Abstract
The goal of the current study was to determine whether sediments from agriculturally-intense watersheds can act as a potential source of anti-estrogenic endocrine-disrupting compounds. The specific objectives of the current study were to determine 1) whether female fathead minnows (Pimephales promelas) experience alterations in endocrine function when exposed to sediments collected from agriculturally-intense watersheds and 2) if these sediments display anti-estrogenic activity in an in vitro assay. In addition, sediment samples were analyzed for the presence of steroid hormones and pesticides associated with local agricultural practices. To accomplish this, sediments and water were collected from three sites within two agriculturally-intense Nebraska watersheds (Bow Creek and the Elkhorn River). In 2009, minnows were exposed to sediment and/or water collected from the two Bow Creek sites (East Bow Creek and the Confluence) in the laboratory, while in 2010, minnows were exposed to sediment and/or water from East Bow Creek, the Confluence and the Elkhorn River. Following the 7-d exposure period, the hepatic mRNA expression of two-estrogen responsive genes, estrogen receptor α (ERα) and vitellogenin (Vtg) was determined. In 2009, females exposed to Confluence sediments, in the presence of laboratory water or Confluence water, experienced significant reductions in ERα expression relative to unexposed and Confluence water-exposed females. The defeminization of these females suggests the presence of a biologically-available anti-estrogenic compound in sediments collected from this site. In 2010, sediments were assessed for anti-estrogenic activity on days 0 and 7 of the exposure period using a four-hour yeast estrogen screen. Lipophilic extracts (LEs) of day 0 sediments collected from the Confluence and the Elkhorn River induced significant reductions in the estrogenic reporter activity of treated yeast cultures suggesting the presence of a lipophilic anti-estrogenic compound in these extracts. Chemical analysis revealed the presence of a variety of steroid hormones, including those associated with the production of beef cattle (ie: β-trenbolone, α-zearalanol and α-zearalenol), in sediments indicating that compounds utilized by local beef cattle operations are capable of entering nearby watersheds. Overall, the results of this study indicate that an environmentally-relevant anti-estrogenic compound is present in sediments from agriculturally-intense watersheds and that this compound is bioavailable to fish. Furthermore, the presence of steroid hormones in sediments from these watersheds provides evidence indicating that steroids are capable of sorbing to sediments. Clearly, sediments are capable of acting as a source of endocrine-disrupting compounds in the aquatic environment.
Keywords: fathead minnow, gene expression, sediment, agrichemicals, anti-estrogen
1. Introduction
The advent of modern-day agriculture has allowed for food production on an unprecedented scale; however, one potential negative consequence of current farming practices is the release of agrichemicals, some of which have known or suspected endocrine-disrupting activity, into the aquatic environment. Two potential sources of endocrine-disrupting compounds in agriculturally-intense watersheds are pesticides from row crop production and steroid hormones from livestock production (Kolok et al., 2007; Sellin et al., 2009; Kolodziej and Sedlak, 2007). Studies conducted within agricultural landscapes have found that a variety of agrichemicals reach surface waters. For example, studies of agriculturally-intense Nebraska (USA) watersheds, including the Elkhorn, Platte and Bow Creek watersheds, found that polar organic chemical integrative samplers (POCISs) deployed in these watersheds contained a variety of agrichemicals including atrazine, deethylatrazine, deisopropylatrazine, acetochlor and metolachlor (Sellin et al., 2009; Sellin Jeffries et al., in review). In addition to pesticides, steroid hormones have also been detected in the surface waters of agriculturally-intense watersheds. For example, estrone (E1), 17β-estradiol (E2), estriol, testosterone, 4-androstenedione, progesterone, and melengestrol acetate were detected in extracts from POCISs deployed at four sites within the Elkhorn River watershed (Kolok et al., 2007). Also, Kolodziej and Sedlak (2007) found detectable levels of estrogens, androgens and progestins in watersheds characterized by rangeland for grazing cattle.
While many agrichemicals have been found in surface waters, these compounds have also been found to be associated with riverbed sediments. Pereira et al. (1996) found appreciable amounts of DDT and the associated metabolites DDD and DDE, as well as trace amounts of chlordane, in riverbed sediments of the intensively-farmed San Joaquin River Basin, California. In addition to legacy pesticides, currently-used pesticides, such as simazine (Villaverde et al., 2008) and atrazine (Hela et al., 2005), have also been detected in bed sediments. Steroid hormones have also been found associated with sediments. For example, androstenedione and progesterone have been detected in sediments from a river receiving paper-mill effluent (Jenkins et al., 2003), while estrogens have been found in sediments collected downstream from a wastewater treatment facility (Peck et al., 2003). In addition, Urbatzka et al. (2007) found that the extracts of sediments collected from an Italian watershed receiving agricultural runoff and domestic/industrial waste water possessed androgenic and anti-androgenic activity. Clearly, the presence of compounds with endocrine-disrupting activity in not only water, but also sediments may pose a significant risk to aquatic organisms.
Previous studies have shown that female fathead minnows (Pimephales Promelas) deployed in agriculturally-intense Nebraska watersheds experience alterations in endocrine function (Sellin et al., 2009; Sellin Jeffries et al., in review). Specifically, field studies revealed that female minnows deployed at two sites in the Bow Creek watershed (East Bow Creek and a site near the confluence of Bow Creek and the Missouri River) and at a site in the Elkhorn River watershed, experience defeminization, as indicated by significant reductions in the mRNA expression of the estrogen-responsive genes, vitellogenin (Vtg) and/or estrogen receptor-alpha (ERα). With regard to the Elkhorn River site, a laboratory study by Sellin et al. (2010) demonstrated that this effect was associated with exposures to the riverbed sediment, rather than exposures to water. Though the specific contaminant was not identified in the Sellin et al (2009) or Sellin et al. (2010) studies; it was hypothesized that the causative agent is anti-estrogenic, as reductions in Vtg levels have been shown to occur following exposures to anti-estrogenic compounds (Panter et al., 2002).
The current study was designed to determine 1) if female fathead minnows exposed to sediments from East Bow Creek, the Confluence or the Elkhorn River display reductions in the mRNA expression of Vtg or ERα and 2) if sediments from these sites display anti-estrogenic activity as determined by in vitro assays. In addition, the sediments from each of the selected sites were tested to determine the presence of agrichemicals associated with regional agricultural practices.
2. Material and methods
2.1. Experimental design and exposure regime
Three sites, each characterized by high densities of cattle feedlots and row crop agriculture, were selected for inclusion in the current study. Two of the sites, East Bow Creek and the Confluence, are located in the Bow Creek watershed, while the third site was located in the Elkhorn River watershed (Fig. 1). Experiments were conducted in the summers of 2009 and 2010. The 2009 study featured sediments from two sites within the Bow Creek watershed, East Bow Creek and the Confluence with the Missouri River. The 2010 study featured sediments collected from the same two sites within the Bow Creek watershed and a site within the Elkhorn River watershed. In the 2009 study, sexually-mature adult male and female fathead minnows were divided into seven groups – unexposed, Confluence water-exposed, Confluence sediment-exposed and Confluence water/sediment-exposed, East Bow water-exposed, East Bow sediment-exposed and East Bow water/sediment-exposed. Exposures were initiated on August 25th, 2009 and ended on September 1st, 2009. The endocrine effects of exposures to sediments and water from each site was assessed by measuring the hepatic mRNA expression of steroid-responsive genes in female fathead minnows.
Figure 1.
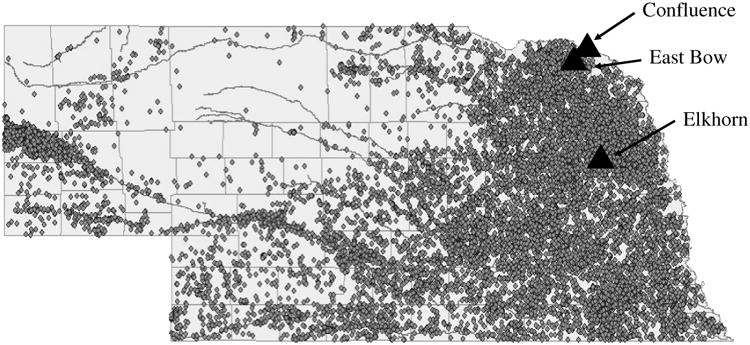
The location of the three field sites from which water and sediments were collected for use in this study. Small, closed circles indicate beef feedlots with over 500 head of cattle.
The 2010 study consisted of a series of three experiments – one featuring East Bow sediments, one featuring Confluence sediments and one featuring Elkhorn sediments. In each of these experiments, sexually-mature adult male and female fathead minnows were divided into four groups – unexposed, water-exposed, sediment-exposed or water/sediment-exposed groups. The unexposed groups served as negative controls and were maintained in laboratory water and not exposed to sediment. The water-exposed groups were exposed to water collected from the appropriate field site in a sediment-free aquarium. Sediment-exposed groups were maintained in an aquaria containing sediment collected from the given field site. Water/sediment-exposed groups served as positive controls and were held in aquaria containing sediment and water collected from the appropriate field site. For each of the experiments, sediment and water was collected from each site on May 24th, 2010. Exposures persisted for 7 days beginning on May 25th and ending on June 1st. In the 2010 study, the endocrine function of female minnows was assessed by measuring the hepatic mRNA expression of steroid responsive genes. In addition, sediments collected from the field on May 24th and from exposure aquaria on June 1st (last day of the exposure period) were analyzed for anti-estrogenic activity using in vitro assays and for the presence of 20 steroids and 46 pesticides using liquid chromatography-tandem mass spectrometry (LC/MS/MS) and gas chromatography-mass spectrometry (GC/MS), respectively.
2.2. Fathead minnow maintenance
Sexually-mature adult male and female fathead minnows were obtained from the University of Nebraska fathead minnow breeding colony. In each experiment, 45-L test aquaria contained 14 minnows (seven males and seven females). All aquaria were aerated, heated to 26°C and kept under a photoperiod of 16:8 light:dark. Fish were fed commercially available high-protein flake food (Aquatic Ecosystems, Apopka, FL, USA) daily. During the exposures, a water exchange (>30% of the total volume) was conducted on day 3 of the exposure period. Water used to replenish the volume of tanks containing water collected from the field sites was obtained no more than 24 hours prior to use. Water quality measures in each aquarium were obtained every other day during the course of exposures. Average temperature, pH and conductivity ranged from 26.2-26.9°C, 8.2-8.5 and 478-965 μS. Aquaria containing sediment held approximately 8 cm of sediment collected from the selected field sites. In 2010, the total organic carbon content of sediments from East Bow, the Confluence and Elkhorn was determined to be 3.0, 3.4 and 2.4%, respectively. Sediments from the Confluence were classified as sandy loam (56% sand, 38% silt, 6% clay), while East Bow (82% sand, 16% silt, 2% clay) and Elkhorn (78% sand, 18% silt, 4% clay) sediments were classified as loamy sand.
2.3. Hepatic mRNA expression of minnows
The results of previous studies showed that female minnows, but not male minnows, deployed at East Bow Creek, the Confluence and the Elkhorn River experienced significant alterations in the mRNA expression of steroid-responsive genes (Sellin et al. 2009, Sellin Jeffries et al. in review). As such, the analysis of hepatic mRNA expression of minnows was restricted to females only. Following the 7-d exposures, females were measured for body mass, liver mass and gonad mass. HSI and GSI were generated by dividing the mass of the tissues into the body mass of the fish, then multiplying by 100. Immediately upon dissection, livers were flash frozen in liquid nitrogen and stored at -80°C until analysis. Hepatic Vtg and ERα mRNA expression was evaluated for a minimum of five females from each group according to the protocol outlined in Kolok et al. (2007). Briefly, total RNA was isolated from each liver using the SV Total RNA Isolation System (Promega Corp. Madison, WI) and then quantified spectrophotometrically at 260 nm. Only samples with absorbance260 to absorbance280 ratios greater than 1.7 were used in subsequent analyses. First-strand cDNA was synthesized using an iScript cDNA Synthesis Kit (Bio-Rad Inc, Hercules, CA). Q-PCR was carried out using a Bio-Rad iCycler equipped with a real-time PCR detection system (MyiQ). The expression of mRNA was determined using a Bio-Rad MyiQ Real-Time Polymerase Chain Reaction Detection System managed by Optical System Software version 1.0. Data were quantified by the standard curve method using series diluted cDNA samples as a standard and the expression of each gene was normalized by the expression of L8 mRNA, a “housekeeping” gene.
2.4. Sediment processing
In the 2010 experiment, sediments were analyzed for in vitro anti-estrogenic activity and for the presence of steroid hormones and pesticide. To accomplish this, these sediments were processed within 12 hours of collection using a modified version of Lorenzen (2005). Each sediment sample was used to generate an aqueous wash (AW) and a lipophilic extract (LE). The AWs were generated by combing 30 g of sediment and 15 mL reverse osmosis filtered water to a 50 mL glass centrifuge tube. The samples were gently rotated for 90 minutes and centrifuged at 2000 rpm for 15 minutes. The supernatant was extracted and evaporated to dryness under a steady stream of nitrogen at 35°C. Samples utilized in the in vitro assays and for chemical analysis were reconstituted in 1 mL of 10% DMSO or 50% methanol, respectively. Reconstituted samples were transferred to amber glass vials and stored at -20°C.
The LEs were created by placing approximately 30g of sediment in a 50 mL glass centrifuge tube with 10 mL of ethyl acetate. Samples were then gently rotated for 15 minutes followed by centrifugation at 2000 rpm for 15 minutes. The resulting supernatant was transferred to a glass container and re-extracted twice, repeating the above procedure with 10 mL acetone (rather than ethyl acetate). Supernatants from each extraction procedure were combined and evaporated to dryness under a steady stream of nitrogen at 35°C. Samples were reconstituted and stored as described above.
Compound recovery in LE was determined by spiking 30 g sediment samples with a solution of 9.9 × 10-5 μCi/mL 14C-labelled 17β-estradiol in methanol, achieving a final concentration of 0.25 μg/g. Spiked sediments were equilibrated for 24 hr and then extracted according to the procedures described above. A 0.2 mL aliquot of the extract was placed in a 20 mL scintillation vial with 5 mL of OptimaGold scintillation cocktail and 14C activity was monitored by scintillation counting (Packard 2500Tr). Recovery of 14C -labeled 17β-estradiol in spiked sediments was determined to be greater than or equal to 80%.
2.5. Assessment of anti-estrogenic activity in sediment extracts
Reconstituted LEs and AWs from each site were shipped, on dry ice, to the University of Texas-El Paso for determination of anti-estrogenic activity using a four-hour yeast bioassay. This in vitro assay features a receptor-mediated β-galactosidase reporter (Balsiger et al., 2010) and was performed in order to establish anti-estrogenic activity of sediments collected from the field prior to initiation of exposures (day 0) and from aquaria upon termination of the exposures (day 7). The parental yeast strain W303α (MATα leu2-112 ura3-1 trp1-1 his3-11,15 ade2-1 can1-100 GAL SUC2) with a deleted pleiotrophic drug resistance gene (PDR5) was used for all assays described. The deletion of PDR5 in this strain was performed according to previously published methods (Gueldener et al., 2002). The parent strain was co-transformed with a TRP1-marked constitutive human ERα expression plasmid (pG/ER) and a URA3-marked estrogen-inducible β-galactosidase reporter plasmid (pUCΔSS-ERE, both plasmids kindly provided by Didier Picard, University of Geneva) and maintained in synthetic complete media lacking uracil and tryptophan (SC-UW) to select for plasmid retention. For all assays described, the yeast reporter strain was cultured overnight in SC-UW at 30°C in a shaking water bath. The next morning the cells were diluted back to an optical density of 0.08 at 600 nm (O.D.600) and incubated in a shaking water bath at 30°C until the culture reached an O.D.600 of 0.1. The growing culture was then aliquoted into an opaque 96-well plate at 100 ul per well. E2, in a final concentration of 0.145 nM (previously determined to be the EC50), was added to the yeast culture in each well which was designated for the addition of AWs or LEs. Prior to analysis, sediment extracts underwent a 10-fold dilution to obtain a 1% DMSO solution. The concentrated samples or hormone standards (E2) in ethanol vehicle were then added directly to the wells and the plate was incubated at 30 °C for 2 h. The amount of DMSO and ethanol added never exceeded 1% of the total culture volume. Yeast cultures containing only DMSO or ethanol were included in the assay to ensure that alterations in reporter expression were unrelated to DMSO or ethanol. After the 2 h incubation in the presence of hormone, 100 μl of Tropix Gal-Screen in Buffer B (Applied Biosystems, Foster City, CA) was added to each well and the plate was incubated for an additional 2 h at room temperature. The hormone-induced chemiluminescent signal was then measured on a Luminoskan Ascent microplate luminometer (Thermo Fisher Scientific Inc., Waltham, MA). All data were normalized to percentages by setting the largest value in each dataset to 100%. In addition, the extract dilutions were back calculated to total sediment mass and the data were plotted as the percent estrogenic activity versus total sediment (g/mL).
2.6. Analysis of pesticides and steroid hormones in sediments
The AWs and LEs of sediments collected from each site (day 0) and from aquaria (day 7) were sent, on dry ice, to the University of Nebraska Water Science Center where they were analyzed for 46 pesticides and 20 steroid hormones (Table 1). Pesticide concentrations were determined using selected ion monitoring GC/MS, while steroid hormone concentrations were determined using LC/MS/MS with atmospheric pressure photoionization (APPI) as described in Sellin et al. (2010). Concentrations of steroids and pesticides are expressed as ng/g dry sediment.
Table 1.
Steroid hormones and pesticides measured in the aqueous-rinsed sediment fractions (AWs) and lipid-soluble sediment extracts (LEs) from each of the study sites. Detection limits for steroid hormones and pesticides in sediments were 1 and 5 ng/sample, respectively.
| Steroid Hormones | Pesticides | |
|---|---|---|
| 4-Androstenedione | Atrazine | Ethyl dipropylthiocarbamate |
| Androsterone | Cyanazine | 2,4,5,6-tetrachloro-m-xylene |
| Androstenedienedione | Deethylatrazine | 4,4-dichlorodiphenyldichloroethane |
| 11-ketotestosterone | Deisopropylatrazine | 4,4-dichlorodiphenyldichloroethylene |
| Epitestosterone | Metribuzin | 4,4-dichlorodiphenyltrichloroethane |
| Testosterone | Prometon | α-benzene hexachloride |
| 17β-trenbolone | Propazine | β- benzene hexachloride |
| 17α-trenbolone | Simazine | δ- benzene hexachloride |
| 17α-ethinylestradiol | Acetochlor | Lindane |
| 17β-estradiol | Alachlor | α-chlordane |
| 17α-estradiol | Dimethenamid | γ-Chlordane |
| Estrone | Propachlor | Aldrin |
| Estriol | Butylate | Decachlorobiphenyl |
| α-zearalonol | Terbufos | Dieldrin |
| α -zearalenol | Norflorazon | Endosulfan I |
| β-zearalonol | Pendimethalin | Endosulfan II |
| β-zearalenol | Trifluralin | Endosulfan sulfate |
| Progesterone | Permethrin | Endrin |
| 17α-hydroxyprogesterone | Telfluthrin | Endrin aldehyde |
| Melengestrol acetate | Chlorothalonil | Endrin ketone |
| Metolachlor | Heptachlor | |
| Tebupirimfos | Heptachlor epoxide B | |
| Chlorpyrifos | Methoxychlor |
2.7. Statistical Analysis
For each of the biological parameters measured, the assumption of homogeneity of variances was met; therefore, differences in body mass, organ indices and hepatic mRNA expression were tested for using single factor analysis of variance (ANOVA, Statview 5.0) followed by Newman-Kuels multiple comparison tests. Differences in reporter expression were test for using single factor analysis of variance (ANOVA, JMP 8.0.2) followed by Tukey-Kramer multiple comparison tests. Statistical significance was assumed at p ≤ 0.05.
3. Results
3.1. Fathead minnows
3.1.1. 2009 Study
In 2009, females were exposed to sediments and water collected from either East Bow Creek or the Confluence. Among females in the East Bow experiment, the average body mass, HSI and GSI ranged from 1.30 to 1.38 g, 3.0 to 4.4 and 9.2 to 11.2, respectively. No significant differences in any of the morphometric endpoints were detected (ANOVA, p > 0.15 in all cases). Hepatic Vtg and ERα mRNA expression among females in the East Bow groups ranged from 0.76 to 1.00 and 0.38 to 0.67, respectively and no significant differences in the expression of either gene were detected (p = 0.58 in both cases).
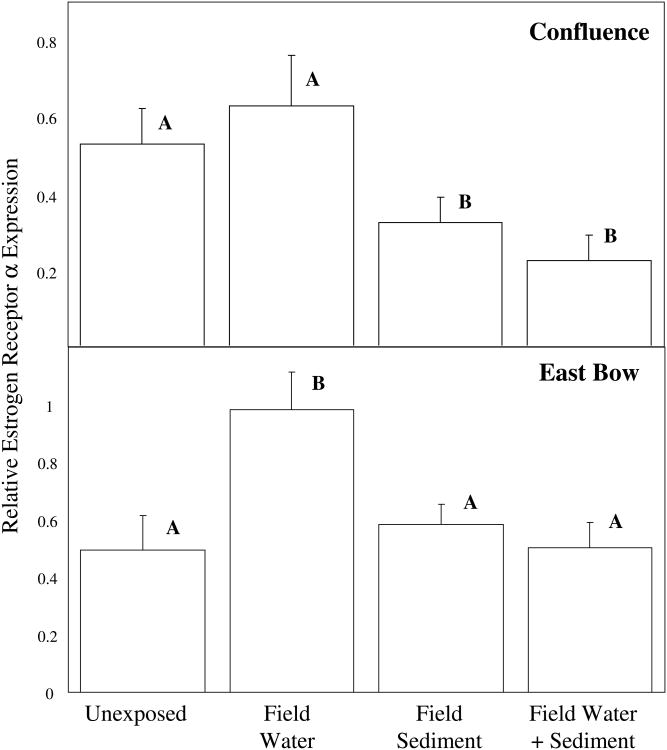
Females in the Confluence exposure groups had average body masses, HSIs and GSIs of ranging from 1.24 to 1.38 g, 2.8 to 4.0 and 10.3 to 11.4, respectively. No significant differences in any of morphometric endpoints were detected (ANOVA, p > 0.50 in all cases). Hepatic Vtg mRNA expression among females in the Confluence groups ranged from 0.76 to 1.0 and no significant differences in expression were detected (p = 0.61). However, significant differences in ERα mRNA expression were detected (Fig. 2, p = 0.03). Specifically, females in the Confluence sediment-exposed and Confluence sediment/water-exposed groups experienced significant reductions in ERα mRNA expression relative to unexposed females and Confluence-water exposed females.
Figure 2.
Relative hepatic estrogen receptor α (ERα) mRNA expression (mean ± standard error) of unexposed (lab water), field water-exposed, field sediment-exposed and field sediment/water-exposed females. The top panel shows ERα mRNA expression of females exposed to Confluence water/sediment, while the bottom panel shows ERα mRNA expression of females exposed to East Bow water/sediment. Letters indicate significant differences between groups.
3.1.2. 2010 Study
In 2010, three sets of experiments were conducted. The first set of experiments featured females exposed to sediments and/or water collected from East Bow Creek. The average body mass of these female minnows ranged from 1.31 to 1.57 g and HSI and GSI ranged from 1.8 to 2.4 and 12.4 to 13.7, respectively. No significant differences in these endpoints were detected (ANOVA, p > 0.33 in all cases). Among females exposed to water and/or sediments from East Bow Creek, Vtg mRNA expression ranged from 0.69 to 0.94 with no significant differences between groups detected (ANOVA, p = 0.56). In contrast, significant differences in the hepatic mRNA expression of ERα were detected (ANOVA, p = 0.01, Fig. 2). Specifically, females in the East Bow water-exposed group had significantly higher ERα mRNA expression than unexposed females and females in the sediment-exposed and water/sediment-exposed groups.
Females involved in the second experiment were exposed to sediments and/or water collected from the Confluence. The average body mass and HSI of these female ranged from 1.32 to 1.68 g and 1.40 to 1.99, respectively and no significant differences in either parameter were detected (ANOVA, p > 0.25 in both cases). Female GSI ranged from 11.5 to 16.5 and significant differences between groups were detected (ANOVA, p < 0.001). Specifically, the mean GSI of females in the Confluence sediment-exposed group was significantly higher than that of females from the water-exposed and water/sediment-exposed groups, but did not differ from that unexposed females. The hepatic Vtg and ERα mRNA expression of females in the Confluence groups ranged from 0.85 to 1.24 (ANOVA, p > 0.3371) and 0.68 to 0.80 (ANOVA, p = 0.8466), respectively. No significant differences in the expression of either gene were detected.
The third experiment featured females exposed to Elkhorn sediment and/or water. The average body mass, HSI and GSI of these females ranged from 1.43 to 1.83 g, 1.7 to 2.7 and 12.1 to 14.0, respectively and no significant differences in any of these morphometric endpoints were detected (ANOVA, p > 0.17 in all cases). Hepatic Vtg and ERα mRNA expression among these females ranged from 0.85 to 1.24 and 0.59 to 1.02, respectively and no significant differences between groups were detected (ANOVA, p > 0.13 in both cases).
3.2. Anti-estrogenic activity of sediment extracts
For the day 0, field-collected sediments, ten-fold serial dilutions of the AWs and LEs (dissolved in 10% DMSO) were performed such that the final concentrations tested, in addition to the original extract, were: 1/10, 1/100, 1/1000, 1/10,000 and 1/100,000 of the original sediment. The reporter expression of 100 μl of the growing yeast culture, with the addition of E2 (0.145 nM final concentration) was determined and used as the basis for the control line in each graph in Figures 3.
Figure 3.
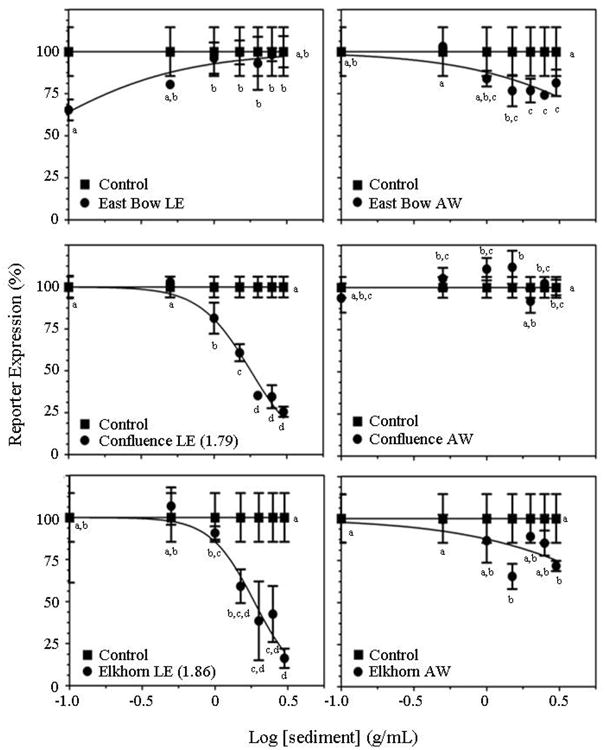
Dose-response curves for aqueous-rinsed sediment fractions (AWs) and lipid-soluble sediment extracts (LEs) from each of the sites on day 0 using the 4-hr yeast bioassay. Control samples containing only 17β-estradiol (E2) are indicated by the line connected by the closed squares. Samples containing sediment extract in the presence of E2 are indicated by closed circles. Each data point represents the mean of 3 independent replicates with error bars indicating standard deviation. All data were normalized to 100% with the highest value being established as either the first or last value, whichever is higher. Letters indicate significant differences between groups.
3.2.1. Day 0, field-collected samples
Significant differences in the reporter activity of cell cultures treated with various concentrations of East Bow LEs were detected (ANOVA, p = 0.0023, Fig. 3); however, in no case was the reporter activity of cells treated with LEs different from that of cultures containing only E2 and DMSO. The addition of East Bow AWs to the cell cultures, in the presence of E2, caused a significant decrease in estrogenic reporter activity (ANOVA, p = 0.0002, Fig. 3). As the concentration of East Bow AWs added to the cell cultures increased, the estrogenic reporter activity decreased with an approximately 20% decrease in activity occurring in the cultures containing the highest concentration of East Bow AWs.
The addition of Confluence LEs to the cell cultures caused significant alterations in reporter activity (ANOVA, p < 0.0001, Fig. 3). Specifically, Confluence LEs caused dose-dependent decreases in activity with an approximate 75% reduction in estrogenic reporter activity (relative to activity in the presence of E2 + DMSO only) at the highest concentration of LE tested. Confluence AWs caused significant alterations in estrogen reporter activity in some of dilutions tested (ANOVA, p < 0.0001, Fig. 3); however, these alterations did not appear to be dose-dependent.
Elkhorn LEs caused significant dose-dependent decreases in the estrogenic reporter activity of cell cultures (ANOVA, p < 0.0001, Fig. 3). Relative to cell cultures containing only E2 and DMSO, cultures containing the highest concentration of Elkhorn LEs tested displayed approximately 90% lower estrogenic reporter activity. Significant alterations in the reporter activity of cultures treated with Elkhorn AWs were observed (ANOVA, p = 0.0011, Fig. 3); yet there was little indication that these alterations were dependent on dose.
3.2.2. Day 7, aquarium-collected samples
Sediment samples were collected from aquaria containing sediments and laboratory water, as well as from those containing sediments and field-collected water, following the termination of exposures on day 7. In general, dose-dependent significant alterations in reporter activity were not observed among yeast cultures treated with LEs and AWs generated from these sediments. The only exception to this was in yeast cultures treated with Elkhorn AWs from aquaria containing laboratory water. Specifically, addition of this sample to the yeast cultures caused significant decreases in estrogenic reporter activity (ANOVA, p < 0.0001, data not shown) with cultures treated with the highest concentration of sediment displaying a reduction of over 65% in reporter activity. These decreases appeared to be dose dependent with reporter activity decreasing as the concentration of sediment added to the cultures increased. It should be noted, that Elkhorn AWs from aquaria containing Elkhorn water did not cause significant alterations in reporter activity (ANOVA, p = 0.2189, data not shown).
3.3. Agrichemicals in sediments
In 2010, pesticide concentrations in the AWs and LEs generated from field collected (day 0) and aquaria collected (day 7) sediments were below the detection limit of 5 ng/sample in all but one case. Atrazine was detected at a concentration of 0.28 ng/g sediment (dry weight) in the LE from Confluence sediments collected from the field (day 0).
Steroid concentrations in the AWs and LEs are reported in Tables 2 and 3. Of the 20 steroid hormones analyzed for, 9 were present at concentrations above the detection limit of 1 ng/sample. Four androgenic steroids (4-androstenedione, epitestosterone, testosterone and 17β-trenbolone), four estrogenic steroids (17α-estradiol, estrone, α-zearalanol and α-zearalenol) and one progestogen (progesterone) were detected.
Table 2.
Steroid concentrations (ng/g dry sediment) of the aqueous washes (AWs) of sediments collected from each of the field sites. Steroids that were measured for, but found to be below the detection limit of 1 ng/sample in each sample are not shown. Dashes represent concentrations below the detection limit.
| Epitestosterone | Testosterone | β-trenbolone | α-estradiol | α-zearalanol | |
|---|---|---|---|---|---|
| East Bow | 0.06 | - | - | - | - |
| Confluence | - | - | 0.19 | - | - |
| Elkhorn | - | 0.04 | 0.10 | 0.13 | 0.22 |
Table 3.
Steroid concentrations (ng/g dry sediment) of the lipophilic extracts (LEs) of sediments from each of the sites. Sediments collected on day 0 were taken directly from the field, while day 7 samples were taken from tanks containing sediments either in combination with laboratory water (sediment only tanks) or field-collected water (sediment/H2O tanks). Steroids that were measured for, but found to be below the detection limit of 1 ng/sample in each sample are not shown. Dashes represent concentrations below the detection limit.
| β-trenbolone | α -estradiol | α -zearalenol | Estrone | Testosterone | Progesterone | 4-androstenedione | |
|---|---|---|---|---|---|---|---|
| East Bow | |||||||
| 0 day, field | 0.49 | - | - | - | - | - | 0.06 |
| 7 day, sediment only tank | 0.28 | - | - | - | - | - | - |
| 7 day, sediment/H2O tank | - | - | - | - | 0.25 | 0.29 | 1.18 |
|
| |||||||
| Confluence | |||||||
| 0 day, field | 0.69 | - | - | 0.17 | - | - | 0.09 |
| 7 day, sediment only tank | - | - | - | - | - | 0.11 | 0.41 |
| 7 day, sediment/H2O tank | - | - | - | - | - | 0.15 | 0.10 |
|
| |||||||
| Elkhorn | |||||||
| 0 day, field | 0.09 | 0.07 | 1.80 | - | - | - | - |
| 7 day, sediment only tank | - | - | - | - | - | - | - |
| 7 day, sediment/H2O tank | - | - | - | - | - | - | - |
4. Discussion
The goal of this study was to test the hypothesis that the previously documented defeminization of caged female fathead minnows deployed at East Bow Creek, the Confluence and Elkhorn River (Sellin et al. 2009, Sellin et al. in review) was related to the presence of antiestrogenic compounds in river bed sediments. This was accomplished by determining if: 1) female minnows experience alterations in endocrine function following exposures to sediments from these sites and 2) sediments from each site possess anti-estrogenic activity in vitro. The findings of this study indicate that: 1) females exposed to sediments collected from the Confluence in 2009 experienced defeminization as indicated by significant reductions in the hepatic expression of ERα, and that 2) sediments collected from the Confluence and the Elkhorn River in 2010 display anti-estrogenic activity as indicated by dose-response reductions in the estrogenic response of yeast cultures. These finding, in conjunction with the results of previous studies, indicate that a compound (or suite of compounds) with anti-estrogenic properties is present in sediments from these agricultural watersheds. Furthermore, steroid hormones, some of which are associated with the production of beef cattle, were present in sediments from each of the field sites; however, the presence of these compounds could not be conclusively linked to the observed defeminization of females or the anti-estrogenic activity of sediments in vitro.
4.1. Fathead minnows
In the 2009 study, the expression of steroid-responsive genes was not different between female minnows exposed to sediment, water or sediment and water collected from East Bow Creek relative to unexposed females suggesting that neither water nor sediment from East Bow Creek acts as a source of endocrine-active compounds. In contrast, females exposed to Confluence sediments (in the presence of laboratory water or Confluence water) had significantly higher ERα mRNA expression than unexposed females, while the ERα expression of females exposed to water collected from the Confluence did not differ from that of unexposed females. This suggests that a bioavailable anti-estrogenic compound is present in the sediments, but not in water, collected from the Confluence.
In 2010, there was no evidence to suggest that females exposed to water or sediments from any of the sites experienced defeminization. However, females exposed to water collected from one of the sites, East Bow Creek, experienced hyper-feminization as indicated by increased hepatic ERα expression relative to females in the other treatment groups. Increases in the expression of estrogen-responsive genes, such as this one, are generally associated with exposures to estrogenic compounds. While it is possible that females were exposed to estrogenic compounds present in the water, this finding is somewhat difficult to interpret given that feminization of males is the standard metric for establishing whether a fish has been exposed to environmental estrogens. Since the hepatic gene expression of males was not measured in the current study, the observed hyper-feminizing effects of East Bow water cannot be supported nor refuted by concomitant feminization of males. However, a previous study in which caged male minnows were deployed in East Bow Creek did not provide any evidence suggesting that males had been feminized (Sellin Jeffries et al. in review).
Interestingly, only females exposed to East Bow water, in the absence of sediments, experienced hyper-feminization. It would be expected that if East Bow water was indeed estrogenic, then females exposed to East Bow water in both the absence and presence of sediment would become hyper-feminized. The lack of increased ERα expression among females exposed simultaneously to East Bow water and sediments may be indicative of a complex relationship between water-borne contaminants and sediments. It is possible that estrogenic compounds present in the water sorb to sediment particles making them biologically unavailable to the fish; however, results from steroid analysis do not provide solid evidence that estrogens are present in East Bow sediments.
4.2. Temporal variation in the occurrence of endocrine-disrupting compounds in sediment
When the results of the current study are compared with the results of previous laboratory and field studies (Table 4), a number of discrepancies become apparent. In previous studies, caged female minnows deployed at each of the sites experienced defeminization (Sellin et al. 2009; Sellin Jeffries et al. in review). Similarly, when females are exposed to sediments collected from the Elkhorn in 2008 (Sellin et al. 2010) and the Confluence in 2009, they become defeminized. However, exposures to sediments collected from each of the three sites in 2010 did not lead to defeminization. The fact that the defeminization of females has been observed in both field and laboratory studies suggests that a chemical agent, rather than an environmental agent, is responsible for the observed defeminization of females. However, the discrepancies between the aforementioned studies suggest that the presence or concentration of this chemical agent varies temporally, as these studies were conducted in different months across multiple years (Table 4). In each of the agricultural watersheds investigated in these studies, the ultimate source of biologically active compound is probably application of the chemicals to the adjacent land surface or administration of steroid hormones to livestock, and the vector by which the compounds reach locations tested in this study is most likely surface runoff. Under these conditions, it is not unreasonable to hypothesize that the presence of the compounds in the environment (as well as their subsequent impacts on aquatic organisms) will vary from season to season and from year to year.
Table 4.
Summary of results from the current study and previous studies investigating endocrine-disruption among female fathead minnows following field deployments or exposures to sediment (s) or water (w) collected from East Bow Creek, the Confluence or the Elkhorn River. The effects of field collected water and sediment on the estrogenic reporter expression of yeast cultures is also summarized. Negative effects include decreases in hepatic mRNA expression of either vitellogenin (Vtg) or estrogen receptor α (ERα) among female minnows and decreased reporter activity of yeast cultures, while positive effects include increased hepatic mRNA expression of Vtg or ERα and increased estrogenic reporter activity in yeast cultures.
| East Bow | Confluence | Elkhorn | |
|---|---|---|---|
| Female minnows deployed in the field | |||
| Early June 2007a | - | - | Negative |
| Early June 2008b | Negative | - | - |
| Early June 2009b | Negative | Negative | - |
|
| |||
| Female Minnows exposed to field-collected water (w) or sediments (s) in the lab | |||
| Late May 2008c | - | - | Negative (s) |
| Late August 2009 | No effect | Negative (s) | - |
| Early June 2010 | Positive (w) | No effect | No effect |
|
| |||
| Yeast cultures treated with lipid extracts of sediments | |||
| Early June 2010 | No effect | Negative | Negative |
| Yeast cultures treated with aqueous rinses of sediments | |||
| Early June 2010 | Negative | No Effect | No Effect |
4.3. In vitro assays for anti-estrogenic activity
The results of the 4-hr yeast assays showed that sediments collected from each of the sites possessed endocrine activity on day 0 indicating that endocrine-active compounds are present in agricultural sediments. Specifically, the addition of Confluence and Elkhorn LEs to the cell cultures caused profound dose-dependent decreases in estrogenic reporter activity suggesting the presence of a lipophilic anti-estrogenic compound in sediments from these two sites. The East Bow AWs also caused significant dose-dependent decreases in reporter activity suggesting the presence of a hydrophilic anti-estrogenic compound in sediments from this site. These findings are generally consistent with the results from the fish bioassays. Though females exposed to sediments in 2010 did not experience defeminization, females exposed to Confluence sediments in 2009 (current study), to Elkhorn sediments in 2009 (Sellin et al., 2010) and deployed at each of the sites in previous years did experience defeminization. It seems likely that the compound(s) responsible for the observed decreases in estrogenic reporter activity are the same as those responsible for the defeminization of females observed in this study and in previous studies; however, the fact that females exposed to Confluence and Elkhorn sediments in 2010 did not experience defeminization demonstrates that the yeast cell cultures may be more sensitive than fathead minnows.
It is interesting to note that East Bow LEs caused an increase in reporter activity. This finding suggests that estrogenic compounds are present in the lipophilic extracts of East Bow sediments. While exposures to East Bow sediments did not cause the hyper-feminization of females, it is worth mentioning that exposures to East Bow water did lead to the hyper-feminization of females. These findings, when taken together, suggest that estrogenic compounds may be present in East Bow Creek. Furthermore, this finding highlights the fact that sediments are likely to contain a variety of contaminants with different modes of action.
4.4. Steroid and pesticide concentrations in sediments
Steroids were detected in several of the sediment extracts analyzed in this study. Sorption of steroids to sediments is expected due to the physico-chemical properties (ie: relatively low polarity) of steroids (Kuster et al., 2004). In contrast, only one sediment sample was found to contain detectable levels of pesticides. This suggests that either pesticides are not reaching local surface waters or that the pesticides reaching surface waters have a lower affinity for sediments than steroid hormones. Given that previous studies have shown that pesticides do indeed reach the surface waters of the Bow Creek and Elkhorn watersheds (Sellin et al., 2009; Sellin et al., 2010; Sellin Jeffries et al., in review), it is reasonable to conclude that the lack of pesticides in sediments from these sites could be related, in part, to physico-chemical properties that favor portioning of these compounds into water, rather than sediments.
Contrary to the results of the current study, previous studies failed to detect the presence of steroid hormones in sediments from the Elkhorn River, East Bow Creek and the Confluence (Sellin et al. 2010 and Sellin Jeffries et al. in review). While it is possible that these compounds were simply not present in sediments collected in previous studies, it is more likely that the methods utilized in these studies were not as effective as those used in the current study. In the previous studies, whole sediments were collected, frozen and then extracted for lipophilic compounds (Sellin et al. 2010, Sellin Jeffries et al. in review), while sediments in this study were processed within 12 hours of collection. Steroid hormones can degrade rapidly under certain conditions, as a result of microbial activity (Jurgens et al., 2002; Ying et al., 2002; Czajka and Londry, 2006; Robinson and Hellou, 2009; Bradley et al., 2009); therefore, processing of the sediments shortly after collection may have been more effective at limiting microbial degradation than the approach of freezing whole sediment samples prior to extractions.
The steroid data generated in the current study provides strong evidence that steroid hormones utilized by the cattle industry reach the surface waters of agriculturally-intense watersheds. Many of the steroids (ie: testosterone, estrone, etc.) detected in sediments from this study are naturally occurring and could arise from a variety of sources; however, α-zearalanol, α-zearalenol and β-trenbolone are synthetic steroids used cattle finishing feedlots. Given land use practices in the Bow Creek and Elkhorn River watersheds, it is reasonable to conclude that either cattle feedlot effluent or runoff from fields treated with cattle manure are the source of α-zearalanol, α-zearalenol and β-trenbolone in these watersheds. While the ultimate source of other, naturally-occurring steroid hormones (viz. 4-androstenedione, testosterone, estrone and progesterone) in sediments from these watersheds cannot be definitely determined, it is likely that cattle feedlots or fields treated with cattle manure are a source of these steroids as well.
4.5. Correlating the occurrence of steroid hormones in sediments to endocrine effects in minnows
In general, a link between the presence of steroid hormones in sediments and the endocrine effects of sediment exposures could not be established. For example, sediments collected from the Elkhorn River were found to contain the estrogenic compounds α-estradiol, α-zearalanol and α-zearalenol, yet female minnows exposed to Elkhorn sediments did not appear to suffer the effects associated with exposures to estrogenic compounds suggesting that these steroids are either not present in concentrations sufficient to induce estrogenic effects or are not biologically available. Furthermore, estrogenic effects may not have been observed due to the presence of other compounds with different modes of action, such as testosterone and β-trenbolone, in Elkhorn sediments.
The synthetic androgen, β-trenbolone, was detected in sediments from each of the field sites in 2010. Laboratory studies have shown that waterborne exposures to β-trenbolone lead to the defeminization of female fish, as indicated by reductions in plasma Vtg levels (Ankley et al., 2003). Despite the fact that β-trenbolone was detected in the 2010 sediments, there was no evidence that females exposed to sediments collected in 2010 had been defeminized. While the defeminization of females observed in the 2009 portion of the current study is consistent with the effects of β-trenbolone exposures, steroid hormones were not measured in the 2009 sediments; therefore, β-trenbolone cannot be definitively linked to the defeminization of these females.
4.6. Sediments act as a source of endocrine-active compounds
The findings of this study add to the growing body of evidence indicating that sediments act as a source of bioavailable endocrine-disrupting compounds. While previous studies have shown that endocrine-active compounds are present in sediments (Johnson et al. 1998; Petrovic et al., 2002; Williams et al., 2003; Peck et al., 2004; Schlenk et al., 2005; Jenkins et al., 2003; Orrego et al., 2005), few of these studies provided any information regarding the bioavailability of these compounds to fish or other aquatic organisms. The results of this study, as well as those from Sellin et al. (2010), provide evidence that endocrine-active compounds in sediments can be bioavailable, as fathead minnows exposed to agricultural sediments experienced alterations in endocrine function.
The vast majority of studies investigating the potential for endocrine-disruption in aquatic systems have focused on estrogenic compounds discharged from wastewater treatment plants (Williams et al., 2003; Aerni et al., 2004; Lee and Rasmussen, 2006) and on androgenic compounds released from pulp and paper mills (Munkittrick et al., 1998; Parrot et al., 2006). In comparison to estrogenic and androgenic compounds, anti-androgens and to an even greater extent, anti-estrogens have received little attention in studies aimed at determining the presence and effects of endocrine-disrupting compounds in aquatic systems. However, the results of this study indicate that an environmentally-relevant compound with ER-mediated anti-estrogenic activity may be of particular importance in agriculturally-intense watersheds.
Acknowledgments
Partial support for this project was provided by the University of South Dakota, Missouri River Institute, and by NSF grant numbers CBET-1040895 and CBET-0966850. M.K.S.J. was supported by the University of Nebraska Medical Center Emley Fellowship Program. H.A.B., A.A.B. and M.B.C. were, in part, supported by grant #5G12RR008124 (to the Border Biomedical Research Center (BBRC)/University of Texas at El Paso) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. A.A.B. was supported by the Research Experience for Undergraduates Program funded by NSF-REU grant number DBI-0851881. J.L.S. was supported by NSF grant #CBET-0966850. Dr. Daniel Snow of the University of Nebraska Water Science Center led the chemical analysis of sediment samples. Kelty Abbott, Kate Oien and Eric Jeffries assisted with sediment and water collections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aerni HR, Kobler B, Rutishauser BV, Wettstein FE, Rischer R, Giger W, Hungerbuhler A, Marazuela MD, Peter A, Schonenberger R, Vogeli AC, Suter MJF, Eggen RIL. Combined biological and chemical assessment of estrogenic activities in wastewater treatment plant effluents. Anal Bioanal Chem. 2004;378:688–696. doi: 10.1007/s00216-003-2276-4. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, Henry TR, Denny JS, Leino RL, Wilson VS, Cardon MC, Hartig PC, Gray LE. Effects of the androgenic growth promoter 17-β-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ Toxicol Chem. 2003;22:1350–1360. [PubMed] [Google Scholar]
- Balsiger HA, de la Torre R, Lee WY, Cox MB. A four-hour yeast bioassay for the direct measure of estrogenic activity in wastewater without sample extraction, concentration, and sterilization. Sci Total Environ. 2010;408:1422–1429. doi: 10.1016/j.scitotenv.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Barber LB, Chapelle FH, Gray JL, Kolpin DW, McMahon PB. Biodegradation of 17b-estradiol, estrone and testosterone in stream sediments. Environ Sci Technol. 2009;43:1902–1910. doi: 10.1021/es802797j. [DOI] [PubMed] [Google Scholar]
- Czajka CP, Londry KL. Anaerobic biotransformation of estrogens. Sci Total Environ. 2006;367:932–941. doi: 10.1016/j.scitotenv.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 2002;30:23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hela DG, Lambropoulou DA, Konstantinou IK, Albanis TA. Environmental monitoring and ecological risk assessment for pesticide contamination and effects in Lake Pamvotis, northwestern Greece. Environ Toxicol Chem. 2005;24:1548–1556. doi: 10.1897/04-455r.1. [DOI] [PubMed] [Google Scholar]
- Jenkins RL, Wilson EM, Angus RA, Howell WM, Kirk M. Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol Sci. 2003;73:53–59. doi: 10.1093/toxsci/kfg042. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Salveson A, Holmes M, Denison MS, Fry DM. Environmental estrogens in agricultural drain water from the Central Valley of California. Bull Envrion Contam Toxicol. 1998;60:609–614. doi: 10.1007/s001289900669. [DOI] [PubMed] [Google Scholar]
- Jurgens MD, Holthaus KIE, Johnson AC, Smith JJL. The potential for estradiol and ethynylestradiol degradation in English rivers. Environ Toxicol Chem. 2002;21:480–488. [PubMed] [Google Scholar]
- Kolodziej EP, Sedlak DL. Rangeland grazing as a source of steroid hormones to surface waters. Environ Sci Technol. 2007;41:3514–3520. doi: 10.1021/es063050y. [DOI] [PubMed] [Google Scholar]
- Kolok AS, Snow DD, Kohno S, Sellin MK, Guillette LJ., Jr Occurrence and biological effect of exogenous steroids in the Elkhorn River, Nebraska. Sci Total Environ. 2007;388:104–115. doi: 10.1016/j.scitotenv.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kuster M, Lopez de Alda MJ, Barcelo D. Analysis and distribution of estrogens and progestogens in sewage sludge, soils and sediments. Trac-Trend Anal Chem. 2004;23:790–798. [Google Scholar]
- Lee CJ, Rasmussen TJ. Occurrence of organic wastewater compounds in effluent-dominated streams in Northeastern Kansas. Sci Total Environ. 2006;371:258–269. doi: 10.1016/j.scitotenv.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Lorenzen A, Hendel JG, Conn KL, Bittman S, Kwabiah AB, Lazarovitz G, Masse D, McAllister TA, Topp E. Survey of hormone activities in municipal biosolids and animal manures. Environ Toxicol. 2004;19:216–225. doi: 10.1002/tox.20014. [DOI] [PubMed] [Google Scholar]
- Munkittrick KR, McMaster ME, McCarthy LH, Servos MR, Van Der Kraak GJ. An overview of recent studies on the potential of pulp-mill effluents to alter reproductive parameters in fish. J Toxicol Environ Health B. 1998;1:347–371. doi: 10.1080/10937409809524558. [DOI] [PubMed] [Google Scholar]
- Orrego R, Moraga-Cid G, Gonzalez M, Barra R, Valenzuela A, Burgos A, Gavilan JF. Reproductive, physiological, and biochemical responses in juvenile female rainbow trout (oncorhynchus mykiss) exposed to sediment from pulp and paper mill industrial discharge areas. Environ Toxicol Chem. 2005;24:1935–1943. doi: 10.1897/04-251r1.1. [DOI] [PubMed] [Google Scholar]
- Panter GH, Hutchinson TH, Lange R, Lye CM, Sumpter JP, Zerulla M, Tyler CR. Utility of a juvenile fathead minnow screening assay for detecting (anti-)estrogenic substances. Environ Toxicol Chem. 2002;21:319–326. [PubMed] [Google Scholar]
- Parrot JL, McMaster ME, Hewitt LM. A decade of research on the environmental impacts of pulp and paper mill effluents in Canada: development and application of fish bioassays. J Toxicol Environ Health B. 2006;9:297–317. doi: 10.1080/15287390500195752. [DOI] [PubMed] [Google Scholar]
- Peck M, Gibson RW, Kortenkamp A, Hill EM. Sediments are major sinks of steroidal estrogens in two United Kingdom rivers. Environ Toxicol Chem. 2004;23:945–952. doi: 10.1897/03-41. [DOI] [PubMed] [Google Scholar]
- Pereira WE, Domagalski JL, Hostettler FD, Brown LR, Rapp JB. Occurrence and accumulation of pesticides and organic contaminants in river sediment, water and clam tissues from the San Joaquin River and tributaries, California. Environ Toxicol Chem. 1996;15:172–180. [Google Scholar]
- Petrovic M, Sole M, Lopez de Alda MJ, Barcelo D. Endocrine disruptors in sewage treatment plants, receiving river waters, and sediments: integration of chemical analysis and biological effects on feral carp. Environ Toxicol Chem. 2002;21:2146–2156. [PubMed] [Google Scholar]
- Robinson BJ, Hellou J. Biodegradation of endocrine disrupting compounds in harbor seawater and sediment. Sci Total Environ. 2009;407:5713–5718. doi: 10.1016/j.scitotenv.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Schlenk D, Sapozhnikova Y, Irwin MA, Xie L, Hwang W, Reddy S, Brownawell BJ, Armstrong J, Kelly M, Montagne DE, Kolodziej EP, Sedlak D, Snyder S. In vivo bioassay-guided fractionation of marine sediment extracts from the Southern California Bight, USA, for estrogenic activity. Environ Toxicol Chem. 2005;24:2820–2826. doi: 10.1897/05-116r.1. [DOI] [PubMed] [Google Scholar]
- Sellin MK, Snow DD, Schwarz M, Carter BJ, Kolok AS. Agrichemicals in Nebraska watersheds: Occurrence and endocrine effects. Environ Toxicol Chem. 2009;92:221–227. doi: 10.1897/09-135.1. [DOI] [PubMed] [Google Scholar]
- Sellin MK, Snow DD, Schwarz M, Kolok AS. Reductions in hepatic vitellogenin and estrogen receptor alpha expression by sediments from an agriculturally impacted waterway. Aquat Toxicol. 2010;96:103–108. doi: 10.1016/j.aquatox.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Sellin Jeffries MK, Abbott KI, Cowman T, Kolok AS. Endocrine disruption in an agricultural watershed: Correlating watershed characteristics with the occurrence and biological effects of agrichemicals. Submitted to Science of the Total Environment In reviw. [Google Scholar]
- Urbatzka R, van Cauwenberge A, Maggioni S, Vigano L, Mandich A, Benfenati E, Lutz I, Kloas W. Androgenic and antiandrogenic activities in water and sediment samples from the river Lambro, Italy, detected by yeast androgen screen and chemical analyses. Chemosphere. 2007;67:1080–1087. doi: 10.1016/j.chemosphere.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Villaverde J, Hildenbrandt A, Martinez E, Lacorte S, Morillo E, Maqueda C, Viana P, Barcel D. Priority pesticides and their degradation products in river sediments from Portugal. Sci Total Environ. 2008;390:507–513. doi: 10.1016/j.scitotenv.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Johnson AC, Smith JJL, Kanda R. Steroid estrogens profiles along river stretches arising from sewage treatment works discharges. Environ Sci Technol. 2003;37:1744–1750. doi: 10.1021/es0202107. [DOI] [PubMed] [Google Scholar]
- Ying GG, Kookana RS, Ru RJ. Occurrence and fate of hormone steroids in the environment. Environ Internation. 2002;28:545–551. doi: 10.1016/s0160-4120(02)00075-2. [DOI] [PubMed] [Google Scholar]