Abstract
Despite keen interest in extra-pair mating in birds, its adaptive significance remains unresolved. Here, we use a multi-year dataset to test whether traits of a female’s social mate influence her propensity to produce extra-pair offspring in a population of house wrens, and whether producing extra-pair young has consequences for a female’s fitness through effects on offspring survival. Females were most likely to produce extra-pair offspring when paired with old males and when paired with males on poor-quality territories, although this latter effect was marginally non-significant. Among offspring, the cutaneous immunity of within-pair young decreased as the age of their sires increased, but cutaneous immunity of extra-pair young was not affected by the age of their extra-pair sires or by the age of the males rearing them. Extra-pair offspring were more likely than within-pair offspring to return as breeding adults to the local population, with extra-pair sons being more likely to return as a breeder for multiple years. Our findings support the hypothesis that females produce extra-pair offspring to enhance their inclusive fitness beyond what they are capable of given the male with which they are socially paired.
Keywords: extra-pair mating, house wren, life history, sex allocation, Troglodytes aedon
Although monogamy is viewed as the predominant mating system across avian taxa, true genetic monogamy is rare, as individuals in many species mate with individuals other than the ones with which they form a social bond (Westneat et al. 1990; Jennions and Petrie 2000; Griffith et al. 2002). Despite the widespread occurrence of extra-pair mating and the high level of research interest it has engendered, the adaptive significance of this behavior remains unresolved. The significance of extra-pair mating from a male’s perspective is obvious, as males that sire extra-pair young increase their reproductive success while forcing the males they cuckold to pay the rearing costs. Females, however, often initiate extra-pair copulations, and the value of mating outside the pair bond from their perspective is much less clear.
Producing extra-pair offspring does not increase female reproductive success the way producing extra-pair young does for males, and often does not yield direct benefits to females (females do not generally receive food or predator defense from their extra-pair mates; Petrie and Kempenaers 1998; but see Slayter et al. 2012). In fact, extra-pair mating can be costly to females, for example, through potential reductions in paternal care provided by their cuckolded mates (Møller and Birkhead 1993; Queller 1997; Whittingham and Dunn 2001; Sheldon 2002) and increasing the risk of contracting sexually transmitted infections for both sexes (Sheldon 1993; Petrie and Kempenaers 1998). Thus, the focus of much research has been on elucidating indirect genetic benefits that females receive from extra-pair sires, and such endeavors have produced mixed results (Arnqvist and Kirkpatrick 2005; Akçay and Roughgarden 2007). While some studies have reported evidence of indirect genetic benefits (Foerster et al. 2003; Fossøy et al. 2008; Reid et al. 2015), others have not (Kleven and Lifjeld 2004; Wilk et al. 2008; Krist and Munclinger 2011). Still others report that extra-pair young have reduced recruitment into local breeding populations relative to their within-pair half-siblings (Schmoll et al. 2005, 2009; Krist and Munclinger 2011; Sardell et al. 2011; Hsu et al. 2014), although such effects may be contingent on environmental conditions (Schmoll et al. 2005). These contradictory findings suggest that variation in extra-pair mating may be subject to multiple selective forces, perhaps acting antagonistically between the sexes.
Recent hypotheses to explain the evolution of extra-pair mating (Arnqvist and Kirkpatrick 2005; Forstmeier et al. 2011, 2014; Eliassen and Jørgensen 2014; Roff and Fairbairn 2015) may explain why extra-pair and within-pair males and their offspring often do not obviously differ in components of phenotypic ‘quality’ that might reflect heritable genetic variation (Krist and Munclinger 2011; Sardell et al. 2011, 2012; Hsu et al. 2015). It is possible that, in the absence of any benefits to females, female extra-pair mating evolved through intersexual antagonistic pleiotropy (Forstmeier et al. 2014), whereby direct selection on males to produce extra-pair young also causes females to seek extra-pair copulations, assuming that genetic variation underlying this behavior is shared between the sexes (Arnqvist and Kirkpatrick 2005; Forstmeier et al. 2011, 2014). The strength of selection on males resulting from extra-pair mating, however, varies widely among species (Webster et al. 1995; Yezerinac et al. 1995; Whittingham and Dunn 2005). For example, in the house wren (Troglodytes aedon), extra-pair paternity contributes only about 10% of the variation in male reproductive success (Whittingham and Dunn 2005) even though extra-pair young occur in 30–35% of nests (Soukup and Thompson 1997; Poirier et al. 2004; Forsman et al. 2008). Hence, selection on males to produce extra-pair young may not be sufficiently strong to maintain this behavior in the face of costs to females associated with mating outside the pair bond (Petrie and Kempenaers 1998; Sardell et al. 2011). It follows that any detectable increase in fitness that females gain by mating outside the pair bond must have a large effect on maintaining this enigmatic behavior. In this study, we test for potential fitness benefits that females might accrue from producing extra-pair young in a wild songbird.
We first test whether the production of extra-pair young by female house wrens is associated with traits of their social mate (age, territory quality, and body mass). We predicted a U-shaped relationship between male age and the occurrence of extra-pair young within broods because, although experienced males may, on average, carry genes that confer higher survival than yearling males, old males often suffer a reduction in sperm quality (Hansen and Price 1995; Radwan 2003; Velando et al. 2011), potentially generating non-linear effects of male age on paternity. We also tested whether the production of extra-pair young varied with the body mass and the quality of the breeding territory that a female’s mate was able to secure and defend from rival males. Because males begin selecting and defending nest sites prior to female arrival from spring migration, and females choose among males based, at least in part, on territory quality (Eckerle and Thompson 2006; Grana et al. 2012), this is a useful indicator of a male’s resource-holding potential. We thus predicted a negative correlation between territory quality and the occurrence of extra-pair young within broods. We then tested whether females obtain potential fitness benefits from extra-pair offspring by analyzing whether paternity and sire age affect offspring traits, including immune responsiveness and body condition. Finally, we test whether extra-pair young have an enhanced probability of returning to breed as adults in future populations relative to their within-pair half-siblings.
Methods
House wrens are secondary-cavity-nesting songbirds with a widespread distribution in North America (Johnson 2014). Clutch sizes typically range from four to eight eggs. Only females incubate the eggs and brood nestlings, but both parents provision young with food after hatching, and fledging occurs 14–16 d post-hatching (Barnett et al. 2012; Bowers et al. 2013b). We studied a population breeding in Illinois, USA (40.665°N, 88.89°W). Nestboxes (N = 820; see Lambrechts et al. 2010 for details) were distributed at a density of 5.4 boxes/ha. The subset of available nestboxes in the present study (N = 302) has been in place since the early 1980s in secondary deciduous forest. Males are highly territorial, with heavier, larger, and more attractive males typically out-competing others for breeding territories and mates and having increased reproductive success (Johnson and Kermott 1990; DeMory et al. 2010; Bowers et al. 2015a). We obtained a proxy of territory quality as the number of broods produced (clutches hatched) in a given nestbox over the ten years preceding this study (Fig. 1), which is a reliable measure of territory quality, as historically productive territories are occupied at a higher rate, and less-productive territories at a lower rate, than predicted by chance (Fig. 1; see also Janiszewski et al. 2013).
Figure 1.
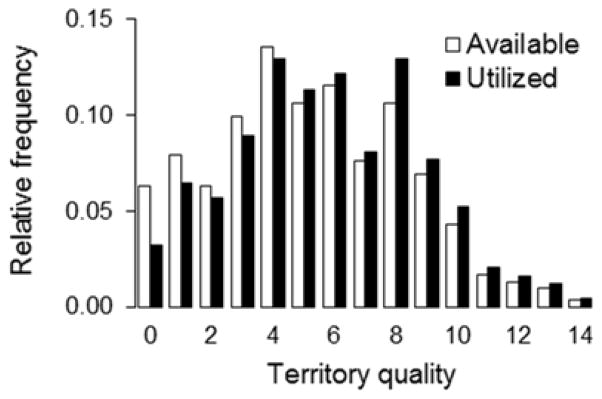
Variation in territory quality, quantified as the number of broods produced at a given nesting site over the ten years prior to this study. Open bars represent available territories (N = 302 nestboxes), and filled bars represent the sites occupied in the present study (N = 247). The average number of broods produced on a territory over the ten years prior to this study was 5.23 ± 0.18 (mean ± SE); thus, during this study, low-quality territories were under-occupied and high-quality territories were over-occupied relative to the null expectation ( , P < 0.001).
The nestlings for which we determined parentage were produced during the 2004–2006 breeding seasons. Eleven days after hatching began within a nest, all nestlings were weighed, banded with a unique aluminum leg band, and had a blood sample drawn for sexing and paternity assignment (details in Forsman et al. 2008; Sakaluk et al. 2014). At this time, we also administered a phytohaemagglutinin (PHA) skin test to obtain a measure of cutaneous immune responsiveness. Injection of PHA into the wing web results in inflammation and swelling, the magnitude of which provides a measure of cutaneous immune activity (Martin et al. 2006) that is positively associated with inter-annual return and lifetime reproductive success in our study population (Bowers et al. 2014a). We measured wing-web thickness (±0.01 mm) as the mean of three measures prior to and 24 h after injection, and used the change in wing-web thickness (the difference between post- and pre-injection means) as a measure of cutaneous immune activity.
We attempted to catch, band, and weigh all breeding adults in each year preceding, during, and following the three seasons during which we genotyped nestlings; we captured nearly every adult on the site and banded all nestlings prior to fledging each year. Although our data for inter-annual return rates do not account for dispersal events, our data suggest that the emigration of young prior to reproduction occurs randomly with respect to nestling traits and environmental conditions, and that variation in recruitment is largely attributable to variation in inter-annual survival (Bowers et al. 2014a). Nestling sex and paternity were determined using DNA extracted from red-blood cells, and we sampled blood from attendant males and females. Paternity was assigned to nestlings using three microsatellite loci (TA-C3 (B)2, Mcyμ4, and LTMR6), and two additional loci (TA-A5-15 and TA-B4-2) when more resolution was needed. We analyzed allele data using Cervus 2.0. For the three-locus set and five-locus set, exclusion probabilities were 0.991 and 0.998, respectively. Overall, the probability of false assignment for nestlings designated as within-pair was < 0.008 (see Sakaluk et al. 2014 for further details). We assigned paternity to 1,772 nestlings (1,482 within-pair and 290 extra-pair) from 361 broods. To assign sires to extra-pair young, we compared extra-pair young against all males for which we obtained blood samples in the population using the five loci above. For 146 extra-pair young, a single sire could be unambiguously assigned as they matched a single male, usually from a nearby territory, at all five loci. For each assignment, we calculated the probability that a randomly selected male from the population would also match the alleles from a given extra-pair sire (Masters et al. 2003); we assigned sires to 32 additional extra-pair young for which the sire could potentially have been one or two other males, but in which we were confident that the sire had been correctly assigned (all P < 0.03). Overall, the probability of incorrect assignment of extra-pair sires was 0.006.
We used SAS (v. 9.3) for all analyses, all tests are two-tailed, and we included year and female identity as random effects in all analyses. We also centered and standardized input variables following Schielzeth (2010), a procedure that removes collinearity between linear and higher-order terms in polynomial regressions (Schielzeth 2010). We first tested whether traits of a female’s social mate influenced the occurrence of extra-pair young within broods using a generalized linear mixed model (GLMM) with a binary response and logit link, similar to a logistic regression. We included effects of male age, body mass, territory quality, and breeding date (clutch-initiation date). We also included a quadratic term for age, as we predicted a U-shaped relationship between male age and rates of extra-pair paternity (see also Ramos et al. 2014). There were no correlations between male age, body mass, or territory quality (all P > 0.1). We identified 252 adult males and knew the exact age of 60 of them because they hatched on the study site; our age estimates for the remaining males, therefore, represent minimum ages, and we included these males because many of them bred on the site in multiple years, which allowed us to use these males in assessing age-related effects. Analysis of a smaller subset of males (N = 202) that excludes immigrant males breeding on the study site for only one year produces qualitatively similar results, as does analysis of the known-age males only (data not shown). We then analyzed the effects of male age on nestling cutaneous immune responsiveness using a linear mixed model with nest identity as an additional random effect to account for non-independence of nestlings within broods. We controlled for nestling condition, as nestling PHA responsiveness often covaries positively with this trait (Forsman et al. 2010; Bowers et al. 2015b). We then used a similar model to analyze whether sire age and paternity affect nestling condition using nestling mass on day 11 post-hatching as the dependent variable and tarsus length as an added covariate. We followed this analysis with a test of whether the age of a male tending a nest affected fledging success (the proportion of eggs that produced fledglings) using a linear mixed model. We did not include the paternity of individual nestlings because survival of nestlings from 11 days post-hatching to fledging was greater than 99.7% (4 of 1482 within-pair and 1 of 290 extra-pair young died in the nest between blood-sampling and fledging). We then analyzed inter-annual return rates of offspring to the breeding population across multiple years as a function of offspring age using a Cox regression (survival analysis; PROC PHREG) in relation to paternity and sex, and we accounted for nonindependence by grouping offspring within their natal nest, maternal identity, and year, similar to the use of random effects in mixed-model ANOVA, following Allison (2010). Among recruits, there was a tendency for their lifetime fecundity as adults to mirror their inter-annual return rates (data not shown), but with substantially fewer extra-pair young than within-pair young (four extra-pair daughters and seven extra-pair sons vs. 24 within-pair daughters and 25 within-pair sons recruited), we lacked sufficient power to compare their fecundity as adults.
Results
The age of a female’s social mate had a J-shaped effect on her probability of producing extra-pair young (Table 1; Fig. 2a). The effect of male age is also significant if the terms for territory quality, body mass, and breeding date are omitted (quadratic effect of male age: estimate ± S.E. = 0.271 ± 0.111, F1, 353 = 5.98, P = 0.015; linear term: estimate ± S.E. = −0.411 ± 0.194, F1, 353 = 4.50, P = 0.035), and if the datum for the six-year-old male is omitted (quadratic effect: F1, 349 = 5.11, P = 0.025; Fig. 2a). Yearling males had more extra-pair young within their broods than did two- or three-year-old males, but females had the highest likelihood of producing extra-pair young when paired with the oldest males (Fig. 2a). Given the average frequency of extra-pair young in the population (35% of broods), the occurrence of extra-pair young in nests attended by males older than four years of age (4 of 4 broods containing extra-pair young; Fig. 2a) was significantly higher than expected by chance (binomial test: P = 0.015), and this was also true for nests of yearling males (binomial test: P = 0.016). There was also a trend for extra-pair paternity to decline with increases in the quality of a male’s territory (Table 1; Fig. 2b). For nests in which females produced at least one extra-pair nestling, we compared the age and body mass of their social mates with that of the extra-pair sires, and these males did not differ in age (F1, 200 = 0.00, P = 0.987) or body mass (F1, 191 = 0.54, P = 0.465). There was also no correlation between the age of within-pair and extra-pair males (r109 = −0.024, P = 0.806).
Table 1.
Effects on the probability of a female producing at least one extra-pair nestling within her brood.
| Source | Estimate ± S.E. | F | df | P |
|---|---|---|---|---|
| Male age | −0.405 ± 0.197 | 4.23 | 1, 350 | 0.041 |
| Male age × male age | 0.273 ± 0.112 | 5.97 | 1, 350 | 0.015 |
| Territory quality | −0.204 ± 0.119 | 2.96 | 1, 350 | 0.086 |
| Male body mass | −0.015 ± 0.118 | 0.02 | 1, 350 | 0.900 |
| Breeding date | 0.021 ± 0.117 | 0.03 | 1, 350 | 0.858 |
| Intercept | −0.781 ± 0.163 |
Figure 2.
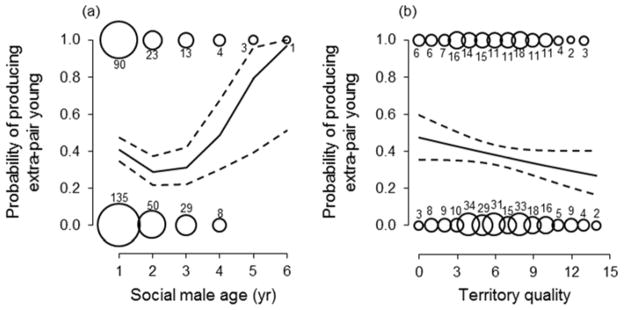
Variation in the production of extra-pair offspring by females in relation to the age and territory quality of their social mate. Bubble sizes are proportional to the number of observations, which are given, and lines are from a generalized linear mixed model ± 95% confidence limits. The total sample for male age (N = 356) is slightly smaller than for territory quality (N = 361), because there were five broods for which we could not identity, and therefore age, the attendant male, but for which we were able to designate nestlings as being either extra-pair or within-pair.
There was an interaction between paternal age and offspring paternity in their effect on offspring immune responsiveness (estimate ± SE = 0.069 ± 0.032, F1, 839 = 4.67, P = 0.031; Fig. 3a,b), while controlling for variation in nestling condition (effect of condition: estimate ± SE = 0.019 ± 0.011, F1, 851 = 2.85, P = 0.092). Follow-up tests revealed that within-pair-male age had a negative effect on the immune responsiveness of their genetic offspring (estimate ± SE = −0.054 ± 0.020, F1, 172 = 7.25, P = 0.008; Fig. 3a), but the age of within-pair males did not affect the immune responses of extra-pair young (estimate ± SE = 0.011 ± 0.032, F1, 45.1 = 0.12, P = 0.732), nor was the immune responsiveness of extra-pair young influenced by the age of their extra-pair sires (estimate ± SE = 0.001 ± 0.038, F1, 49.2 = 0.00, P = 0.974; Fig. 3b). Contrary to the effects of sire age and paternity on nestling immune responses, body condition at this age was not affected by sire age (F1, 874 = 0.69, P = 0.407), paternity (F1, 877 = 0.74, P = 0.391), or an interaction between these effects (F1, 856 = 0.21, P = 0.646). The proportion of offspring fledged from a nest declined with increases in the age of a female’s social mate (estimate ± S.E. = −0.121 ± 0.051, F1, 401 = 5.53, P = 0.019). We partitioned this result between an effect on hatching success of eggs or post-hatching survival; hatching success was not correlated with male age (estimate ± S.E. = 0.025 ± 0.051, F1, 393 = 0.25, P = 0.620), but the proportion of hatchlings fledged was negatively correlated with male age (estimate ± S.E. = −0.156 ± 0.051, F1, 400 = 9.36, P = 0.002).
Figure 3.
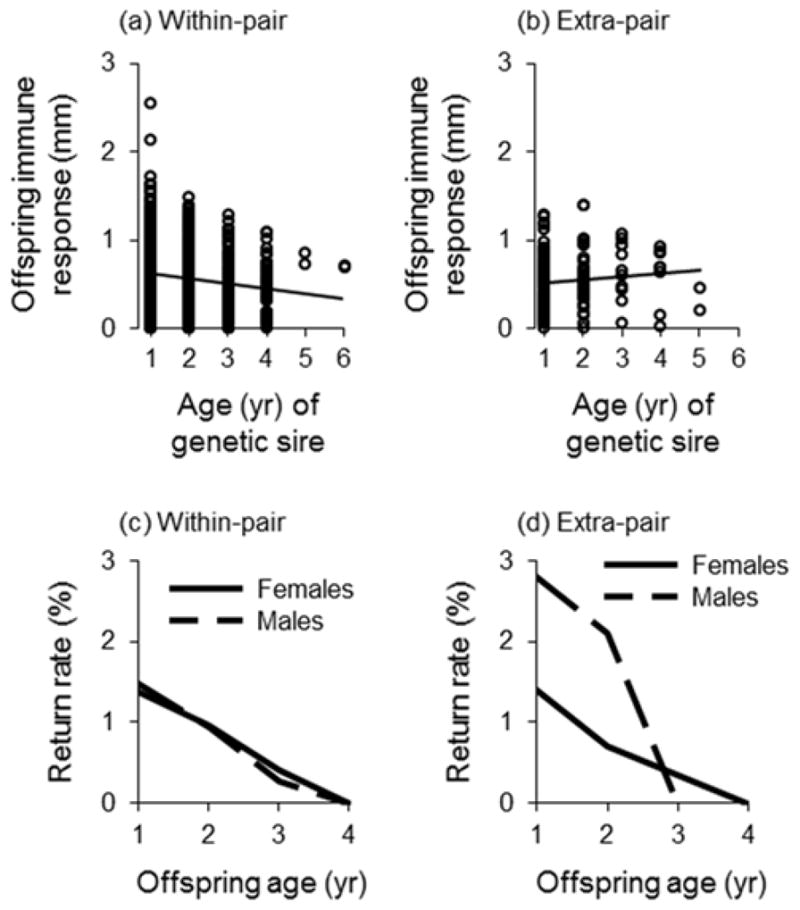
(a,b) Offspring immune responsiveness to PHA injection in relation to paternity and the age of their genetic sire. (c,d) Rates at which male and female within-pair and extra-pair offspring returned to breed in the study population when age 1 and older.
Among offspring, extra-pair young had higher probability of recruiting to the breeding population than within-pair young and then breeding through at least two years of age, and there was a trend for this effect to be manifested primarily by increased return rates of extra-pair sons (paternity: estimate ± S.E. = 0.113 ± 0.045, , P = 0.013; sex: estimate ± S.E. = 0.0005 ± 0.026, , P = 0.985; paternity × sex: estimate ± S.E. = 0.141 ± 0.076, , P = 0.061; Fig. 3c,d).
Discussion
Females paired with yearling males were more likely to produce extra-pair young than those paired with two- or three-year-old males, but females had the highest likelihood of producing extra-pair young when paired with the oldest males (see also Ramos et al. 2014). While experienced males may carry genes that confer increased survival relative to yearling males, increasingly older males may suffer a reduction in sperm quantity or quality (increased germline mutations or reduced sperm motility), potentially favoring the increased production of extra-pair young by females paired with older males (Radwan 2003; Velando et al. 2011). Our results are inconsistent with those of a number of studies reporting that extra-pair males are older than a female’s social mate (Akçay and Roughgarden 2007; Hsu et al. 2015). The life history of our study species may contribute to this apparent inconsistency, as house wrens are short-lived with most adults breeding in only one or two years (Johnson 2014). Thus, a live-fast-die-young life history may accelerate the rate of senescence among male house wrens, thereby selecting for increased occurrence of extra-pair young in broods of aging males. In contrast, the finding that yearling males also had increased extra-pair young in their broods relative to two- and three-year old males is consistent with previous findings (Akçay and Roughgarden 2007; Hsu et al. 2015). Our results are also consistent with previous findings that gains and losses in paternity are often distributed non-randomly among males within populations (Dunn and Cockburn 1999; Richardson and Burke 1999; Griffith 2007; Whittingham and Dunn 2014). Females in the current study also tended to be less likely to produce extra-pair young when paired with males on high-quality territories, suggesting that a male’s resource-holding potential or intrasexual competitive ability influences a female’s propensity to produce extra-pair young independent of male age.
Collectively, these results suggest that females produce extra-pair young in response to age- or condition-dependent sexual signals or cues of male condition or quality (Kokko 1997; Evans et al. 2011; Adamson 2013). Theory suggests that females may evolve a preference for intermediate-aged males in relatively short-lived species (Beck et al. 2002), consistent with the finding that two- and three-year-old males had the lowest incidence of extra-pair paternity within their broods (Fig. 2A). In reality, females may not be as choosy of their mates as traditionally thought, at least early within breeding seasons, if choosiness might delay a breeding attempt. Considering the fact that females in a wide range of taxa prospect among a limited number of potential mates (median = 2.9 in Roff and Fairbairn 2014), and the high costs associated with delayed breeding in short-lived, seasonally breeding species (Verhulst and Nilsson 2008), females may often settle initially with males that are not their most preferred, but which control limiting nest cavities that are critical for breeding, and then engage in extra-pair copulations if they encounter a high-quality sire (Roff and Fairbairn 2015). House wrens are sexually monochromatic, and males do not possess conspicuous plumage characteristics; indeed, the ability of males to secure and defend suitable nest sites from rival males is a more important determinant of male pairing success than other components of the male phenotype (Eckerle and Thompson 2006). Therefore, pairing as early as possible within breeding seasons, albeit perhaps with older or non-preferred males, may allow females to breed under high-quality conditions and still produce high-quality extra-pair young if they encounter a potential sire of higher quality than their social mate.
Consistent with the finding that females were more likely to produce extra-pair young when socially paired with older males, mating with older males was also associated with a reduction in immune responsiveness of within-pair young (see also Saino et al. 2002) and a reduction in the number of fledglings produced per egg laid and hatched. However, the immune responsiveness of extra-pair offspring was not affected by the age of either the within-pair male or extra-pair sire. We did not detect effects of paternity or sire age on nestling body condition, which is correlated with the cutaneous immune response (Forsman et al. 2010), suggesting an effect of sire per se on offspring immune responsiveness. A reduction in male reproductive effort with age might explain the reduction in hatchling survival, although, if this were the case, we might also expect this to affect nestling body condition. An increase in germ-line mutations could still explain the reduced hatchling survival, even in the absence of an effect on hatching success (Hercus and Hoffmann 2000; Priest et al. 2002; Preston et al. 2015). Intriguingly, Schroeder et al. (2015) recently found that offspring of older parents produced fewer recruits to future breeding populations than those of younger parents, even though offspring of relatively younger and older parents had similar longevity. This effect was robust to cross-fostering, thus representing an epigenetically inherited effect of parental age on offspring fitness that was not caused by the rearing environment or levels of parental care (Schroeder et al. 2015). In our study population, older males breed earlier and obtain more-preferable breeding sites than younger males, on average (DeMory et al. 2010); thus, a reduction in male virility, intrasexual competitive ability, or general reproductive effort with increased age seems unlikely to be fully responsible for the effects we detected on nestling immune responsiveness and survival within the nest.
We also found that extra-pair young, particularly males, had the highest probability of returning as breeding adults over multiple years, which is a major determinant of fitness in wild populations (McCleery et al. 2004). The finding that extra-pair young were more likely to recruit to future breeding populations is consistent with the hypothesis that females secure genetic benefits from their extra-pair mates, but may also reflect maternal effects via differential allocation in relation to paternity (Tschirren et al. 2012), or a combination of both. We know, for example, that extra-pair offspring are more likely to occur in earlier-laid eggs (Johnson et al. 2009a; see also Magrath et al. 2009; Krist and Munclinger 2011 for examples in other species), that extra-pair young in the study population, including those in the current study, are more likely to be male than female (Johnson et al. 2009b), and that females hatching their eggs asynchronously bias their first-laid eggs in favor of sons, leading to heavier and larger nestlings that are more likely to recruit to the breeding population (Bowers et al. 2011, 2015a). Whether eggs that produce extra-pair or within-pair young receive differing levels of maternal resources (e.g., yolk or steroids that promote growth) needs further study, but considering that extra-pair young are more likely to occur among earlier-laid eggs within clutches, and that these eggs are smaller and contain lower amounts of yolk and yolk-testosterone than later-laid eggs (Bowers et al. 2015b), it is unlikely that extra-pair young received significantly greater allocation of these resources than their siblings, on average. Moreover, we did not detect an effect of paternity on nestling body condition, as would be expected if offspring received differential allocation from either parent on the basis of paternity. It is also worth noting that females not producing any extra-pair young also produce males among earlier-hatching, competitively advantaged positions within their broods (Bowers et al. 2011), suggesting that the increased return rate of extra-pair males is attributable, at least in part, to genetic effects. This finding is also consistent with predictions of non-random sex allocation in relation to male quality, as females should overproduce the sex with the greatest fitness potential under prevailing conditions (Trivers and Willard 1973; Weatherhead and Robertson 1979; Calsbeek and Sinervo 2004; Pryke and Griffith 2009; Bowers et al. 2011, 2014b, 2015; but see Dietrich-Bischoff et al. 2006; Bowers et al. 2013a).
It is worth noting that the production of extra-pair offspring may not directly reflect a female’s propensity to seek extra-pair copulations (Dunn and Lifjeld 1994; Griffith 2007). For example, a reduction in motility or competitiveness of the sperm produced by older males (Møller et al. 2009) may account, at least in part, for increased rates of extra-pair paternity within their broods. However, such a process does not explain the effect of male age on nestling immune responsiveness and survival within the nest, nor the increased rate of return as breeding adults for extra-pair relative to within-pair young. Regardless of the underlying mechanism, these results, to our knowledge, are the first to document enhanced rates of return to future breeding populations for extra-pair young. It seems unlikely, therefore, that the production of extra-pair young by females is maintained solely as an incidental, non-adaptive consequence of selection acting on male extra-pair mating, although it is important to note that the possibility of such a process contributing to female extra-pair mating in this species remains. Given the non-random production of extra-pair offspring and effects on their recruitment detected in the current study, our results suggest that females produce extra-pair young as part of an adaptive mating strategy to enhance their fitness beyond what they are capable of given the male with which they are socially paired.
Acknowledgments
We thank the numerous Wren Crews that collected data and the ParkLands Foundation, the Illinois Great Rivers Conference of the United Methodist Church, and the Sears and Butler families for the use of their properties. We also thank Derek Roff, Joel Adamson, and an anonymous reviewer for helpful and constructive comments that improved the manuscript. Financial support was provided by NSF grant IBN-0316580; NIH grant R15HD076308-01; the School of Biological Sciences, Illinois State University; and the Beta Lambda Chapter of the Phi Sigma Biological Sciences Honor Society. All activities complied with Illinois State University Institutional Animal Care and Use Committee Protocols 17-2003, 15-2006, 10-2009, 05-2010 and with United States Geological Survey banding permit 09211.
Contributor Information
Anna M. Forsman, Email: amf226@cornell.edu.
Brian S. Masters, Email: bmasters@towson.edu.
Bonnie G. P. Johnson, Email: bgjohnson@towson.edu.
L. Scott Johnson, Email: sjohnson@towson.edu.
Scott K. Sakaluk, Email: sksakal@ilstu.edu.
Charles F. Thompson, Email: wrens@ilstu.edu.
Literature Cited
- Adamson JJ. Evolution of male life histories and age-dependent sexual signals under female choice. PeerJ. 2013;1:e225. doi: 10.7717/peerj.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akçay E, Roughgarden J. Extra-pair paternity in birds: review of the genetic benefits. Evol Ecol Res. 2007;9:855–868. [Google Scholar]
- Allison PD. Survival analysis using SAS: a practical guide. 2. SAS Institute; Cary, NC: 2010. [Google Scholar]
- Arnqvist G, Kirkpatrick M. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior. Am Nat. 2005;165:S26–S37. doi: 10.1086/429350. [DOI] [PubMed] [Google Scholar]
- Barnett CA, Thompson CF, Sakaluk SK. Aggressiveness, boldness and parental food provisioning in male house wrens (Troglodytes aedon) Ethology. 2012;118:1–10. [Google Scholar]
- Beck CW, Shapiro B, Choksi S, Promislow DEL. A genetic algorithm approach to study the evolution of female preference based on male age. Evol Ecol Res. 2002;4:275–292. [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Adaptive sex allocation in relation to hatching synchrony and offspring quality in house wrens. Am Nat. 2011;177:617–629. doi: 10.1086/659630. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Munclinger P, Bureš S, Kučerová L, Nádvorník P, Krist M. Cross-fostering eggs reveals that female collared flycatchers adjust clutch sex ratios according to parental ability to invest in offspring. Mol Ecol. 2013a;22:215–228. doi: 10.1111/mec.12106. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Sibling cooperation influences the age of nest-leaving in an altricial bird. Am Nat. 2013b;181:775–786. doi: 10.1086/670244. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014a;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Offspring sex ratio varies with clutch size for female house wrens induced to lay supernumerary eggs. Behav Ecol. 2014b;25:165–171. [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J Anim Ecol. 2015a;84:473–786. doi: 10.1111/1365-2656.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. Am Nat. 2015b;185:769–783. doi: 10.1086/681017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsbeek R, Sinervo B. Within-clutch variation in offspring sex determined by differences in sire body size: cryptic mate choice in the wild. J Evol Biol. 2004;17:464–470. doi: 10.1046/j.1420-9101.2003.00665.x. [DOI] [PubMed] [Google Scholar]
- DeMory ML, Thompson CF, Sakaluk SK. Male quality influences male provisioning in house wrens independent of attractiveness. Behav Ecol. 2010;21:1156–1164. [Google Scholar]
- Dietrich-Bischoff V, Schmoll T, Winkel W, Krackow S, Lubjuhn T. Extra-pair paternity, offspring mortality and offspring sex ratio in the socially monogamous coal tit (Parus ater) Behav Ecol Sociobiol. 2006;60:563–571. [Google Scholar]
- Dunn PO, Lifjeld JT. Can extra-pair copulations be used to predict extra-pair paternity in birds? Anim. Behav. 1994;47:983–985. [Google Scholar]
- Dunn PO, Cockburn A. Extrapair mate choice and honest signaling in cooperatively breeding superb fairy-wrens. Evolution. 1999;53:938–946. doi: 10.1111/j.1558-5646.1999.tb05387.x. [DOI] [PubMed] [Google Scholar]
- Eckerle KP, Thompson CF. Mate choice in house wrens: nest cavities trump male characteristics. Behaviour. 2006;143:253–271. [Google Scholar]
- Eliassen S, Jørgensen C. Extra-pair mating and the evolution of cooperative neighbourhoods. PLoS One. 2014;9:e99878. doi: 10.1371/journal.pone.0099878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SR, Gustafsson L, Sheldon BC. Divergent patterns of age-dependence in ornamental and reproductive traits in the collared flycatcher. Evolution. 2011;65:1623–1636. doi: 10.1111/j.1558-5646.2011.01253.x. [DOI] [PubMed] [Google Scholar]
- Foerster K, Delhey K, Johnsen A, Lifjeld JT, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. [DOI] [PubMed] [Google Scholar]
- Forsman AM, Vogel LA, Sakaluk SK, Johnson BG, Masters BS, Johnson LS, Thompson CF. Female house wrens (Troglodytes aedon) increase the size, but not immunocompetence, of their offspring through extra-pair mating. Mol Ecol. 2008;17:3697–3706. doi: 10.1111/j.1365-294X.2008.03860.x. [DOI] [PubMed] [Google Scholar]
- Forsman AM, Sakaluk SK, Thompson CF, Vogel LA. Cutaneous immune activity, but not innate immune responsiveness, covaries with mass and environment in nestling house wrens (Troglodytes aedon) Physiol Biochem Zool. 2010;83:512–518. doi: 10.1086/649894. [DOI] [PubMed] [Google Scholar]
- Forstmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B. Female extrapair mating behavior can evolve via indirect selection on males. Proc Natl Acad Sci USA. 2011;108:10608–10613. doi: 10.1073/pnas.1103195108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W, Nakagawa S, Griffith SC, Kempenaers B. Female extra-pair mating: adaptation or genetic constraint? Trends Ecol. Evol. 2014;29:456–464. doi: 10.1016/j.tree.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Fossøy F, Johnsen A, Lifjeld JT. Multiple genetic benefits of female promiscuity in a socially monogamous passerine. Evolution. 2008;62:145–156. doi: 10.1111/j.1558-5646.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- Grana SC, Sakaluk SK, Bowden RM, Doellman MA, Vogel LA, Thompson CF. Reproductive allocation in female house wrens is not influenced by experimentally altered male attractiveness. Behav Ecol Sociobiol. 2012;66:1247–1258. [Google Scholar]
- Griffith SC. The evolution of infidelity in socially monogamous passerines: neglected components of direct and indirect selection. Am Nat. 2007;169:274–281. doi: 10.1086/510601. [DOI] [PubMed] [Google Scholar]
- Griffith SC, I, Owens PF, Thuman KA. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- Hansen TF, Price DK. Good genes and old age: Do old males provide superior genes? J Evol Biol. 1995;8:759–778. [Google Scholar]
- Hercus MJ, Hoffmann AA. Maternal and grandmaternal age influence offspring fitness in Drosophila. Proc R Soc Lond B. 2000;267:2105–2110. doi: 10.1098/rspb.2000.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YH, Schroeder J, Winney I, Burke T, Nakagawa S. Costly infidelity: low lifetime fitness of extra-pair offspring in a passerine bird. Evolution. 2014;68:2873–2884. doi: 10.1111/evo.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YH, Schroeder J, Winney I, Burke T, Nakagawa S. Are extra-pair males different from cuckolded males? A case study and a meta-analytic examination. Mol Ecol. 2015;24:1558–1571. doi: 10.1111/mec.13124. [DOI] [PubMed] [Google Scholar]
- Janiszewski T, Minias P, Wojciechowski Z. Occupancy reliably reflects territory quality in a long-lived migratory bird, the white stork. J Zool. 2013;291:178–184. [Google Scholar]
- Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- Johnson LS. House wren (Troglodytes aedon) In: Poole A, editor. The birds of North America online. 2. 380. Cornell Lab of Ornithology; Ithaca, NY, USA: 2014. [DOI] [Google Scholar]
- Johnson LS, Kermott LH. Possible causes of territory takeovers in a north-temperate population of house wrens. Auk. 1990;107:781–784. [Google Scholar]
- Johnson LS, Brubaker JL, Johnson BGP, Masters BS. Evidence for a maternal effect benefiting extra-pair offspring in a songbird, the house wren Troglodytes aedon. J Avian Biol. 2009a;40:248–253. [Google Scholar]
- Johnson LS, Thompson CF, Sakaluk SK, Neuhäuser M, Johnson BGP, Soukup SS, Forsythe SJ, Masters BS. Extra-pair young in house wren broods are more likely to be male than female. Proc R Soc B. 2009b;276:2285–2289. doi: 10.1098/rspb.2009.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven O, Lifjeld JT. Extrapair paternity and offspring immunocompetence in the reed bunting, Emberiza schoeniclus. Anim Behav. 2004;68:283–289. [Google Scholar]
- Kokko H. Evolutionarily-stable strategies of age-dependent sexual advertisement. Behav Ecol Sociobiol. 1997;41:99–107. [Google Scholar]
- Krist M, Munclinger P. Superiority of extra-pair offspring: maternal but not genetic effects as revealed by a mixed cross-fostering design. Mol Ecol. 2011;20:5074–5091. doi: 10.1111/j.1365-294X.2011.05337.x. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, Barba E, Bouvier JC, Camprodon J, Cooper CB, et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol. 2010;45:1–26. [Google Scholar]
- Magrath MJL, Vedder O, van der Velde M, Komdeur J. Maternal effects contribute to the superior performance of extra-pair offspring. Curr Biol. 2009;19:792–797. doi: 10.1016/j.cub.2009.03.068. [DOI] [PubMed] [Google Scholar]
- Martin LB, II, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol. 2006;20:290–299. [Google Scholar]
- Masters BS, Hicks BG, Johnson LS, Erb LA. Genotype and extra-pair paternity in the house wren: a rare-male effect? Proc. R Soc Lond B. 2003;270:1393–1397. doi: 10.1098/rspb.2003.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery RH, Pettifor RA, Armbruster P, Meyer K, Sheldon BC, Perrins CM. Components of variance underlying fitness in a natural population of the great tit Parus major. Am Nat. 2004;164:E62–E72. doi: 10.1086/422660. [DOI] [PubMed] [Google Scholar]
- Møller AP, Birkhead TR. Certainty of paternity covaries with paternal care in birds. Behav Ecol Sociobiol. 1993;33:261–268. [Google Scholar]
- Møller AP, Mousseau TA, Rudolfsen G, Balbontín J, Marzal A, Hermosell I, de Lope F. Senescent sperm performance in old male birds. J Evol Biol. 2009;22:334–344. doi: 10.1111/j.1420-9101.2008.01650.x. [DOI] [PubMed] [Google Scholar]
- Petrie M, Kempenaers B. Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol Evol. 1998;13:52–58. doi: 10.1016/s0169-5347(97)01232-9. [DOI] [PubMed] [Google Scholar]
- Poirier NE, Whittingham LA, Dunn PO. Males achieve greater reproductive success through multiple broods than through extra-pair mating in house wrens. Anim Behav. 2004;67:1109–1116. [Google Scholar]
- Preston BT, Jalme MS, Hingrat Y, Lacroix F, Sorci G. The sperm of aging male bustards retards their offspring’s development. Nature Comm. 2015;6:6146. doi: 10.1038/ncomms7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest NK, Mackowiak B, Promislow DEL. The role of parental age effects on the evolution of aging. Evolution. 2002;56:927–935. doi: 10.1111/j.0014-3820.2002.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Pryke SR, Griffith SC. Genetic incompatibility drives sex allocation and maternal investment in a polymorphic finch. Science. 2009;323:1605–1607. doi: 10.1126/science.1168928. [DOI] [PubMed] [Google Scholar]
- Queller DC. Why do females care more than males? Proc. R Soc Lond B. 1997;264:1555–1557. [Google Scholar]
- Radwan J. Male age, germline mutations and the benefits of polyandry. Ecol Lett. 2003;6:581–586. [Google Scholar]
- Ramos AG, Nunziata SO, Lance SL, Rodríguez C, Faircloth BC, Gowaty PA, Drummond H. Interactive effects of male and female age on extra-pair paternity in a socially monogamous seabird. Behav Ecol Sociobiol. 2014;68:1603–1609. [Google Scholar]
- Reid JM, Arcese P, Keller LF, Germain RR, Duthie AB, Losdat S, Wolak ME, Nietlisbach P. Quantifying inbreeding avoidance through extra-pair reproduction. Evolution. 2015;69:59–74. doi: 10.1111/evo.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DS, Burke T. Extra-pair paternity in relation to male age in Bullock’s orioles. Mol Ecol. 1999;8:2115–2126. doi: 10.1046/j.1365-294x.1999.00832.x. [DOI] [PubMed] [Google Scholar]
- Roff DA, Fairbairn DJ. The evolution of phenotypes and genetic parameters under preferential mating. Ecol Evol. 2014;4:2759–2776. doi: 10.1002/ece3.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff DA, Fairbairn DJ. Bias in the heritability of preference and its potential impact on the evolution of mate choice. Heredity. 2015;114:404–412. doi: 10.1038/hdy.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino N, Ambrosini R, Martinelli R, Møller AP. Mate fidelity, senescence in breeding performance and reproductive trade-offs in the barn swallow. J Anim Ecol. 2002;71:309–319. [Google Scholar]
- Sakaluk SK, Wilson AJ, Bowers EK, Johnson LS, Masters BS, Johnson BGP, Vogel LA, Forsman AM, Thompson CF. Genetic and environmental variation in condition, cutaneous immunity, and haematocrit in house wrens. BMC Evol Biol. 2014;14:242. doi: 10.1186/s12862-014-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardell RJ, Arcese P, Keller LF, Reid JM. Sex-specific differential survival of extra-pair and within-pair offspring in song sparrows, Melospiza melodia. Proc R Soc B. 2011;278:3251–3259. doi: 10.1098/rspb.2011.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardell RJ, Arcese P, Keller LF, Reid JM. Are there indirect fitness benefits of female extra-pair reproduction? Lifetime reproductive success of within-pair and extra-pair offspring. Am Nat. 2012;179:779–793. doi: 10.1086/665665. [DOI] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010;1:103–113. [Google Scholar]
- Schmoll T, Dietrich V, Winkel W, Epplen JT, Schurr F, Lubjuhn T. Paternal genetic effects on offspring fitness are context dependent within the extrapair mating system of a socially monogamous passerine. Evolution. 2005;59:645–657. [PubMed] [Google Scholar]
- Schmoll T, Schurr FM, Winkel W, Epplen JT, Lubjuhn T. Lifespan, lifetime reproductive performance and paternity loss of within-pair and extra-pair offspring in the coal tit Periparus ater. Proc R Soc B. 2009;276:337–345. doi: 10.1098/rspb.2008.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J, Nakagawa S, Rees M, Mannarelli ME, Burke T. Reduced fitness in progeny from old parents in a natural population. Proc Natl Acad Sci USA. 2015;112:4021–4025. doi: 10.1073/pnas.1422715112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC. Sexually transmitted disease in birds: occurrence and evolutionary significance. Philos Trans R Soc Lond B. 1993;339:491–497. doi: 10.1098/rstb.1993.0044. [DOI] [PubMed] [Google Scholar]
- Sheldon BC. Relating paternity to paternal care. Philos Trans R Soc Lond B. 2002;357:341–350. doi: 10.1098/rstb.2001.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter RA, Mautz BS, Backwell PRY, Jennions MD. Estimating genetic benefits of polyandry from experimental studies: a meta-analysis. Biol Rev. 2012;87:1–33. doi: 10.1111/j.1469-185X.2011.00182.x. [DOI] [PubMed] [Google Scholar]
- Soukup SS, Thompson CF. Social mating system affects the frequency of extra-pair paternity in house wrens. Anim Behav. 1997;54:1089–1105. doi: 10.1006/anbe.1997.0556. [DOI] [PubMed] [Google Scholar]
- Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Tschirren B, Postma E, Rutstein AN, Griffith SC. When mothers make sons sexy: maternal effects contribute to the increased sexual attractiveness of extra-pair offspring. Proc R Soc B. 2012;279:1233–1240. doi: 10.1098/rspb.2011.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velando A, Noguera JC, Drummond H, Torres R. Senescent males carry premutagenic lesions in sperm. J Evol Biol. 2011;24:693–697. doi: 10.1111/j.1420-9101.2010.02201.x. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Nilsson J-Å. The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Phil Trans R Soc B. 2008;363:399–410. doi: 10.1098/rstb.2007.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherhead PJ, Robertson RJ. Offspring quality and the polygyny threshold: “the sexy son hypothesis”. Am Nat. 1979;113:201–208. [Google Scholar]
- Webster MS, Pruett-Jones S, Westneat DF, Arnold SJ. Measuring the effects of pairing success, extra-pair copulations and mate quality on the opportunity for sexual selection. Evolution. 1995;49:1147–1157. doi: 10.1111/j.1558-5646.1995.tb04441.x. [DOI] [PubMed] [Google Scholar]
- Westneat DF, Sherman PW, Morton ML. The ecology and evolution of extra-pair copulations in birds. Curr Onithol. 1990;7:331–369. [Google Scholar]
- Whittingham LA, Dunn PO. Male parental care and paternity. Curr Ornithol. 2001;16:257–298. [Google Scholar]
- Whittingham LA, Dunn PO. Effects of extra-pair and within-pair reproductive success on the opportunity for selection in birds. Behav Ecol. 2005;16:138–144. [Google Scholar]
- Whittingham LA, Dunn PO. Extra-pair mating and sexual selection on male traits across populations. Wilson J Ornithol. 2014;126:9–18. [Google Scholar]
- Wilk T, Cichoń M, Wolff K. Lack of evidence for improved immune response of extra-pair nestlings in collared flycatcher Ficedula albicollis. J Avian Biol. 2008;39:546–552. [Google Scholar]
- Yezerinac SM, Weatherhead PJ, Boag PT. Extra-pair paternity and the opportunity for sexual selection in a socially monogamous bird (Dendroica petechia) Behav Ecol Sociobiol. 1995;37:179–188. [Google Scholar]


