributed, at least in part, to the insulin-induced up-regulation of the carbohydrate-responsive element-binding protein (ChREBP) in the liver. This factor exerts a negative control on ARNT/HIF-1β, an essential element in the AhR regulatory system [65].
Stimulation of GH secretion from the anterior pituitary lobe by dopamine is well documented [42]. STAT5b, is the major GH pulse-activated transcription factor, which is involved in the regulation of several P450s in the liver [43,68]. Therefore, the possible involvement of the GH/STAT5b pathway in the SULP-mediated repression of CYP1A and CYP1B1 was assessed. Treatment with SULP resulted in decreased serum GH levels and inactivation of the GH/STAT5b signalling pathway. Thyroid hormone levels were also decreased by SULP. Based on the fact that both, GH and thyroid hormones, hold a negative control on CYP1A regulation [43,69,70], it is assumed that the SULP-induced repression of CYP1A and CYP1B1 is not mediated by inactivation of the GH/STAT5b- and thyroid hormone-related pathways.
Other regulatory pathways are potentially involved including PRL, as SULP increased serum PRL concentration, which has a down-regulating effect on various P450 isoforms [69,71]. Glucocorticoid-related pathways could be also involved, as SULP reduced serum corticosterone concentration, which is a positive regulator of CYP1A1/2 and CYP1B1 in the rat liver [72].
In conclusion, inhibition of dopamine D2-receptors results in significant repression of constitutive and B[a]P-induced CYP1A1, CYP1A2 and CYP1B1 expression in the rat liver. The activation of the AhR-related regulatory system was reduced following D2-inhibition, indicating a cross-talk between the D2-receptor- and AhR-regulatory pathways. Previous studies indicated that D2-receptor-linked signalling pathways potentially cross-talk also with a broad array of cellular transcription systems that regulate various P450 genes encoding isozymes that metabolize a plethora of prescribed drugs and toxicants [22], indicating that the down-regulating effect of D2-receptor inhibition is not specific for the AhR-related regulatory system. The down-regulating effect of SULP on constitutive CYP1A and CYP1B expression appears to be mediated by activation of the insulin/PI3K/AKT pathway, which though has a less significant contribution in the SULP-mediated reduction of CYP1 inducibility by B[a]P. The PRL-activated negative regulatory pathway and inactivation of the glucocorticoid-linked up-regulating pathway may also contribute in the SULP-mediated down-regulation of CYP1A and CYP1B. As dopamine D2-receptors serve as targets for various prescribed drugs, patients following a relative treatment may have altered drug toxicity and efficacy outcomes due to reduced enzymatic activity when exposed to substrates of the CYP1A1/2 and the CYP1B1. These findings potentially indicate that the regulatory pathways involving D2-receptors could be considered as targets of the pharmaceutical strategy for the protection of individuals heavily exposed to environmental toxicants and pre-carcinogens that are activated upon CYP1A- and CYP1B-catalyzed metabolism. It should be noted though that carcinogenesis is a complex and multi-factorial process that involves various regulatory systems, and not only those related to CYP1A1/2 and CYP1B1 enzymes [73,74]. Therefore, further investigation is needed in order to clarify the outcome of D2-receptor inhibition in carcinogenesis, such as assessment of the B[a]P-DNA-adduct formation in the liver and other tissues.
Supporting Information
Alterations in CYP1A1, CYP1A2 and CYP1B1 relative mRNA expression following sulpiride (SULP, 10μM) exposure of primary hepatocytes for different time periods was assessed. Control cells were treated with DMSO. SULP1: incubation of hepatocytes with sulpiride for 1 hr; SULP6: incubation of hepatocytes with sulpiride for 6 hr; SULP12: incubation of hepatocytes with sulpiride for 12 hr; SULP24: incubation of hepatocytes with sulpiride for 24 hr; ***P<0.001.
(DOC)
Alterations in CYP1A1, CYP1A2 and CYP1B1 relative mRNA expression following exposure of primary hepatocytes with either 10μM or 25μM sulpiride (SULP) for 24 hr. Control cells were treated with DMSO.
(DOC)
***P<0.001.
(DOC)
These primers were used for the quantitation of the mRNA levels of the genes tested using q-PCR.
(DOC)
Acknowledgments
This research project has been co-financed by 1) the European Union (European Regional Development Fund- ERDF) and Greek national funds through the Operational Program “THESSALY- MAINLAND GREECE AND EPIRUS-2007-2013” of the National Strategic Reference Framework (NSRF 2007–2013, Grant 346985/80753) and 2) the E.U.-European Social Fund (80%) and the Greek Ministry of Development-GSRT (20%) PENED (03EΔ/957). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
European Regional Development Fund- ERDF NSRF 2007-2013, Grant 346985/80753, MK and Greek Ministry of Development-GSRT (20%) PENED (03EΔ/957, MK.
References
- 1. Kilic T, Ural D, Ural E, Yumuk Z, Agacdiken A, Sahin T et al. (2006) Relation between proinflammatory to anti-inflammatory cytokine ratios and long-term prognosis in patients with non-ST elevation acute coronary syndrome. Heart 92: 1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conney AH (1982) Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res 42: 4875–4917. [PubMed] [Google Scholar]
- 3. Fuhr U, Rost KL (1994) Simple and Reliable Cyp1a2 Phenotyping by the Paraxanthine/Caffeine Ratio in Plasma and in Saliva. Pharmacogenetics 4: 109–116. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez FJ (1988) The molecular biology of cytochrome P450s. Pharmacol Rev 40: 243–288. [PubMed] [Google Scholar]
- 5. Ioannides C, Parke DV (1993) Induction of Cytochrome P4501 as an Indicator of Potential Chemical Carcinogenesis. Drug Metabolism Reviews 25: 485–501. [DOI] [PubMed] [Google Scholar]
- 6. Kawajiri K, Fujii-Kuriyama Y (1991) P450 and human cancer. Jpn J Cancer Res 82: 1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nebert DW (1989) The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol 20: 153–174. [DOI] [PubMed] [Google Scholar]
- 8. Omiecinski CJ, Redlich CA, Costa P (1990) Induction and developmental expression of cytochrome P450IA1 messenger RNA in rat and human tissues: detection by the polymerase chain reaction. Cancer Res 50: 4315–4321. [PubMed] [Google Scholar]
- 9. Ioannides C, Parke DV (1990) The cytochrome P450 I gene family of microsomal hemoproteins and their role in the metabolic activation of chemicals. Drug Metabolism Reviews 22: 1–85. [DOI] [PubMed] [Google Scholar]
- 10. Lewis DF, Ioannides C, Parke DV (1993) Validation of a novel molecular orbital approach (COMPACT) for the prospective safety evaluation of chemicals, by comparison with rodent carcinogenicity and Salmonella mutagenicity data evaluated by the U.S. NCI/NTP. Mutat Res 291: 61–77. [DOI] [PubMed] [Google Scholar]
- 11. IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Polynuclear Aromatic Compounds, Part 1, Chemical, Environmental and Experimental Data; 1983; Lyon. [PubMed] [Google Scholar]
- 12. Pelkonen O, Nebert DW (1982) Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev 34: 189–222. [PubMed] [Google Scholar]
- 13. Konstandi M, Johnson EO, Marselos M, Kostakis D, Fotopoulos A, Lang MA. (2004) Stress-mediated modulation of B(alpha)P-induced hepatic CYP1A1: role of catecholamines. Chem Biol Interact 147: 65–77. [DOI] [PubMed] [Google Scholar]
- 14. Konstandi M, Marselos M, Radon-Camus AM, Johnson E, Lang MA (1998) The role of stress in the regulation of drug metabolizing enzymes in mice. Eur J Drug Metab Pharmacokinet 23: 483–490. [DOI] [PubMed] [Google Scholar]
- 15. Konstandi M, Kostakis D, Johnson E, Lang MA, Marselos M (1998) Evidence of alpha2-adrenoceptor involvement in B[alpha]P induction processes of drug-metabolizing enzymes: the effect of stress. Eur J Drug Metab Pharmacokinet 23: 491–495. [DOI] [PubMed] [Google Scholar]
- 16. Konstandi M, Johnson E, Lang MA, Malamas M, Marselos M (2000) Noradrenaline, dopamine, serotonin: different effects of psychological stress on brain biogenic amines in mice and rats. Pharmacol Res 41: 341–346. [DOI] [PubMed] [Google Scholar]
- 17. Konstandi M, Johnson E, Lang MA, Camus-Radon AM, Marselos M (2000) Stress modulates the enzymatic inducibility by benzo[alpha]pyrene in the rat liver. Pharmacol Res 42: 205–211. [DOI] [PubMed] [Google Scholar]
- 18. Konstandi M (2013) Psychophysiological stress: a significant parameter in drug pharmacokinetics. Expert Opin Drug Metab Toxicol 9: 1317–1334. 10.1517/17425255.2013.816283 [DOI] [PubMed] [Google Scholar]
- 19. Flint MS, Hood BL, Sun M, Stewart NA, Jones-Laughner J, Conrads TP (2010) Proteomic analysis of the murine liver in response to a combined exposure to psychological stress and 7,12-dimethylbenz(a)anthracene. J Proteome Res 9: 509–520. 10.1021/pr900861j [DOI] [PubMed] [Google Scholar]
- 20. Konstandi M, Kostakis D, Harkitis P, Marselos M, Johnson EO, Adamidis K et al. (2005) Role of adrenoceptor-linked signaling pathways in the regulation of CYP1A1 gene expression. Biochem Pharmacol 69: 277–287. [DOI] [PubMed] [Google Scholar]
- 21. Konstandi M, Kostakis D, Harkitis P, Johnson EO, Marselos M, Adamidis K et al. (2006) Benzo(alpha)pyrene-induced up-regulation of CYP1A2 gene expression: role of adrenoceptor-linked signaling pathways. Life Sci 79: 331–341. [DOI] [PubMed] [Google Scholar]
- 22. Daskalopoulos EP, Lang MA, Marselos M, Malliou F, Konstandi M (2012) D(2)-dopaminergic receptor-linked pathways: critical regulators of CYP3A, CYP2C, and CYP2D. Mol Pharmacol 82: 668–678. [DOI] [PubMed] [Google Scholar]
- 23. Konstandi M, Harkitis P, Kostakis D, Marselos M, Johnson EO, Lang MA (2008) D2-receptor-linked signaling pathways regulate the expression of hepatic CYP2E1. Life Sci 82: 1–10. [DOI] [PubMed] [Google Scholar]
- 24. Woodcroft KJ, Hafner MS, Novak RF (2002) Insulin signaling in the transcriptional and posttranscriptional regulation of CYP2E1 expression. Hepatology 35: 263–273. [DOI] [PubMed] [Google Scholar]
- 25. Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63: 182–217. 10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- 26. Konstandi M, Johnson EO, Lang MA (2014) Consequences of psychophysiological stress on cytochrome P450-catalyzed drug metabolism. Neurosci Biobehav Rev 45C: 149–167. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez FJ, Yu A-M (2006) Cytochrome P450 and xenobiotic receptor humanized mice. Annu Rev Pharmacol Toxicol 46: 41–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cransac H, Cottet-Emard JM, Pequignot JM, Peyrin L (1996) Monoamines (norepinephrine, dopamine, serotonin) in the rat medial vestibular nucleus: endogenous levels and turnover. J Neural Transm 103: 391–401. [DOI] [PubMed] [Google Scholar]
- 29. Cooper JR, Bloom FE, Roth RH (2003) The Biochemical Basis of Neuropharmacology: Oxford University Press. Adrenergic Receptors p. [Google Scholar]
- 30. Elo HA, MacDonald E (1989) Effects of 2,4-dichlorophenoxyacetic acid (2,4-D) on biogenic amines and their acidic metabolites in brain and cerebrospinal fluid of rats. Arch Toxicol 63: 127–130. [DOI] [PubMed] [Google Scholar]
- 31. Mefford IN (1981) Application of high performance liquid chromatography with electrochemical detection to neurochemical analysis: measurement of catecholamines, serotonin and metabolites in rat brain. J Neurosci Methods 3: 207–224. [DOI] [PubMed] [Google Scholar]
- 32. Stephanou P, Konstandi M, Pappas P, Marselos M (1998) Alterations in central monoaminergic neurotransmission induced by polycyclic aromatic hydrocarbons in rats. Eur J Drug Metab Pharmacokinet 23: 475–481. [DOI] [PubMed] [Google Scholar]
- 33. Lang MA, Gielen JE, Nebert DW (1981) Genetic evidence for many unique liver microsomal P-450-mediated monooxygenase activities in heterogeneic stock mice. J Biol Chem 256: 12068–12075. [PubMed] [Google Scholar]
- 34. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275. [PubMed] [Google Scholar]
- 35. Burke MD, Mayer RT (1974) Ethoxyresorufin: direct fluorimetric assay of a microsomal O-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab Dispos 2: 583–588. [PubMed] [Google Scholar]
- 36. Omura T, Sato R (1964) The Carbon Monoxide-Binding Pigment of Liver Microsomes. Ii. Solubilization, Purification, and Properties. J Biol Chem 239: 2379–2385. [PubMed] [Google Scholar]
- 37. Klaunig JE (1991) Alterations in intercellular communication during the stage of promotion. Proc Soc Exp Biol Med 198: 688–692. [DOI] [PubMed] [Google Scholar]
- 38. Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP et al. (2000) Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 6: 909–919. [DOI] [PubMed] [Google Scholar]
- 39. Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M et al. (2003) Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem 278: 31277–31285. [DOI] [PubMed] [Google Scholar]
- 40. Vorrink SU, Severson PL, Kulak MV, Futscher BW, Domann FE (2014) Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines. Toxicol Appl Pharmacol 274: 408–416. 10.1016/j.taap.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu J, Li J, Zhang S, Xu X, Zheng M, Jiang G et al. (2012) IGF-1 induces hypoxia-inducible factor 1alpha-mediated GLUT3 expression through PI3K/Akt/mTOR dependent pathways in PC12 cells. Brain Res 1430: 18–24. 10.1016/j.brainres.2011.10.046 [DOI] [PubMed] [Google Scholar]
- 42. Tuomisto J, Mannisto P (1985) Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev 37: 249–332. [PubMed] [Google Scholar]
- 43. Waxman DJ, O'Connor C (2006) Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20: 2613–2629. [DOI] [PubMed] [Google Scholar]
- 44. Wiwi CA, Waxman DJ (2004) Role of hepatocyte nuclear factors in growth hormone-regulated, sexually dimorphic expression of liver cytochromes P450. Growth Factors 22: 79–88. [DOI] [PubMed] [Google Scholar]
- 45. Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH (1991) Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci U S A 88: 6868–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pampori NA, Shapiro BH (1999) Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinology 140: 1245–1254. [DOI] [PubMed] [Google Scholar]
- 47. Legraverend C, Mode A, Westin S, Strom A, Eguchi H, Zaphiropoulos PG et al. (1992) Transcriptional regulation of rat P-450 2C gene subfamily members by the sexually dimorphic pattern of growth hormone secretion. Mol Endocrinol 6: 259–266. [DOI] [PubMed] [Google Scholar]
- 48. Charmandari E, Kino T, Souvatzoglou E, Chrousos GP (2003) Pediatric stress: hormonal mediators and human development. Horm Res 59: 161–179. [DOI] [PubMed] [Google Scholar]
- 49. Daskalopoulos EP, Malliou F, Rentesi G, Marselos M, Lang MA, Konstandi M (2012) Stress is a critical player in CYP3A, CYP2C, and CYP2D regulation: role of adrenergic receptor signaling pathways. Am J Physiol Endocrinol Metab 303: 40–54. [DOI] [PubMed] [Google Scholar]
- 50. Bonci A, Hopf FW (2005) The dopamine D2 receptor: new surprises from an old friend. Neuron 47: 335–338. [DOI] [PubMed] [Google Scholar]
- 51. Craig TJ, Lin SP (1981) Cancer and mental illness. Compr Psychiatry 22: 404–410. [DOI] [PubMed] [Google Scholar]
- 52. Kabbani N, Woll MP, Nordman JC, Levenson R (2012) Dopamine receptor interacting proteins: targeting neuronal calcium sensor-1/D2 dopamine receptor interaction for antipsychotic drug development. Curr Drug Targets 13: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang N, Walker MK (2007) Crosstalk between the aryl hydrocarbon receptor and hypoxia on the constitutive expression of cytochrome P4501A1 mRNA. Cardiovasc Toxicol 7: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP (2000) Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol 59: 65–85. [DOI] [PubMed] [Google Scholar]
- 55. Okey AB (1990) Enzyme induction in the cytochrome P-450 system. Pharmacol Ther 45: 241–298. [DOI] [PubMed] [Google Scholar]
- 56. Fitzgerald CT, Nebert DW, Puga A (1998) Regulation of mouse Ah receptor (Ahr) gene basal expression by members of the Sp family of transcription factors. DNA Cell Biol 17: 811–822. [DOI] [PubMed] [Google Scholar]
- 57. Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y (1999) Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev 13: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hahn ME, Allan LL, Sherr DH (2009) Regulation of constitutive and inducible AHR signaling: complex interactions involving the AHR repressor. Biochem Pharmacol 77: 485–497. 10.1016/j.bcp.2008.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH (2008) The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr 18: 207–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rubi B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, et al. (2005) Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem 280: 36824–36832. [DOI] [PubMed] [Google Scholar]
- 61. Woodcroft KJ, Novak RF (1997) Insulin effects on CYP2E1, 2B, 3A, and 4A expression in primary cultured rat hepatocytes. Chem Biol Interact 107: 75–91. [DOI] [PubMed] [Google Scholar]
- 62. Yoshida Y, Kimura N, Oda H, Kakinuma A (1996) Insulin suppresses the induction of CYP2B1 and CYP2B2 gene expression by phenobarbital in adult rat cultured hepatocytes. Biochem Biophys Res Commun 229: 182–188. [DOI] [PubMed] [Google Scholar]
- 63. Kodama S, Koike C, Negishi M, Yamamoto Y (2004) Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24: 7931–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim SK, Novak RF (2007) The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther 113: 88–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Noordeen NA, Khera TK, Sun G, Longbottom ER, Pullen TJ, da Silva Xavier G, et al. (2010) Carbohydrate-responsive element-binding protein (ChREBP) is a negative regulator of ARNT/HIF-1beta gene expression in pancreatic islet beta-cells. Diabetes 59: 153–160. 10.2337/db08-0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wood SM, Gleadle JM, Pugh CW, Hankinson O, Ratcliffe PJ (1996) The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J Biol Chem 271: 15117–15123. [DOI] [PubMed] [Google Scholar]
- 67. Wu R, Zhang L, Hoagland MS, Swanson HI (2007) Lack of the aryl hydrocarbon receptor leads to impaired activation of AKT/protein kinase B and enhanced sensitivity to apoptosis induced via the intrinsic pathway. J Pharmacol Exp Ther 320: 448–457. [DOI] [PubMed] [Google Scholar]
- 68. Waxman DJ, Holloway MG (2009) Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 76: 215–228. 10.1124/mol.109.056705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamazoe Y, Shimada M, Murayama N, Kato R (1987) Suppression of levels of phenobarbital-inducible rat liver cytochrome P-450 by pituitary hormone. J Biol Chem 262: 7423–7428. [PubMed] [Google Scholar]
- 70. Jones SN, Jones PG, Ibarguen H, Caskey CT, Craigen WJ (1991) Induction of the Cyp1a-1 dioxin-responsive enhancer in transgenic mice. Nucleic Acids Res 19: 6547–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fitzgerald P, Dinan TG (2008) Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol 22: 12–19. 10.1177/0269216307087148 [DOI] [PubMed] [Google Scholar]
- 72. Meredith C, Scott MP, Renwick AB, Price RJ, Lake BG (2003) Studies on the induction of rat hepatic CYP1A, CYP2B, CYP3A and CYP4A subfamily form mRNAs in vivo and in vitro using precision-cut rat liver slices. Xenobiotica 33: 511–527. [DOI] [PubMed] [Google Scholar]
- 73. Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, et al. (2004) Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol 65: 1225–1237. [DOI] [PubMed] [Google Scholar]
- 74. Endo K, Uno S, Seki T, Ariga T, Kusumi Y, Mitsumata M, et al. (2008) Inhibition of aryl hydrocarbon receptor transactivation and DNA adduct formation by CYP1 isoform-selective metabolic deactivation of benzo[a]pyrene. Toxicol Appl Pharmacol 230: 135–143. 10.1016/j.taap.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 75. Hay N (2011) Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta 1813: 1965–1970. 10.1016/j.bbamcr.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP, et al. (1998) Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem Res Toxicol 11: 1048–1056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alterations in CYP1A1, CYP1A2 and CYP1B1 relative mRNA expression following sulpiride (SULP, 10μM) exposure of primary hepatocytes for different time periods was assessed. Control cells were treated with DMSO. SULP1: incubation of hepatocytes with sulpiride for 1 hr; SULP6: incubation of hepatocytes with sulpiride for 6 hr; SULP12: incubation of hepatocytes with sulpiride for 12 hr; SULP24: incubation of hepatocytes with sulpiride for 24 hr; ***P<0.001.
(DOC)
Alterations in CYP1A1, CYP1A2 and CYP1B1 relative mRNA expression following exposure of primary hepatocytes with either 10μM or 25μM sulpiride (SULP) for 24 hr. Control cells were treated with DMSO.
(DOC)
***P<0.001.
(DOC)
These primers were used for the quantitation of the mRNA levels of the genes tested using q-PCR.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.