Abstract
Leaves of C4 crops usually have higher radiation, water and nitrogen use efficiencies compared to the C3 species. Engineering C4 traits into C3 crops has been proposed as one of the most promising ways to repeal the biomass yield ceiling. To better understand the function of C4 photosynthesis, and to identify candidate genes that are associated with the C4 pathways, a comparative transcription network analysis was conducted on leaf developmental gradients of three C4 species including maize, green foxtail and sorghum and one C3 species, rice. By combining the methods of gene co-expression and differentially co-expression networks, we identified a total of 128 C4 specific genes. Besides the classic C4 shuttle genes, a new set of genes associated with light reaction, starch and sucrose metabolism, metabolites transportation, as well as transcription regulation, were identified as involved in C4 photosynthesis. These findings will provide important insights into the differential gene regulation between C3 and C4 species, and a good genetic resource for establishing C4 pathways in C3 crops.
Introduction
With growing population and increasing urbanization, humanity faces a looming food crisis, which to prevent, will require yields to be increased by at least 50% over the next 40 years [1, 2]. In addition, extreme climate changes, decreasing availability of water and energy resources, and competitions between grains for bio-fuels and food could worsen the situation. One of the most promising solutions is to introduce the C4 photosynthetic pathways into C3 crops such as rice and soybeans, to improve their water, radiation, and nitrogen use efficiency [1–3], resulting in higher yields than present day C3 crops [4].
In the past few decades, numerous efforts have been made to introduce C4 traits into C3 plants, for example the work carried out by the C4 Rice Consortium (http://irri.org/c4rice). Although none of them have so far demonstrated significantly enhanced photosynthetic properties in transgenic plants, the expression patterns and activities of many known C4 associated genes and proteins were thoroughly studied, and can be used as the basis for future engineering attempts [5–7]. Several features of studied C4 genes are: (1) The C4 pathway independently evolved more than 60 times from C3 plants [8]. Orthologues of genes encoding classic C4 enzymes preexisted in their C3 ancestors but are usually lowly expressed in C3 plants, while in C4 plants these genes are highly expressed and co-regulated by multiple stimuli, e.g., light [5, 9]; (2) Many proteins, encoded by multi-gene families and thought to fulfill housekeeping functions in C3 species [7, 9], are recruited into the C4 pathway after a neo-function is acquired for the C4 paralog [10], which may change its gene expression pattern [11]; (3) C4 genes are often expressed in a cell-type specific manner, i.e. bundle sheath (BS) or mesophyll (ME) cells. These characteristics could be exploited to identify novel C4 genes as well as their regulatory networks, which in practice could provide guidance for strategies of establishing the C4 cycle in C3 plants, e.g., by transferring a group of genes instead of a single gene into C3 crops [5].
With recent advances in sequencing technologies, genome assemblies of multiple C4 species including maize [12], sorghum [13] and new C4 model species, foxtail millet (Setaria Italica) [14, 15], are currently available, providing a good opportunity to dissect the C4 pathway using system biology approaches. To date, several transcriptomics and proteomics studies have provided insight into C4 gene expression and protein accumulation by comparative analysis of BS and ME cells in maize [16–21], green foxtail (Setaria viridis) [22], and rice [23], transcriptional profiling along a leaf development gradient in maize [24, 25] and between maize and rice [26], and between both distantly and closely related C3 and C4 species [27, 28]. Hundreds of differentially accumulated genes and proteins were identified and functionally characterized. Little, however, is known about the downstream regulatory networks of genes and protein interactions responsible for the fundamental anatomical features in both C4 and C3 species [29], as well as the mechanisms controlling the expression and function of well characterized C4 genes[9]. Systems biology analysis of multiple lineages of C3 and C4 species [7, 9], and comparative studies across species could provide great promise for identifying unknown genes that control many, yet unknown, C4 functions [30]. Recently, novel cell type-specific cis-regulatory elements and candidate transcription factors of C4 photosynthesis have been identified, by comparing sets of leaf gradient transcriptome data from maize and rice [26]. In that study, transcriptome data from anatomically and developmentally different leaf sections of maize and rice were projected to a unified gradient to facilitate cross-species clustering analysis on orthologue gene sets. The limitation of this approach is that when studying tissues from two species that are very divergent, it is not always feasible to project a unified gradient.
Gene co-expression network analysis, which uses transcriptomic data (either microarray or RNA-seq data) to group genes according to the similarity of their expression profiles [31], is one of the most powerful methods to explore genes relationships and to predict their function. It is presumed that genes that exhibit similar expression profiles across various tissues/samples are often functionally related [32]. The identified groups are referred to as modules, while the gene relationships within groups are referred to as networks, where nodes represent genes, and edges represent the correlations between pairs of genes [31, 33]. In plants, this method has been successfully applied many times to identify new members of biological processes [34–36]. When multiple species data are available, this process can be refined by extracting the gene co-expression networks found independently in each species, as biologically irrelevant associations caused by noise are not likely to be repeatedly observed in the co-expression networks in different species [32]. Two different strategies can be used for multi-species comparisons: (1) To find conserved modules across species with common gene orthologues [37] and then compare their expression patterns and expression levels, (2) to detect differentially co-expressed modules in which gene orthologues show different network structures between species.
In the present study, expression profiles of segments along a leaf developmental gradient [29], was used to conduct gene co-expression analysis in one C3 and three C4 species. The goal of our study was to identify C4 candidate genes, which are light-regulated and functionally different between C4 and C3 species (e.g., different expression levels, different expression patterns, present in C4 species but absent in C3 species), through genome-wide transcriptome data comparisons. Such C4 candidate genes may be used in the future as a useful source for engineering C4 traits into C3 crops for production improvement.
Material and Methods
Plant material
In total, leaf gradient transcription data from four species, including one C3 (rice) and three C4 (maize, green foxtail and sorghum), were used in this study. Of which, 15 sections of maize (Zea mays, inbred B73) and 11 sections of rice (Oryza sativa var. Nipponbare) data were derived from Wang et al. [26], and 13 sections of sorghum (Sorghum bicolor var. BTx623) and 10 sections of green foxtail (Setaria viridis, ecotype A10.1) data, which derived in each case from 10-day-old third leaves were generated. The growth conditions of these four species were described previously [24, 26], in detail, under an 80:20 mix of metal halide, with capsylite halogen lamps at light intensity of 550 μmol/m2/sec, 12:12 L/D, 31°C L/22°C D and 50% relative humidity. All samples were harvested three hours after light on in the morning, pooled from at least seven plants (seven for maize, rice and sorghum, twenty for green foxtail) per biological replicate and have at least three biological replicates (five for maize, four for rice and three for sorghum) except green foxtail, which has only one replicate. We believed the data from green foxtail is reliable based on following reasons: (1) the samples of green foxtail showed very similar clustering patterns as maize and sorghum throughout all the sections (S1 Fig); (2) the expression patterns of classical C4 genes (e.g., PPDK, NADP-MDH, NADP-ME), which were used as case control in our study, were the same as maize and sorghum; (3) additional two C4 species (maize and sorghum) were also used, the cross species validation designed in the study can minimize the false-positive results due to lack of replication in green foxtail.
Sequence analysis
Total RNA was extracted using TRIzol (Invitrogen, CA) following the manufacturer's suggestion from four species and subsequent RNA-seq libraries were constructed according to Wang et al. [24, 26]. 169M, 332M, 141M and 364M raw reads were generated by single end 35 bp, 51 bp, 51 bp and 35 bp sequencing with Illumina HiSeq 2000 machine from maize, sorghum, green foxtail and rice. After sequence quality examination, reads were mapped to the reference genomes (B73_AGPv2 for maize via MaizeSequence.org, rice_v6 for rice via rice.plantbiology.msu.edu, and JGIv2.0.21 for green foxtail and Sorbi1.22 for sorghum via plants.ensembl.org) using Tophat v2.0.10 [38] with most default settings (e.g., mismatches = 2, threads = 6) but without novel junctions detection (—no-novel-juncs). Because green foxtail reference genome is not currently available, the reads were mapped to its domesticated cultivar, foxtail millet (setaria italica). Reads counting and calculation of RPKM were described previously [26], and gene expression level was finally expressed as mean of RPKM across replicates. The reliability of RNA-seq was validated by qPCR ([39], S1 Table).
Before gene co-expression network analysis, low expressed loci were filtered by using RPKM > 1 in more than 10% sections (i.e., genes with RPKM > 1 in more than 2 out of 15 maize, 13 sorghum, 10 green foxtail and 11 rice sections are kept), and outliers were detected by clustering of samples with the correlation of gene expression [39]. Section 1, harvested from leaf base that displays very different expression profiling from other sections in three C4 species, was detected as outlier and removed from the analysis to avoid the network structures being dominated by difference between section 1 and the others.
Gene co-expression network analysis
Gene co-expression networks were constructed by WGCNA [33] in each species respectively. In order to render the network scale free, different soft thresholding powers (e.g., 10 for maize, 12 for green foxtail, 18 for sorghum and 16 for rice) were chosen to transform Pearson similarity matrix into an adjacency matrix. Modules were determined by the dynamic tree cut method, and modules with high correlated genes (e.g., Pearson correlation > = 0.9) were merged. Modules were named as the first upper letter of each species then followed by their module colors, e.g., M.red and R.black represent red module in maize and black module in rice, respectively. Functional categories enrichment was conducted as previously described [24] based on MapMan annotation [40]. Overlapped modules were detected by using the codes adapted from WGCNA tutorials, and Fisher's exact test was used to calculate p-value for each of the pairwise overlaps.
For species comparison, syntenic orthologues [41] were used, with manual correction for a small number of C4 genes. For examples, selection of function orthologues from tandem repeated gene families (e.g., NADP-MDH and PPDK-RP) was adjusted by expression pattern similarity between species; missing orthologues (e.g., PEPC) due to lack of syntenies in rice were added based on a combination of sequence similarity and expression pattern.
Differentially gene co-expression network analysis
Differentially co-expressed gene modules were identified by DiffCoEx with modification [42]. DiffCoEx was originally designed to cluster genes using a novel dissimilarity measurement computed from the topological overlap of the correlation changes between biological conditions. In this study, this method was adapted to detect gene correlation changes between species. Intuitively, it would detect genes that are significantly co-expressed in one species but not the other. The original code did not separate the positive and negative correlations. It was modified in this study to separate the two types of correlations by an extra step of clustering. The threshold of differentially co-expression module detection was increased, so that only genes with high contrast of connectivity between species were included. More specifically, the cutoff was set as difference of correlation greater than 0.7 in more than 10% gene pairs, and the modules with gene number less than 30 were discarded.
Identification of C4 candidates
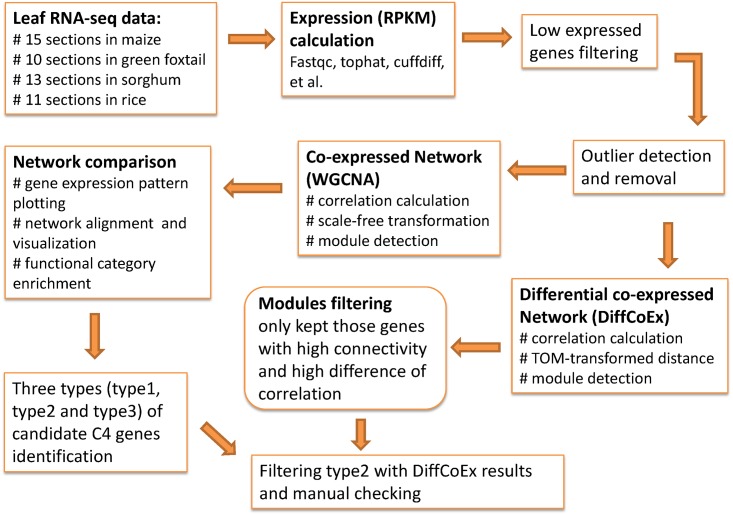
C4 candidate genes were defined as those found in C4 modules (defined by co-expression modules which showed similar gene expression patterns as classic C4 genes, see below) of at least two C4 species in consideration of species specification/divergence, and then categorized into three sub-types by comparing with rice: (I) genes showed similar expression pattern as C4 modules but were lowly expressed in rice, e.g., the expression levels (mean of one third of all sections from the tip) were > = 1.5-fold lower in rice compared to C4 species; (II) genes showed different expression patterns in rice comparing with C4 species; and (III) genes whose syntenic orthologues were present in all three C4 species but absent in rice. We presume that genes should be differentially co-expressed once their expression patterns were changed between species, thus we then filtered the type II of C4 candidate genes with the DiffCoEx results, and only retained those that were differentially co-expressed in at least two out of three comparisons (maize vs. rice, green foxtail vs. rice, and sorghum vs. rice) with the same direction, either lower or higher correlated in C4 species than in rice. Finally, the expression patterns of C4 candidate genes were manually checked. The workflow chart of this study was showed in Fig 1.
Fig 1. Workflow chart of this study.
Results
Gene co-expression network and modules comparison
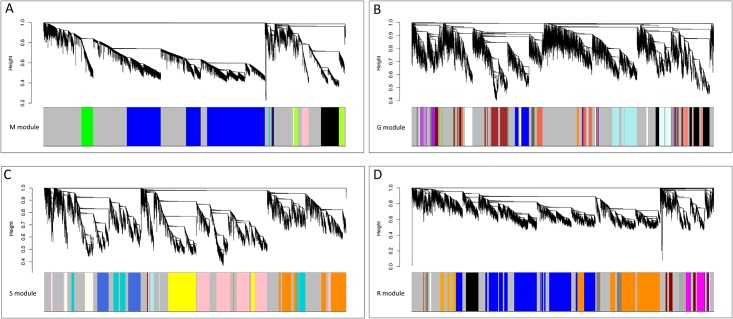
After removing the outlier (leaf section 1) and filtering out low expressed loci, genes of developing leaves from maize (18916 genes, 14 segments), green foxtail (17253 genes, 9 segments), sorghum (18119 genes, 12 segments) and rice (15964 genes, 11 sections), were used for co-expression network construction. Following the standard procedure of WGCNA, genes were assigned into different modules in each species according to their expression patterns along leaf gradients, and genes in the same module showed similar tendency due to a high Pearson correlation. Overall, we identified 11 modules in maize, 32 modules in green foxtail, 12 modules in sorghum and 14 modules in rice (Fig 2 and S2–S5 Figs), which account for 48%, 50%, 39% and 34% genes in each species, respectively.
Fig 2. WGCNA co-expression network in maize (A), green foxtail (B), sorghum (C) and rice (D).
Each line in the dendrogram represented an individual gene, and genes were assigned into modules with different colors in each species, respectively. Genes within grey module were unassigned genes that are not part of any module.
We compared modules between species by following three criteria: gene expression patterns, overlapping orthologous genes, and overlapping enriched function categories based on MapMan annotation (S2 Table). We assumed that modules that show similar expression patterns and were enriched in the same function categories may have the same biological functions, and thus should have significantly overlapped orthologous gene pairs. On the other hand, cross-species modules with significantly overlapped orthologous gene pairs do not always have similar expression patterns and enriched functional categories, and may thus carry out different biological functions among species.
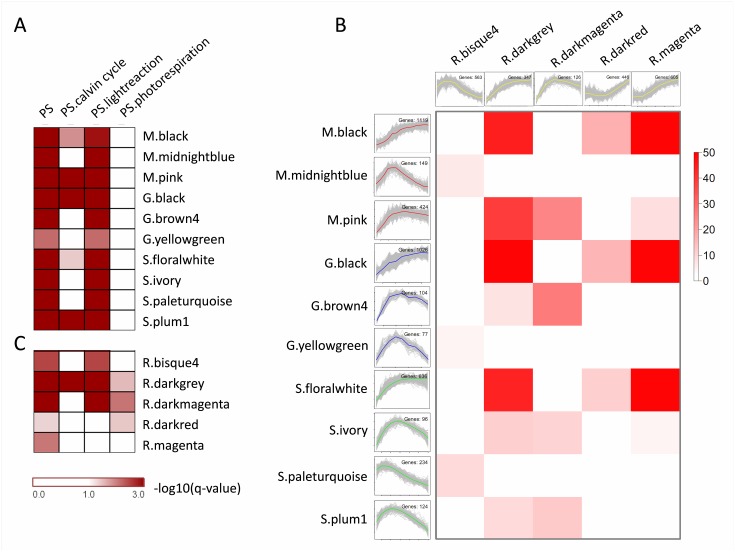
In this study, we aimed to discover genes that were involved in C4 photosynthesis, and thus focused mainly on the photosynthesis (PS) enriched modules in C4 species, specifically, M.black, M.pink and M.midnightblue in maize, S.floralwhite, S.ivory, S.paleturquoise and S.plum1 in soghurm, G.black, G.brown4 and G.yellowgreen in green foxtail (Fig 3A and S2 Table). We found that they were significantly overlapped in orthologous gene pairs (S6–S8 Figs). To examine the expression patterns of rice orthologues of those genes in PS enriched modules from C4 species, pairwise module comparison was performed between C4 species and rice (S9–S11 Figs). Interestingly, many rice modules (e.g., R.bisque4, R.darkgrey, R.darkmagenta, R.darkred and R.magenta) that showed significant overlaps with modules of C4 species (Fig 3B), were also enriched in PS related pathways (Fig 3C). Among them, R.darkgrey, overlapped with M.black in maize, S.floralwhite in sorghum and G.black in green foxtail, and showed similar expression patterns (Fig 3B and S9–S11 Figs), suggesting that the genes in these modules may be functionally important for photosynthesis and thus conserved among all four species. However, the expression patterns of these overlapping modules between rice and C4 species were different in some cases, e.g., genes in R.magenta and R.darkred, which significantly overlapped with M.black, S.floralwhite and G.black but showed different expression patterns (Fig 3B and S9–S11 Figs), may have changed their function as the species diverged.
Fig 3. Photosynthesis enriched modules and their expression patterns in C3 and C4 species.
A. PS modules enriched in C4 species; B. in C3 rice. C. Expression patterns and overlap conditions of PS modules. Modules of each species were prefixed with a capital letter respectively: maize (M), green foxtail (G), sorghum (S) and rice (R). Coloring of the modules overlap table encodes -log(p), with p being a Fisher's exact test p-value for the overlap of the two modules.
It is worth noting that, in these PS enriched modules, three (R.darkgrey, R.darkmagenta and R.darkred) were photorespiration enriched in rice, while none in C4 species (Fig 3A and 3C), suggesting that photorespiration genes were co-expressed in rice but they were not co-expressed in C4 species.
Identification of C4 modules based on classical C4 genes
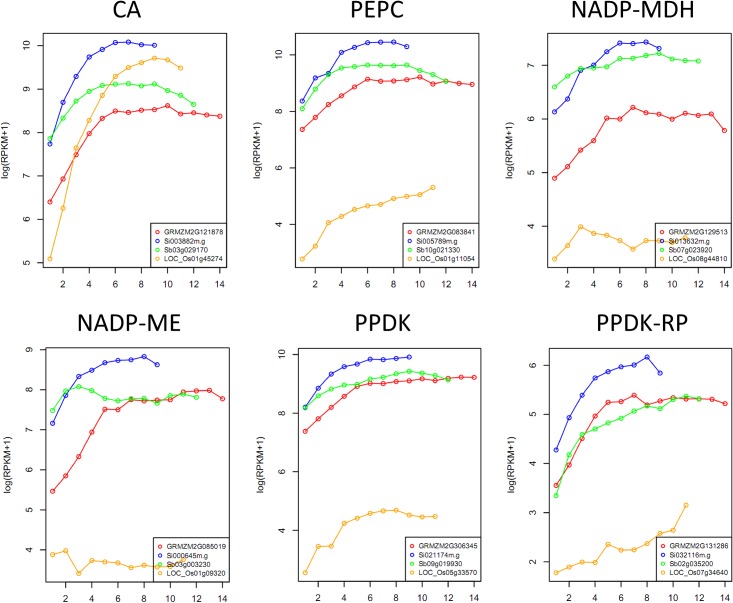
We noticed that classical C4 genes, e.g., carbonic anhydrase (CA), phosphoenolpyruvate carboxylase (PEPC), NADP-malate dehydrogenase (NADP-MDH), NADP-malic enzyme (NADP-ME), pyruvate orthophosphate dikinase (PPDK) and PPDK regulatory protein (PPDK-RP), were grouped in the same module that was enriched in PS related genes in maize (M.pink) and green foxtail (G.black), and had an increasing expression profile from the base to tip along the leaf (Fig 4). In addition, in sorghum, four of them were found in S.floralwhite, and two (NADP-MDH and NADP-ME) in S.grey. The separation of NADP-MDH and NADP-ME from S.floralwhite to grey (genes that are not clustered into modules) may be due to the fact that both of them have tandem duplicated paralogs in the genome (e.g., Sb07g023910 vs. Sb07g023920 and Sb03g003220 vs. Sb03g003230). Based on these observations, we assumed that genes in PS modules, e.g. M.black and M.pink in maize, G.black and G.brown4 in green foxtail, S.floralwhite in sorghum, which contained and showed similar expression patterns of classical C4 genes, contained C4-related candidate genes, and will thus be referred to as “C4 modules” hereafter. In rice, orthologues of CA, PEPC and PPDK showed an expression pattern in R.darkgrey that was similar to the C4 modules. Although CA showed a similar expression level in both C3 and C4 species, the expression of PEPC and PPDK was much lower in rice than in the other three C4 species (Fig 4). The remaining three rice orthologues of classical C4 genes (NADP-MDH, NADP-ME and PPDK-RP), which were grouped in the R.grey module, had a much lower expression level, and showed different expression pattern compared with their C4 orthologues. These results suggest that classical C4 genes either decreased in their expression level or changed their expression pattern in rice. Thus, the characteristics of classical C4 genes can be used as a criterion to define other C4-related candidate genes (see Materials and Methods). Three major categories of C4 genes were characterized: similar expression pattern with higher expression in C4 than in C3 species (type I); different expression patterns in rice compared to C4 species (type II), and syntenic orthologues present in all three C4 species but absent in rice (type III). Based on these criteria and the expression pattern examination of syntenic orthologues in overlapping modules between C3 and C4 species, 478 genes, including 25 type I, 417 type II and 36 type III were identified as C4 candidate genes. We further inspected the type III genes using SynFind in CoGe (https://genomevolution.org/CoGe/SynMap.pl), and found that six of these genes had “no syntenic regions” in the rice genome, while seven of them had "hits" within the rice syntenic regions, but the “hits” were not annotated as genes. These 13 genes were excluded because of the difficulty in verifying whether they were indeed present or absent in rice. The remaining 23 type III genes, which are positioned within all three C4 genomic regions that were in synteny with rice and had no rice homologs detected in these regions, were kept (S3 Table).
Fig 4. Expression pattern of classical C4 genes in four species.
Gene IDs were plotted with different colors, e.g., red, blue, green and yellow for maize, green foxtail, sorghum and rice, respectively. CA: carbonic anhydrase, PEPC: phosphoenolpyruvate carboxylase, NADP-MDH: NADP-malate dehydrogenase, NADP-ME: NADP-malic enzyme, PPDK: pyruvate orthophosphate dikinase, and PPDK-RP: PPDK regulatory protein.
Differential gene co-expression network
To identify genes that had differential co-expression network patterns in rice compared with C4 species, we use DiffCoEx, an algorithm that divides genes into different modules by calculating the correlation difference between rice and C4 species. This method allows the detection of orthologous modules in which genes show high correlation in one species, but low or no correlation in another species (S12–S14 Figs). In total 4249, 2965 and 7517 genes, which were clustered into 12, 11 and 18 modules respectively, were identified as differentially co-expressed by comparing rice with the other three C4 species (maize, sorghum and green foxtail) (S15–S17 Figs).
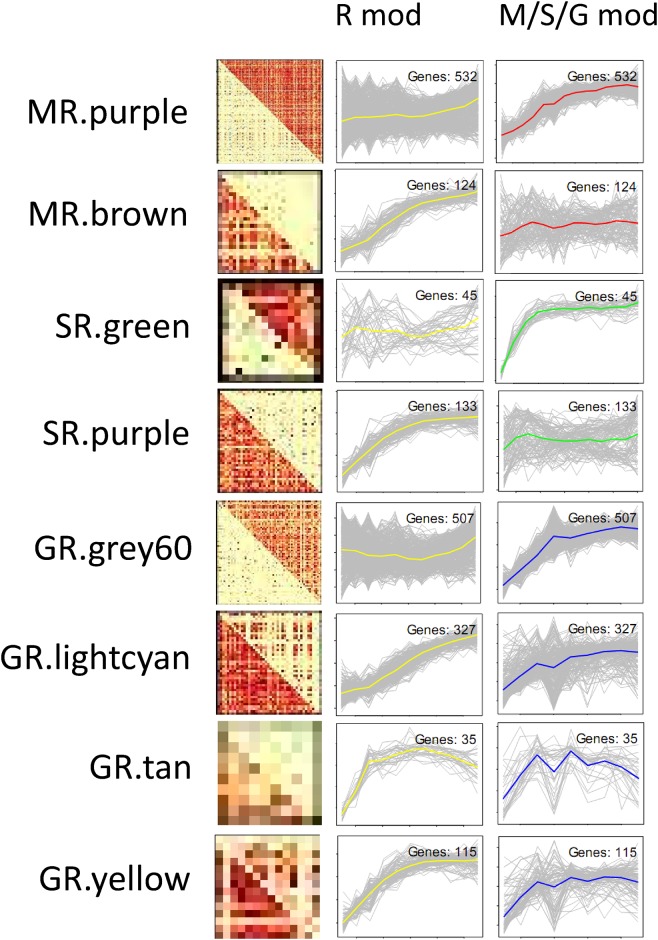
Here, we also focused on eight modules whose expression patterns were similar to the C4 modules, e.g. the gene expression was increased from base to tip, similar to PS genes (MR.purple and MR.brown; SR.green and SR.purple; GR.grey60, GR.lightcyan, GR.tan and GR.yellow) were identified (Fig 5). Of which, MR.purple, SR.green and GR.grey60 were more correlated, while the remaining five were less correlated, in C4 species than in rice. As expected, most of them were significantly enriched in PS related genes (S4 Table). To identify additional genes that may be important for C4 photosynthesis, we selected 155 genes that showed increased correlation in at least two out of three C4 species and 172 genes that showed decreased correlation in at least two C4 species compared to rice.
Fig 5. Differential co-expressed photosynthesis enriched modules between rice (R) and C4 species of maize (M), green foxtail (G) and sorghum (S).
The first column represents heatmaps (the red color indicates positive correlations and blue indicates negative) of the modules, the second column (R Mod) indicated the corresponding gene expression profiles in rice; and the third column (M/S/G Mod) represented corresponding gene expression profiles in maize (red line), sorghum (green line) and green foxtail (blue line).
Identification of C4 candidate genes
We filtered 124 genes from the type II C4 candidates identified via DiffCoEx, assuming that genes that had different expression pattern between rice and C4 species in the co-expression network (WGCNA), should be differentially co-expressed. After manually inspecting the expression patterns between C3 and C4 species, 128 C4 candidate genes (S5 Table), including 25 type I, 80 type II and 23 type III, were identified. As expected, these included many classical C4 genes. For example, PEPC and PPDK were identified as type I, and NADP-ME, NADP-MDH and PPDK-RP were identified as type II C4 genes (S5 Table). In addition, other well-known important C4 genes, such as aspartate aminotransferase (AST, GRMZM5G836910, type II) were also identified. The expression level of AST increased from base to tip in three C4 species, but, the expression trend of its rice orthologue (grouped in R.blue) was reversed (S18 Fig). In summary, the majority (63%) of C4 candidate genes had different expression patterns between C4 and rice, while a smaller proportion had either an elevated (20%) or a novel (18%) expression pattern or in C4 plants, possibly to accommodate the evolutionary transition from C3 to C4 photosynthesis in these species.
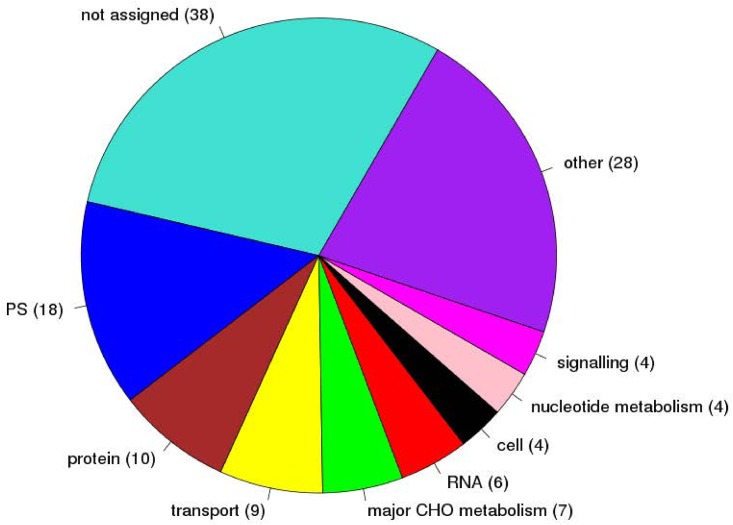
Based on the MapMan category annotation, 70% (90/128) of the genes were assigned to known biological processes or pathways. The three most abundant functional groups, excluding genes classified as “not assigned”, were photosynthesis (PS) (18 genes), protein metabolism (10 genes) and transport (9 genes), followed by major carbohydrate (CHO) metabolism (7 genes) and RNA regulation (6 genes) (Fig 6). Among these 128 C4 candidate genes: 45% (57/128) were differentially expressed between BS and ME cells in both maize and green foxtail [22, 24], and another 49% (63/128) were identified as enriched in one cell type [18, 19, 22, 24] (S5 Table). The fact that the majority (94%, 120/128) of our C4 candidate genes were either BS- or ME-enriched, highly suggests that these C4 genes may play an important roles in C4 metabolism. In addition, by comparing with Wang et al. [21], 50% (64/128) of these C4 candidates were differentially expressed between maize foliar leaf blade (Kranz) and husk leaf sheath (non-Kranz), and 89% (57/64) of them were significant highly expressed in foliar expanded (FE) leaf, not the leaf primodia (S5 Table), indicated these candidates were mainly involved in C4 photosynthesis in leaves. Moreover, 81% (104/128) of these C4 candidates homologous were found to be differentially expressed in leaf gradients of Cleome gynandra [43], a NAD-ME type C4 dicot in an independent C4 lineages, and 45% (57/128) of them were found to show similar expression patterns between C. gynandra and maize (S5 Table). These may indicate the conservation role of these genes in C4 evolution. In addition to the well-known classic C4 genes, we also identified a set of genes, involved in carbohydrate metabolism, that seem to play a role in C4 photosynthesis. For example, GRMZM2G070605 and GRMZM2G066413, two triosephosphate phosphate translocators that transport Calvin cycle derived triosephosphates from the stroma to the cytosol for use in sucrose synthesis and other biosynthetic processes [44], and FBA, FBP and SBP, important enzymes controlling the metabolite flux in the Calvin cycle. Interestingly, six genes related to starch degradation were identified as C4 candidates, e.g. phosphoglucan phosphatase (SEX4, GRMZM2G052546), whose mutation partially blocked the starch degradation process and then influence the plant growth in Arabidopsis [45], and beta-amylases (GRMZM2G082034, GRMZM2G007939, GRMZM2G035749 and GRMZM2G347708), which play a central role in the complete degradation of starch to maltose [46]. Except for starch degradation related genes, one gene (GRMZM2G121612), responsible for starch biosynthesis, was identified. We also identified several sugar transporters that may take part in C4 photosynthesis. For example, SUT1/2 (GRMZM2G087901 and GRMZM2G034302) and STP1 (GRMZM5G801949), which are crucial for efficient phloem loading of sucrose in maize leaves [47].
Fig 6. Functional distribution of 128 C4 candidate genes.
Categories with gene number less than four were assigned into other.
In addition, we identified eight transcription factors that might participate in C4 photosynthesis (S5 Table), including MYBs, ARFs, and G2-like TF (GRMZM2G052544). GRMZM2G052544 is a homolog of APL (ALTERED PHLOEM DEVELOPMENT), which is involved in promoting phloem differentiation and repressing xylem differentiation during vascular development in Arabidopsis [48]. GRMZM2G052544 and its syntenic orthologue (Si017608m.g) were BS-enriched (S5 Table), showed high expression in expanded leaf in C4 species and low expression in rice, which indicated it may be essential for C4 photosynthesis.
According to Gene Ontology (GO), 23 type III C4 candidates, involved in many biological processes such as photosynthesis (GO:0015979), oxidation-reduction process (GO:0055114), cellular component such as membrane (GO:0016020), chloroplast (GO:0009507), chloroplast thylakoid membrane (GO:0009535), and plasma membrane (GO:0005886), and chloroplast envelope (GO:0009941), molecular function such as DNA binding (GO:0003677) and protein binding (GO:0005515) (S6 Table) were annotated. These genes may also play an important role in the evolution of C4.
Discussion
C4 photosynthesis is a complex metabolic pathway that relies on tight collaboration of many enzymes. The identity of many of the genes required for the proper function of C4, as well as their regulatory mechanisms, however, remain elusive. In this study, we combined gene co-expression and gene differentially co-expression networks, to identify candidate genes that may be necessary for C4 photosynthesis. Unlike previous studies that focused on the differential expression between bundle sheath and mesophyll at the gene [19, 20, 22, 24] or protein [16–18] level, we focused on the comparison of gene co-expression and differential gene co-expression relationships along a developmental leaf gradient among multiple C3 and C4 species.
Possible evolution of C4 candidate genes
C4 photosynthesis is thought to have evolved mainly through gene/genome duplication, and subsequent functional innovation of pre-existing genes in C3 species [49, 50]. Leaf gradient RNA-seq data from C3 and C4 species provide us with a good opportunity to dissect the possible evolution of C4 candidate genes by tracking changes of gene expression patterns [51, 52]. Overall, the expression levels and patterns of C4 genes were similar within the three C4 species differed in C3 rice, suggesting that long term adaptive selection may have encouraged the formation of C4 photosynthesis, (e.g., adaptation to high temperature and low CO2) [4, 53–55], as previously suggested using different algorithms [50].
Based on our results, we suggest three possible evolution scenarios for the recruitment of genes into the C4 pathway (1) Genes that have expression patterns similar to known and well characterized photosynthetic genes, could have increased their expression levels in C4 species (e.g., type I), because C4 photosynthesis requires light-regulated high expression of genes in leaves [9, 56]; (2) Genes that exhibit an expression patterns different from photosynthetic genes, may have altered their expression patterns to obtain a novel function for C4 photosynthesis (e.g., type II). This explanation is consistent with a recent study, which demonstrated that C4 expression patterns were not present in the C3 ancestors, but were acquired during the evolutionary transition from C3 to C4 photosynthesis [11]; (3) Genes that have syntenic orthologues in C4 species but were absent from C3 rice (e.g., type III), may represent genes that were newly formed in C4 species, after the divergence of the C3 and C4 lineages, and may thus participate in new biological functions that do not exist in C3 plants.
New insights into C4 photosynthesis genes
Using our selection criteria, some characterized C4 genes, such as carbonic anhydrase (CA, GRMZM2G121878) and phosphoenolpyruvate carboxykinase (PEPCK) were eliminated from the candidate list. CA, the first enzyme of the C4 carbon shuttle, was proposed to be a necessary enzyme for C4 photosynthesis. However, our results showed that, both its expression pattern and expression level were very similar among the examined C3 and C4 species, suggesting, as have recently been shown [57], that CA may not be a rate limiting enzyme for photosynthesis in C4 species.
Maize has two PEPCK genes. The expression of GRMZM2G001696 (max RPKM = 2573) was higher than that of GRMZM5G870932 (max RPKM = 791). However, only one PEPCK gene was identified in sorghum, green foxtail and rice, and the expression level of PEPCK in these species (max RPKM 158 in sorghum, 28 in green foxtail and 34 in rice) was much lower than that in maize, although the peak of their expression was at the leaf tip, as expected from C4 genes. Moreover, the expression patterns of PEPCK in green foxtail (G.floralwhite) and rice (R.darkred) were quite similar, and very different from maize (M.black) and sorghum (S.floralwhite, S2–S5 Figs). These results suggest that green foxtail, like rice, may not have a PEPCK regulated decarboxylation reaction as maize or sorghum. Another possibility is the function of PEPCK was limited in the NADP-ME type C4 photosynthesis species examined in this study, consistent with Zhu et al. [58] that hypothesized that only the NAD-ME type and NADP-ME type should be considered as distinct C4 subtypes, with the PEPCK pathway serving only as a supplement.
Identification of additional C4 genes
Engineering C4 photosynthesis into C3 crops requires a deep understanding of the essential components in the C4 pathways. Despite the progresses that have been made in recent years, with the functional characterization of many important C4 genes, the number of genes that are essential for establishing C4 metabolism in C3 crops is still unknown [6]. Comparative transcriptomics has extensively proved to be a useful approach to identify novel C4 candidate genes [19, 22, 24]. By comparing the gene expression pattern among C3 and C4 species, we identified 38 novel C4 candidates, which were previously classified as functional unknown or not-assigned by the MapMan annotation. The vast majority of these newly identified genes (94.7%) were differentially expressed between the BS and ME cells (S5 Table). These candidate genes were highly co-expressed with classical C4 genes, and may thus require further characterization to discover their exact function during C4 photosynthesis. Twenty six of these genes were annotated by GO, as involved in biological processes such as photosynthesis (GO:0015979), chlorophyll biosynthetic process (GO:0015995), maltose metabolic process (GO:0000023), and molecular functions such as catalytic activity (GO:0003824), hydrolase activity (GO:0016787), phosphotransferase activity (GO:0016776), and many cellular component ontology associated with chloroplasts (GO:0009507, GO:0009570, GO:0009534, GO:0009535) and membranes (GO:0016020, GO:0005886, GO:0042651) (S7 Table).
In addition, a set of carbon metabolism related genes, including FBA, FBP and SBP that control the carbon flux during the Calvin cycle; TPT that transport triosephosphate out of the chloroplast, as well as starch synthase that control starch biosynthesis, were identified as essential for C4 metabolism, and should thus be considered when engineering C4 photosynthesis into C3 crops.
Very little is known about genes that are associated with Kranz anatomy and metabolite transportation in C4 leaves [59, 60]. Our method identified two sucrose transporters (SUT1 and SUT2) and two triosephosphate phosphate translocators (TPT1 and TPT2), that were shown to have important roles in the C4 carbon shuttle [27, 44], as well as several transporters associated with K+ efflux, transmembrane transporter activity and others (S4 Table). Due to the low number of developmental stages in our dataset, however, we could not identify any Kranz anatomy associated genes. With the growing availability of high resolution tissue/cell specific data, our method will be very useful in identifying and characterizing additional C4 candidate genes, and assist in our efforts to engineer C4 traits into C3 crops, to improve yield and feed the growing population of the world.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(XLSX)
Numbers are—log10 transformed q values.
(XLSX)
(XLSX)
Numbers are—log10 transformed q values.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. James Schnable for his helps on species syntenic orthologue list. This work was supported by the National Science Foundation of China grant 31271393 to P.L., the National Science Foundation grant IOS-1127017 to T.P.B. and Q.S., ITC ITF Support to the Partner State Key Laboratories in Hong Kong.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Science Foundation of China (http://www.nsfc.gov.cn/) grant 31271393 to PL the National Science Foundation (www.nsf.gov) grant IOS-1127017 to TPB and QS.
References
- 1. Hibberd JM, Sheehy JE, Langdale JA. Using C4 photosynthesis to increase the yield of rice—rationale and feasibility. Current opinion in plant biology. 2008;11(2):228–31. 10.1016/j.pbi.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 2. Mitchell P, Sheehy J. Supercharging rice photosynthesis to increase yield. New Phytologist. 2006;171(4):688–93. [DOI] [PubMed] [Google Scholar]
- 3. Zhu X-G, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Annual review of plant biology. 2010;61:235–61. 10.1146/annurev-arplant-042809-112206 [DOI] [PubMed] [Google Scholar]
- 4. Sage RF. The evolution of C4 photosynthesis. New phytologist. 2004;161(2):341–70. [DOI] [PubMed] [Google Scholar]
- 5. Peterhansel C. Best practice procedures for the establishment of a C4 cycle in transgenic C3 plants. Journal of experimental botany. 2011;62(9):3011–9. 10.1093/jxb/err027 [DOI] [PubMed] [Google Scholar]
- 6. Miyao M, Masumoto C, Miyazawa S-I, Fukayama H. Lessons from engineering a single-cell C4 photosynthetic pathway into rice. Journal of experimental botany. 2011;62(9):3021–9. 10.1093/jxb/err023 [DOI] [PubMed] [Google Scholar]
- 7. Kajala K, Covshoff S, Karki S, Woodfield H, Tolley BJ, Dionora MJA, et al. Strategies for engineering a two-celled C4 photosynthetic pathway into rice. Journal of experimental botany. 2011;62(9):3001–10. 10.1093/jxb/err022 [DOI] [PubMed] [Google Scholar]
- 8. Sage RF, Christin P-A, Edwards EJ. The C4 plant lineages of planet Earth. Journal of Experimental Botany. 2011;62(9):3155–69. 10.1093/jxb/err048 [DOI] [PubMed] [Google Scholar]
- 9. Hibberd JM, Covshoff S. The regulation of gene expression required for C4 photosynthesis. Annual review of plant biology. 2010;61:181–207. 10.1146/annurev-arplant-042809-112238 [DOI] [PubMed] [Google Scholar]
- 10. Aubry S, Brown NJ, Hibberd JM. The role of proteins in C3 plants prior to their recruitment into the C4 pathway. Journal of experimental botany. 2011;62(9):3049–59. 10.1093/jxb/err012 [DOI] [PubMed] [Google Scholar]
- 11. Christin P-A, Boxall SF, Gregory R, Edwards EJ, Hartwell J, Osborne CP. Parallel recruitment of multiple genes into C4 photosynthesis. Genome biology and evolution. 2013;5(11):2174–87. 10.1093/gbe/evt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, et al. The B73 maize genome: complexity, diversity, and dynamics. science. 2009;326(5956):1112–5. 10.1126/science.1178534 [DOI] [PubMed] [Google Scholar]
- 13. Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457(7229):551–6. 10.1038/nature07723 [DOI] [PubMed] [Google Scholar]
- 14. Zhang G, Liu X, Quan Z, Cheng S, Xu X, Pan S, et al. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nature biotechnology. 2012;30(6):549–54. 10.1038/nbt.2195 [DOI] [PubMed] [Google Scholar]
- 15. Bennetzen JL, Schmutz J, Wang H, Percifield R, Hawkins J, Pontaroli AC, et al. Reference genome sequence of the model plant Setaria. Nature biotechnology. 2012;30(6):555–61. 10.1038/nbt.2196 [DOI] [PubMed] [Google Scholar]
- 16. Majeran W, Cai Y, Sun Q, van Wijk KJ. Functional differentiation of bundle sheath and mesophyll maize chloroplasts determined by comparative proteomics. The Plant Cell Online. 2005;17(11):3111–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Majeran W, Zybailov B, Ytterberg AJ, Dunsmore J, Sun Q, van Wijk KJ. Consequences of C4 differentiation for chloroplast membrane proteomes in maize mesophyll and bundle sheath cells. Molecular & Cellular Proteomics. 2008;7(9):1609–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friso G, Majeran W, Huang M, Sun Q, Van Wijk KJ. Reconstruction of metabolic pathways, protein expression, and homeostasis machineries across maize bundle sheath and mesophyll chloroplasts: large-scale quantitative proteomics using the first maize genome assembly. Plant physiology. 2010;152(3):1219–50. 10.1104/pp.109.152694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang Y-M, Liu W-Y, Shih AC-C, Shen M-N, Lu C-H, Lu M-YJ, et al. Characterizing regulatory and functional differentiation between maize mesophyll and bundle sheath cells by transcriptomic analysis. Plant physiology. 2012;160(1):165–77. 10.1104/pp.112.203810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tausta SL, Li P, Si Y, Gandotra N, Liu P, Sun Q, et al. Developmental dynamics of Kranz cell transcriptional specificity in maize leaf reveals early onset of C4-related processes. Journal of experimental botany. 2014:eru152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang P, Kelly S, Fouracre JP, Langdale JA. Genome-wide transcript analysis of early maize leaf development reveals gene cohorts associated with the differentiation of C4 Kranz anatomy. The Plant Journal. 2013;75(4):656–70. 10.1111/tpj.12229 [DOI] [PubMed] [Google Scholar]
- 22. John CR, Smith-Unna RD, Woodfield H, Covshoff S, Hibberd JM. Evolutionary convergence of cell-specific gene expression in independent lineages of C4 grasses. Plant Physiol. 2014;165(1):62–75. 10.1104/pp.114.238667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiao Y, Tausta SL, Gandotra N, Sun N, Liu T, Clay NK, et al. A transcriptome atlas of rice cell types uncovers cellular, functional and developmental hierarchies. Nature genetics. 2009;41(2):258–63. 10.1038/ng.282 [DOI] [PubMed] [Google Scholar]
- 24. Li P, Ponnala L, Gandotra N, Wang L, Si Y, Tausta SL, et al. The developmental dynamics of the maize leaf transcriptome. Nature genetics. 2010;42(12):1060–7. 10.1038/ng.703 [DOI] [PubMed] [Google Scholar]
- 25. Pick TR, Bräutigam A, Schlüter U, Denton AK, Colmsee C, Scholz U, et al. Systems analysis of a maize leaf developmental gradient redefines the current C4 model and provides candidates for regulation. The Plant Cell Online. 2011;23(12):4208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Czedik-Eysenberg A, Mertz RA, Si Y, Tohge T, Nunes-Nesi A, et al. Comparative analyses of C4 and C3 photosynthesis in developing leaves of maize and rice. Nature biotechnology. 2014. [DOI] [PubMed] [Google Scholar]
- 27. Bräutigam A, Hoffmann-Benning S, Weber AP. Comparative proteomics of chloroplast envelopes from C3 and C4 plants reveals specific adaptations of the plastid envelope to C4 photosynthesis and candidate proteins required for maintaining C4 metabolite fluxes. Plant physiology. 2008;148(1):568–79. 10.1104/pp.108.121012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bräutigam A, Kajala K, Wullenweber J, Sommer M, Gagneul D, Weber KL, et al. An mRNA blueprint for C4 photosynthesis derived from comparative transcriptomics of closely related C3 and C4 species. Plant Physiology. 2011;155(1):142–56. 10.1104/pp.110.159442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nelson T. The grass leaf developmental gradient as a platform for a systems understanding of the anatomical specialization of C4 leaves. Journal of experimental botany. 2011;62(9):3039–48. 10.1093/jxb/err072 [DOI] [PubMed] [Google Scholar]
- 30. Sage RF, Zhu X-G. Exploiting the engine of C4 photosynthesis. Journal of Experimental Botany. 2011;62(9):2989–3000. 10.1093/jxb/err179 [DOI] [PubMed] [Google Scholar]
- 31. Usadel B, Obayashi T, Mutwil M, Giorgi FM, Bassel GW, Tanimoto M, et al. Co-expression tools for plant biology: opportunities for hypothesis generation and caveats. Plant, cell & environment. 2009;32(12):1633–51. [DOI] [PubMed] [Google Scholar]
- 32. Hansen BO, Vaid N, Musialak-Lange M, Janowski M, Mutwil M. Elucidating gene function and function evolution through comparison of co-expression networks of plants. Frontiers in plant science. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC bioinformatics. 2008;9(1):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han X, Yin L, Xue H. Co-expression Analysis Identifies CRC and AP1 the Regulator of Arabidopsis Fatty Acid Biosynthesis. Journal of integrative plant biology. 2012;54(7):486–99. 10.1111/j.1744-7909.2012.01132.x [DOI] [PubMed] [Google Scholar]
- 35. Persson S, Wei H, Milne J, Page GP, Somerville CR. Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(24):8633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ficklin SP, Luo F, Feltus FA. The association of multiple interacting genes with specific phenotypes in rice using gene coexpression networks. Plant physiology. 2010;154(1):13–24. 10.1104/pp.110.159459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ficklin SP, Feltus FA. Gene coexpression network alignment and conservation of gene modules between two grass species: maize and rice. Plant Physiology. 2011;156(3):1244–56. 10.1104/pp.111.173047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller JA, Horvath S, Geschwind DH. Divergence of human and mouse brain transcriptome highlights Alzheimer disease pathways. Proceedings of the National Academy of Sciences. 2010;107(28):12698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Usadel B, Poree F, Nagel A, Lohse M, CZEDIK-EYSENBERG A, Stitt M. A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, Maize. Plant, cell & environment. 2009;32(9):1211–29. [DOI] [PubMed] [Google Scholar]
- 41. Schnable JC, Freeling M, Lyons E. Genome-wide analysis of syntenic gene deletion in the grasses. Genome biology and evolution. 2012;4(3):265–77. 10.1093/gbe/evs009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tesson BM, Breitling R, Jansen RC. DiffCoEx: a simple and sensitive method to find differentially coexpressed gene modules. BMC bioinformatics. 2010;11(1):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aubry S, Kelly S, Kümpers BM, Smith-Unna RD, Hibberd JM. Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of Trans-Factors in Two Independent Origins of C4 Photosynthesis. PLoS genetics. 2014;10(6):e1004365 10.1371/journal.pgen.1004365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Majeran W, Friso G, Ponnala L, Connolly B, Huang M, Reidel E, et al. Structural and metabolic transitions of C4 leaf development and differentiation defined by microscopy and quantitative proteomics in maize. The Plant Cell Online. 2010;22(11):3509–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niittylä T, Comparot-Moss S, Lue W-L, Messerli G, Trevisan M, Seymour MD, et al. Similar protein phosphatases control starch metabolism in plants and glycogen metabolism in mammals. Journal of Biological Chemistry. 2006;281(17):11815–8. [DOI] [PubMed] [Google Scholar]
- 46. Smith AM, Zeeman SC, Smith SM. Starch degradation. Annu Rev Plant Biol. 2005;56:73–98. [DOI] [PubMed] [Google Scholar]
- 47. Slewinski TL, Meeley R, Braun DM. Sucrose transporter1 functions in phloem loading in maize leaves. Journal of Experimental Botany. 2009;60(3):881–92. 10.1093/jxb/ern335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bonke M, Thitamadee S, Mähönen AP, Hauser M-T, Helariutta Y. APL regulates vascular tissue identity in Arabidopsis. Nature. 2003;426(6963):181–6. [DOI] [PubMed] [Google Scholar]
- 49. Monson RK. Gene duplication, neofunctionalization, and the evolution of C4 photosynthesis. International Journal of Plant Sciences. 2003;164(S3):S43–S54. [Google Scholar]
- 50. Wang X, Gowik U, Tang H, Bowers JE, Westhoff P, Paterson AH. Comparative genomic analysis of C4 photosynthetic pathway evolution in grasses. Genome Biol. 2009;10(6):R68 10.1186/gb-2009-10-6-r68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garfield DA, Wray GA. The evolution of gene regulatory interactions. BioScience. 2010;60(1):15–23. [Google Scholar]
- 52. Ranz JM, Machado CA. Uncovering evolutionary patterns of gene expression using microarrays. Trends in Ecology & Evolution. 2006;21(1):29–37. [DOI] [PubMed] [Google Scholar]
- 53. Babu MM, Aravind L. Adaptive evolution by optimizing expression levels in different environments. Trends in microbiology. 2006;14(1):11–4. [DOI] [PubMed] [Google Scholar]
- 54. López-Maury L, Marguerat S, Bähler J. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nature Reviews Genetics. 2008;9(8):583–93. 10.1038/nrg2398 [DOI] [PubMed] [Google Scholar]
- 55. Yamori W, Hikosaka K, Way DA. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynthesis research. 2014;119(1–2):101–17. 10.1007/s11120-013-9874-6 [DOI] [PubMed] [Google Scholar]
- 56. Sheen J. Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. The Plant Cell Online. 1991;3(3):225–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Studer AJ, Gandin A, Kolbe AR, Wang L, Cousins AB, Brutnell TP. A limited role for carbonic anhydrase in C4 photosynthesis as revealed by a ca1ca2 double mutant in maize. Plant physiology. 2014;165(2):608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Y, Bräutigam A, Weber AP, Zhu X-G. Three distinct biochemical subtypes of C4 photosynthesis? A modelling analysis. Journal of experimental botany. 2014:eru058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Majeran W, van Wijk KJ. Cell-type-specific differentiation of chloroplasts in C4 plants. Trends in plant science. 2009;14(2):100–9. 10.1016/j.tplants.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 60. Weber AP, von Caemmerer S. Plastid transport and metabolism of C< sub> 3 and C< sub> 4 plants—comparative analysis and possible biotechnological exploitation. Current opinion in plant biology. 2010;13(3):256–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
Each row and column of the table corresponds to one module (labeled by color as well as text) from two species, respectively. Numbers in the table indicate overlapped gene counts in the intersection of corresponding modules. Coloring of the table encodes -log(p), with p being the Fisher's exact test p-value for the overlap of the two modules. The more significant the overlap, the stronger the red color is.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(XLSX)
Numbers are—log10 transformed q values.
(XLSX)
(XLSX)
Numbers are—log10 transformed q values.
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.