Abstract
It is impossible to predict exactly who will develop a cancer and who will not. We know that several “risk factors” may increase the chance of getting cancer and that risk increases with age. However, even with that in mind we seem to be able to explain only a certain number of cancers. Recently, Tomasetti and Vogelstein published a provocative article in Science stating that a large percentage of cancers may be due to “bad luck” (stochastic mutation events during DNA replication) and only a few to carcinogens, pathogens, or inherited genes and that this should impact public health policies. However, their intriguing analysis has numerous limitations, some of which have already been commented upon, including the likely biased subset of cancers and that finding a correlation does not signify a cause-effect mechanism. Here, we point out that there may also be an alternative explanation for the data, the cancer stem cell hypothesis, which postulates that cancers are derived from tissue stem cells and not from somatic differentiated cells. We also highlight the importance of the tissue microenvironment in the growth of transformed cells and outline a table of concurrent factors for several cancers. The message communicated to the public should not be one of helplessness in avoiding cancers, particularly given the now extensive knowledge of known risk factors and several agents/behaviors that can lower risk for specific cancers. While some tumors will still be due to chance, prevention should still be a primary goal for public health policies.
Cancer incidence varies among populations, organs, and tissues, and sometimes we are not able to explain tumor occurrence by known potential determinants, such as environmental exposure, pathogens, or inherited genes. In their secondary analysis of published data, Tomasetti and Vogelstein (1) state in the abstract that most cancers are: “due to bad luck,” that is, “random mutations arising during DNA replication in normal, noncancerous stem cells.”
When oncologists talk about risk of cancer, they are referring to a probability, the chance that a tumor may occur (and not the actual fact that it will). As an example, if you throw a die, any of the six different faces is equally likely to show. So the probability of getting one particular number from 1 to 6 is 1/6. It gets more complicated with two or more dice. With two dice the probability of getting a certain number (from 2 to 12) ranges from 1/35 to 6/35; depending on the number, one can calculate which combinations are more likely. This is a simplified view of probability. Because there are several combinations that can produce 7, there is a higher probability (6/35, 17%) of obtaining that number as compared with getting 12 (6 and 6, 1/35 or 3%); thus, in everyday language, 12 is considered “luck” because it has a low probability, like a lottery win (whereas the term “bad luck” is used for events with low probability, but considered as unpleasant). So there is a difference between chance/risk and “luck” (or “bad luck”). In cancer many mutational events are required, and very few follow the “two hit hypothesis” of Knudsen (2), but most are the consequences of several hits. Furthermore, in the real world, or even in a “simulated real world,” cancer does not match completely with calculated probability. There are many known factors (Table 1) that can influence cancer incidence, which can be summarized as age, sex, ethnic origin, geographic location, inheritance of susceptibility genes, overweight and obesity, life style, exposure to carcinogens (chemical, physical and infectious agents [viruses or bacteria]), and, for breast cancer, age at menarche, parity, hormonal status, and lactation. Many influential factors are still unknown, as are potential interactions among multiple factors.
Table 1.
Modifiable and treatable risk factors for cancer
| Unmodifiable risk factors | Modifiable risk factors | Treatable risk factors |
|---|---|---|
| Age | Tobacco | Chronic inflammation |
| Genetics | Overweight and Obesity | Viral infections |
| Hereditary and somatic mutations | Nutrition | Bacterial infections |
| Sex | Physical activity | Diabetes |
| Ethnicity | Exposure to carcinogens | Irradiation |
| Family history | Alcohol | Hormonal status |
| Personal history | Lactation* | |
| Reproductive history*,† | ||
| World region† | ||
* For women.
†In some cases, people can choose their reproductive history and world region; for others, this is not a specific choice.
Risk estimates for cancer and other diseases are determined in epidemiology by studying large cohorts to see what kinds of populations will develop the disease over a certain period of time and also what characteristics or behaviors are associated with increased or decreased risk. For many cancers, we know that bad habits (smoking, alcohol, poor diet, lack of exercise), physical comorbidities (overweight and obesity, diabetes, metabolic syndrome, certain inflammatory syndromes), and exposure to carcinogens all contribute an additional risk to a pure mathematical probability. Therefore, decreasing exposure to risk factors can reduce incidence and hedge the “bad luck.” While we know the etiological agents or hereditary factors for several neoplasms, for others we have less knowledge and therefore cannot explain them through deterministic factors. The history of research shows that previously unknown etiologic factors have emerged to explain epidemiology of certain tumors, for example, Helicobacter pylori for gastric cancer, HPV for cervical, vulvar, anal, and several head and neck cancers, asbestos for mesothelioma, as well as tobacco use for a number of pathologies (Table 2).
Table 2.
Risk factors associated with replicative and deterministic tumors
| Risk enhancers* | Replicative tumors (1) | Deterministic tumors (1) |
|---|---|---|
| UV irradiation | Basal cell carcinoma | |
| UV irradiation | Melanoma | |
| Smoking/HPV | Head and neck squamous cell carcinoma (non-HPV) | Head and neck squamous cell carcinoma (HPV) |
| Smoking | Lung (NSCLC adenocarcinomas) nonsmokers | Lung (NSCLC adenocarcinomas) smokers |
| Smoking/obesity | Esophageal squamous cell carcinoma (38% of all esophageal cancers) | |
| Duodenal adenocarcinoma (non-FAP) | Duodenal adenocarcinoma (FAP) | |
| Small intestinal adenocarcinoma | ||
| Alcohol/HCV/HBV/cirrhosis | Hepatocellular carcinoma | Hepatocellular carcinoma (HCV) |
| Gallbladder nonpapillary adenocarcinoma | ||
| Obesity/smoking | Pancreatic (ductal adenocarcinoma and endocrine) | |
| Obesity/smoking/diet | Colorectal adenocarcinoma (including FAP and HNPCC) | |
| Glioblastoma multiforme | ||
| Medulloblastoma | ||
| Osteosarcoma | ||
| Medullary thyroid carcinoma | Thyroid papillary and follicular carcinoma | |
| Testicular germ cell cancer (95% of all testicular cancers) | ||
| Ovarian germ cell cancer (3% of all ovarian cancers) | ||
| Major tumors missing from (1): | ||
| Obesity/diet? | Breast (˜30% of all cancers in women) | |
| Obesity/inflammation?/ diet? |
Prostate (˜30% of all cancers in men) | |
| Smoking | Lung SCLC (˜15% of all lung cancers) | |
| Smoking | Lung squamous (˜25.5% of all lung cancers) | |
| Asbestos particulate exposure | Malignant mesothelioma† | |
| Smoking/obesity/diet? | Esophageal adenocarcinoma (62% of all esophageal cancers) | |
| Smoking/obesity/ Helicobacter pylori/diet? | Stomach | |
| Smoking/obesity | Renal | |
| Smoking | Bladder | |
| HPV | Anal | |
| Obesity | Epithelial ovarian cancer (90% of all ovarian cancers) | |
| HPV | Cervix uteri | |
| Endometrial | ||
| HPV | Vulvar | |
* Risk enhancers are epidemiologically associated with increased risk of specific tumors. Hematologic tumors were not included. FAP = familial adenomatous polyposis; HBV = hepatitis B virus; HCV = hepatitis C virus; HNPCC = hereditary non-polyposis colorectal cancer; HPV = human papillomavirus; NSCLC = non–small cell lung cancer; HNPCC = hereditary non-polyposis colorectal cancer; SCLC = small cell lung cancer.
† A rare cancer but strongly associated with exposure to asbestos particulates.
In the Science article (1) the authors, using data extrapolated with a degree of approximation from a systematic review of the literature, correlated the number of lifetime stem cell replications within a tissue, an indicator of DNA replication, with lifetime cancer incidence in various tissues selected from those on which data on stem cells were available. The authors examined the correlation between two variables, the estimated number of replicative events and cancer risk, and found that the predictor—the calculated number of lifetime tissue stem cell replications—explained about 65% of the variability in cancer risk among certain types of cancers. Based on this assumption, Tomasetti and Vogelstein (1) concluded that DNA damage during replication is the single factor influencing cancer incidence for 22 of the 31 specific tumors they examined. These cancers were termed by the authors as “replicative” (R) tumors, whereas the remaining third, in which environmental mutagens, infectious agents, or hereditary predispositions strongly influence risk, were termed “deterministic” (D). The authors, translating the amount of variability explained by univariate regression into epidemiologic evidence, went on to suggest that prevention should only be applied to deterministic tumors, while replicative tumors, for which the number of replications correlates with lifetime risk, cannot be prevented and only early detection can be used to reduce mortality. These data were given substantial attention in the press, largely because of the provocative use of the term “bad luck” in the abstract (1). The authors chose to use the term “bad luck” because of the unjustified guilt experienced by many oncology patients and their families when a cancer with no apparent cause occurs. However, the downside of employing that term is that the lay population is likely to decide that leading a healthy lifestyle and avoiding carcinogen exposure will not prevent many cancers and that getting cancer or not is more or less like rolling dice. We are not sure that this should be the message conveyed by this study.
An interesting scientific debate followed the publication of the Tomasetti and Vogelstein article (1). Several comments challenged some technical aspects of the analysis and the authors’ conclusions and recommendations (3–10). Most criticisms focused on the inclusion criteria for tumor sites that took into account only a small proportion of cancer cases in the United States (8), the accuracy of the literature review that did not have the methodological characteristics of a systematic review (4), and the underestimation of other risk factors that may contribute to carcinogenesis (6), including those related to environmental and lifestyle factors (8). Almost all of the comments and criticisms underline the fact that the authors underestimated the limitations of their data and analysis, which resulted in overestimation of the role of adverse chance (bad luck).
Tomasetti and Vogelstein replied to criticisms, adding further information supporting the validity of their data, analysis, and conclusions (11,12), and basically confirmed their point of view: “We found evidence for a surprisingly large role of these mutations, henceforth called replicative mutations.”
Here we add some further reflections to the debate. The fact that stochastic effects, ie, accumulation of random mutations within specific pathways in particular cell types, play a role in cancer etiology has long been known. Accumulation of mutations is part of the replicative process during a person’s lifetime and increases with longevity; thus an increase in cancer risk occurs over time, which can be accelerated when enhanced DNA damage has occurred. While the analysis of Tomasetti and Vogelstein (1) allowed an estimate of the effects of stochastic events in stem cell replication, the emphasis on stochastic effects alone is misleading. There are several caveats and limitations to the Tomasetti and Vogelstein study (1), and the same data can have different interpretations that should be noted to bring their results into perspective. We therefore propose a reflection on and analysis of several aspects of carcinogenesis.
Tissue Stem Cells as Origin of Cancer Cells
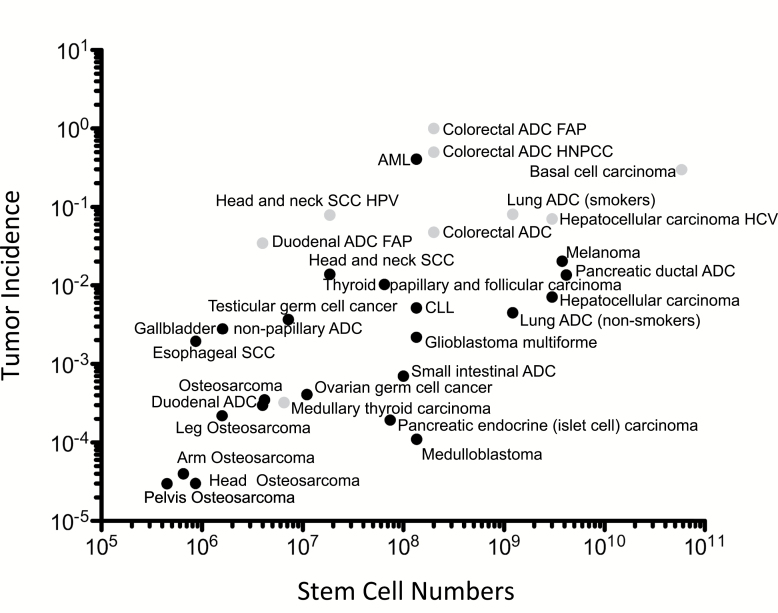
The data of Tomasetti and Vogelstein (1) can be interpreted in a different manner from that proposed by the authors. The data seem to sustain the theory, originally proposed by Julius Cohnheim in 1867 (13), that cancer cells can only arise in tissue stem cells, a hypothesis with increasing support in recent years. The role of stem cells in tumor etiology is clear for some tissues, in particular retinoblastoma, in which after the age of four years this tumor is not formed because there are no replicating retinoblasts left in the tissue. For other types of cells, as Tomasetti and Vogelstein (1) point out for melanocytes, all cells could be considered “stem cells.” The authors estimated the number of stem cell replications within tissues as an indicator of total cell proliferations, finding that tissues with more stem cell replicative events have higher cancer incidence. However, the tissues with higher incidence of neoplasms also have higher numbers of stem cells; if we use a log-log plot similar to that used by Tomasetti and Vogelstein (1) but simply plot cancer incidence against the estimated number of tissue stem cells for each tissue (from Supplementary Table 1 of [1]), a similar “correlation” appears (Figure 1) to that of the log-log plot against estimated stem cell lifetime replicative events. This would suggest that tissues with more stem cells simply have more cells at risk for developing cancer, and thus higher incidence, independent of replication events. These data could be interpreted to support the hypothesis that tissue stem cells are the cells of origin for tumor cells, which could also explain the “cancer stem cell” phenomenon that has been booming in recent years (14,15). This hypothesis may also explain the robustness of the correlation to different estimates of lifetime replications, as indicated by the authors.
Figure 1.
Log-log plot of the stem cell number within a specific tissue (adapted from Supplementary Table 1 of [1]) and the calculated cancer risk for that tissue. There appears to be a correlation between stem cell number within a tissue and the likelihood of developing a cancer in that tissue. The dots in black represent the tumors considered replicative (1); the gray dots indicate those considered deterministic. ADC = adenocarcinoma; AML = acute myeloid leukemia; CLL = chronic lymphocytic leukemia; FAP = familial adenomatous polyposis; HBV = hepatitis B virus; HCV = hepatitis C virus; HNPCC = hereditary non-polyposis colorectal cancer; HPV = human papillomavirus; SCC = squamous cell carcinoma.
The hypothesis that cancer cells are derived from tissue stem cells was further modified by John Cairns in his “immortal strand” hypothesis (16). His hypothesis suggested that during asymmetric division the stem cells retain one strand of their DNA as a template; this strand is never replicated, while the other strand synthesized from the template is given to the committed cells. There is increasing experimental support for this hypothesis using DNA labeling methods (see [17] for brief overview), which would imply that stem cell replications are not the source of mutations in tissue stem cells leading to cancer, but rather errors in DNA repair. This would be in keeping with the many germ-line mutations predisposing to cancer that compromise DNA repair mechanisms.
How Many Cancer Types?
The authors focus on only a few cancer types for which they found sufficient literature on stem cell numbers, thus introducing a selection bias. The analyzed tumor types include specific subtypes based on cells of origin. As already pointed out (5), these cancers represent a relatively small portion of all tumors, and two of the most frequent cancers in terms of incidence, breast and prostate, were not included in the analysis (Table 2). Thus, stating that two thirds of “all cancer types” are due to “bad luck” is an overstatement; rather, the proportion is two thirds of the relatively few cancer types investigated. Many of the tumors studied represent a limited portion of total cases of cancer occurring, and many have known risk factors (Table 2) that have not been taken into account. In addition, the relative number of lifetime stem cell population doublings determined is based on averages of a wide range of variables and a sum of several successive approximations for each tissue (described in the additional material provided by Tomasetti and Vogelstein), reducing the confidence in the data. Although as proof of robustness the authors varied their estimations for lifetime stem cell replications, they did not consider incidence itself as a variable (see below). Further, as pointed out by Weinberg and Zaykin (7), a correlation does not mean a cause-effect relationship and would not reveal effects of prevention or increased risk that would affect all cancers.
Stem Cell Number, Tumor Site, Sex, and Ethnicity
One would assume that most humans would have equal numbers of stem cells in their tissues. Yet we find that incidence for some cancers varies widely in different regions of the world, including esophageal carcinoma, a tumor type indicated as being mostly replicative by Tomasetti and Vogelstein (1), but which is instead known to be influenced by tobacco use; moreover, the risk of some subtypes can be reduced by aspirin use (18). Further, there are also differences in cancer incidence between individuals with divergent ethnicity living in the same region (ie, African Americans vs Americans of European origin) and between males and females (excluding sex-specific tissues). Adipose tissue is a rich source of stem cells that has become clinically important for reconstructive and aesthetic surgery (19,20); clearly, adipose stem cells undergo more replications in an obese individual as compared with an individual who has maintained ideal body weight throughout life. Yet we are not aware of increased incidence of adipose-derived mesenchymal tumors in obese individuals, while incidence of several epithelial cancers appears to be enhanced (21). Further, one would suspect that multiparity and breast feeding in women would increase the number of breast stem cell divisions as compared with nulliparous women, yet these are known to be protective factors, rather than conferring increased risk.
Finally, in some cases the authors go to great lengths to separate out specific tissue areas, eg, osteosarcomas, while for other tissues they lump many tumor histotypes and organ subsites together. For example, it is well known that most cancers of the large bowel occur on the left side; there is decreasing incidence from the ascending colon to the descending colon (22); nearly half of all colon cancers are found in the sigmoid colon and rectum, which represent a relatively small portion of the large bowel. Do tissue stem cell replications in these locations explain these differences? This seems unlikely. There are now several subsets of colorectal cancers recognized that reflect on clinical aspects (23–25). Recent data now suggest an increasing shift toward colon cancer incidence in the ascending colon (22), again suggesting the influence of environment rather than that of stem cell replications.
If most cancers were due to random errors during stem cell replication, then one would expect to see the same cancers with increasing probability over time. Yet the incidence of childhood and adolescent cancers is quite different from those of adults (26). Finally, if stem cell replications are a key factor, then how is it that the prostate, a very tiny organ with a clearly minor subset of stem cells (27), is responsible for 30% of tumors in Western males, has a wide variance of incidence across the world (26), and may be prevented by dietary interventions (28)? The prostate may be like the breast, where there is extensive plasticity allowing normal and neoplastic cells to re-enter into a stem-like phenotype (29).
Replicative Tumors With Known Risk Factors
The authors (1) analyzed separately several tumors known to be based on environmental influence, separating lung cancers in nonsmoking from smoking individuals, HCV-associated from non-HCV liver cancer, and Lynch and familial adenomatous polyposis (FAP) syndrome colorectal cancer from “sporadic” colon cancer. This clearly suggests a bias toward those tissues for which we know of clear risk factors. It would have been interesting to analyze these tumors all together only based on tissue of origin to observe where these would fall in the scheme proposed by Tomasetti and Vogelstein (1). It would appear that for many cancers for which environmental input plays a role for a small subset of tumors as compared with stochastic events in the same tissue the approximative analysis of Tomasetti and Vogelstein (1) might not be sensitive enough to identify these tumors. Because smoking causes most lung cancer cases, it is likely that lung cancer would remain in the “deterministic” category if all lung cancers were analyzed together. In the case of liver cancer, this assumption might not be so clear. Alcohol abuse, chronic infections with HCV or HBV, and combinations of the above lead to liver cirrhosis, which is highly associated with hepatocellular carcinoma (HCC). However, because only a minor portion of HCCs are due to HCV, when HCV-HCC is placed together with other HCC, it is likely that all HCC would remain in the “replicative” category (1). Thus the authors created a bias by selecting out those tumors for which there was a clear determining factor. However, how many unknown determining factors are out there? Further, the authors assumed that in tissues exposed to tumor-promoting effects (ie, in smokers, individuals with hepatitis, or those with a genetic predisposition) the number of tissue stem cell replications is constant; however, one might consider that constant damage to tissues would increase the number of stem cell divisions and eventually lead to stem cell exhaustion. For example, it is probable that there are more hepatic stem cell replications in patients with cirrhosis, followed by stem cell depletion. As our knowledge of nonstochastic causes for tumors increases, the subgroups of deterministic tumors as defined by Tomasetti and Vogelstein are likely to increase for many tissues.
Prevention to Curb Luck in Replicative Tumors
Tomasetti and Vogelstein (1) propose that prevention efforts are appropriate only for those tumors that are deterministic, while for replicative tumors the emphasis should be on early detection. If we take a look at ductal pancreatic cancer, which Tomasetti and Vogelstein placed among the replicative tumors for which prevention should not be applied, in a meta-analysis of over 20 000 patients followed for up to 20 years, Rothwell et al. found a statistically significant reduction in pancreatic cancers among those who took an aspirin a day vs those who took aspirin sporadically (18). Rothwell et al. also found a remarkable suppression of tumors of the esophagus in the same cohort, limited to adenocarcinoma, while Tomasetti and Vogelstein estimated the risk only for esophageal squamous carcinoma. These data, therefore, shed doubt on the first conclusion of Tomasetti and Vogelstein, that primary prevention should not be used in cancers defined by them as replicative. There are several prevention agents that have been epidemiologically shown to reduce cancer incidence below that of baseline, in particular, aspirin for several tumors, metformin in type 2 diabetes, fenretinide and hormone antagonists in breast cancer (for review see [30]).
Over- and Underdiagnosis, What to Fear
A further point is the second conclusion of Tomasetti and Vogelstein (1), that screening interventions should be the focus for replicative cancers. This leads to possible overdiagnosis and overtreatment, two increasing concerns among oncologists as the power of tissue imaging and diagnosis increases. Among the tumors that we can detect very early, many of these might not have created any complications for the patient if left undetected within the lifespan of the individual. Autopsy studies have found that approximately 40% of women between 40 and 49 years old have occult breast cancers (31) yet are diagnosed in only 2% of women of this age. Similarly, in situ prostate cancer has been found in 24% of men age 60 to 70 years (32) yet is diagnosed in only 8% of these men. Studies also estimate that microscopic thyroid cancers are present in 98% of individuals by the age of 70 years (33,34), yet these cancers become clinically relevant in less than 0.5% of these individuals. These clinically indolent, microscopic foci of cancers are likely due to the inability of the tumor itself to activate/inactivate the host dependent hallmarks of cancer (35), angiogenesis, tumor promoting inflammation, and suppression of immune surveillance (30) within the tumor microenvironment (36). Furthermore, the impact on world health is related to mortality rather than incidence. Certain common tumors such as basal cell carcinoma have high survival rates; others like pancreatic carcinomas are often rapidly mortal. We need to not only determine whether a cancer is present or not, but whether and when it will become clinically meaningful to the patient and eventually fatal.
The Tumor Microenvironment
Carcinogenesis and tumor growth are phenomena that occur in the entire neoplastic tissue, not in individual cancer cells, and the microenvironment is an integral, essential part of the cancer. Therefore, it is necessary to consider the microenvironment of a cancer and its associated abnormal epithelium as a functional whole. Tissue organization field theory indicates that stromal, inflammatory, and endothelial cells have crucial roles in the developing tumor and might account for different tumor risk even in the presence of the same amount of tissue stem cells (37). A disruption of homeostasis, often associated with chronic inflammation, is linked to carcinogenesis (38,39). Microenvironment cells (immune, endothelial, and stromal) that normally maintain tissue homeostasis can react paradoxically to permit and promote transformed (stem) cell survival and replication. The microenvironment can be a primary factor in determining whether stem cells after a transformation event, stochastic or deterministic, will continue to grow and become a cancer or remain as an indolent micro-hyperplasia or even be cleared by the organism. A classic example of this is the monoclonal gammopathy of undetermined significance (MGUS) conversion to multiple myeloma; the same genetic abnormalities are present in both cases (40), yet MGUS is indolent while multiple myeloma is frequently deadly. Epigenetics also appears to play a key role in permitting a mutated cell to become a tumor or remain in a tissue homeostatic state (41). Therefore “bad luck” can be also prevented by protecting the tumor microenvironment, curbing inflammation, or stimulating antitumor adaptive immune responses, as can be seen from the recent success of immune checkpoint blockade agents (42).
Estimation Uncertainty
Finally, given the selection bias and the sample size, as well as the correlational nature of the evidence, confidence about the cause and effect of the relationship between stem cell replications and lifetime cancer risk remains to be determined (7). The results and the authors’ conclusions are based on a simple univariate correlation between only two variables in a very small sample and on a log-log scale. Several points merit attention: the 95% confidence interval (39% to 81%) that the authors calculated for the amount of variance explained (65%) is quite wide, indicating that the estimate is unstable. An alternative interpretation of the results is that the variability in the total number of stem cell replications could explain “only” a small amount of the total variability. The authors also took into consideration the estimation uncertainty of only one variable, that is, the error in estimation of the number of stem cell replications during a lifetime. Incidence derived from the Surveillance, Epidemiology, and End Results Program (SEER) database (http://www.seer.cancer.gov) is affected by estimation errors as well. Furthermore, as mentioned above, incidence rates vary across many regions of the world and can change with time (26). The correlation of incidence with lifetime stem cell replications was performed using log10 values, rather than on the raw data (for which a simple linear regression shows much lower correlation), suggesting a nonlinear relationship and/or high variability that cannot be accounted for using a simple linear model. The high variability may be explained by numerous external unknown factors that do in fact increase or decrease the relative risk for a specific cancer. The generalizability of these data is hampered by the very simple statistical approach, because most of the potential additional risk factors were not taken into account.
Conclusion
The inability to explain variation of cancer incidence among populations, individuals, and tissues does not mean that most of the causes are due to “bad luck”; it only means that we still do not know enough about cancer(s) and do not do enough to prevent it. We would argue that, for most tumors, while stochastic “bad luck” has a role, one can and should seek to modify their risk, hedging one’s bets against the “bad luck” mutational events that favor tumor etiology. This includes avoiding tobacco (not only for respiratory, but for most gastrointestinal and urinary tract tumors), maintaining an active lifestyle and balancing caloric input and output, thus keeping obesity at bay, as well as reducing exposure to sunlight for those at risk for basal cell carcinoma and melanoma. Avoiding and controlling chemical agents (excessive alcohol, asbestos, and other workplace carcinogens), as well as infectious agents, is also important; indeed, we will soon see the effects of vaccines and new therapies for infectious agents associated with cancer risk. Moreover, cancer chemoprevention has been shown to be an important approach, eventually targeting the host-dependent hallmarks inflammation and angiogenesis (30,36). Cancer chemoprevention agents may influence tissue stem cell biology, further reducing the cells at risk, and there is increasing evidence that many chemoprevention agents target cancer stem cells (43,44). Attention to these behaviors and measures is unlikely to completely eliminate the risk of cancer. Some individuals leading a healthy lifestyle and/or using chemoprevention will still be diagnosed with cancer because of stochastic “bad luck,” but on the average they will be diagnosed less frequently for many cancers than those leading an unhealthy lifestyle or those exposed to known carcinogens.
The authors were supported by grants from the Associazione Italiana per la Ricerca sul Cancro (IG10228 and IG15895), the Ministero della Salute Ricerca Finalizzata (2010–2011), and Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (2010NECHBX003).
References
- 1. Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347 (6217):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68 (4):820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gotay C, Dummer T, Spinelli J. Cancer risk: prevention is crucial. Science. 2015;347 (6223):728. [DOI] [PubMed] [Google Scholar]
- 4. O’Callaghan M. Cancer risk: accuracy of literature. Science. 2015;347 (6223):729. [DOI] [PubMed] [Google Scholar]
- 5. Potter JD, Prentice RL. Cancer risk: tumors excluded. Science. 2015;347 (6223):727. [DOI] [PubMed] [Google Scholar]
- 6. Song M, Giovannucci EL. Cancer risk: many factors contribute. Science. 2015;347 (6223):728–729. [DOI] [PubMed] [Google Scholar]
- 7. Weinberg CR, Zaykin D. Is bad luck the main cause of cancer? J Natl Cancer Inst. 2015;107 (7):djv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wild C, Brennan P, Plummer M, et al. Cancer risk: role of chance overstated. Science. 2015;347 (6223):728. [DOI] [PubMed] [Google Scholar]
- 9. Most types of cancer not due to ‘bad luck’ IARC responds to scientific article claiming that environmental and lifestyle factors account for less than one third of cancers. Cent Eur J Public Health. 2015;23 (1):87. [PubMed] [Google Scholar]
- 10. Luzzatto L, Pandolfi PP. Causality and Chance in the Development of Cancer. N Engl J Med. 2015;373 (1):84–88. [DOI] [PubMed] [Google Scholar]
- 11. Tomasetti C, Vogelstein B. Cancer risk: role of environment-response. Science. 2015;347 (6223):729–731. [DOI] [PubMed] [Google Scholar]
- 12. Tomasetti C, Vogelstein B. Technical Report: Musings on the theory that variation in cancer risk among tissues is explained by the number of divisions of the normal stem cells. http://arxiv.org/abs/1501.05035. [DOI] [PMC free article] [PubMed]
- 13. Cohnheim J. Ueber entzundung und eiterung. Path Anat Physiol Klin Med. 1867;40:1–79. [Google Scholar]
- 14. Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66 (4):1883–1890; discussion 1895–1896. [DOI] [PubMed] [Google Scholar]
- 15. Albini A, Cesana E, Noonan DM. Cancer stem cells and the tumor microenvironment: soloists or choral singers. Curr Pharm Biotechnol. 2011;12 (2):171–181. [DOI] [PubMed] [Google Scholar]
- 16. Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255 (5505):197–200. [DOI] [PubMed] [Google Scholar]
- 17. Zeps N, Hemmings C. Chasing the immortal strand: evidence for nature’s way of protecting the breast genome. Breast Cancer Res. 2011;13 (1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377 (9759):31–41. [DOI] [PubMed] [Google Scholar]
- 19. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100 (9):1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30 (5):804–810. [DOI] [PubMed] [Google Scholar]
- 21. Park J, Morley TS, Kim M, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10 (8):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu C, Bassig BA, Zaridze D, et al. A birth cohort analysis of the incidence of ascending and descending colon cancer in the United States, 1973–2008. Cancer Causes Control. 2013;24 (6):1147–1156. [DOI] [PubMed] [Google Scholar]
- 23. Hugen N, van de Velde CJ, de Wilt JH, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25 (3):651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marisa L, de Reynies A, Duval A, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med. 2013;10 (5):e1001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sadanandam A, Lyssiotis CA, Homicsko K, et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med. 2013;19 (5):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61 (2):69–90. [DOI] [PubMed] [Google Scholar]
- 27. Huang Y, Hamana T, Liu J, et al. Prostate sphere-forming stem cells are derived from the P63-expressing basal compartment. J Biol Chem. 2015; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brausi M, Rizzi F, Bettuzzi S. Chemoprevention of human prostate cancer by green tea catechins: two years later. A follow-up update. Eur Urol. 2008;54 (2):472–473. [DOI] [PubMed] [Google Scholar]
- 29. Chaffer CL, Brueckmann I, Scheel C, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108 (19):7950–7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albini A, Tosetti F, Li VW, et al. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012;9 (9):498–509. [DOI] [PubMed] [Google Scholar]
- 31. Nielsen M, Thomsen JL, Primdahl S, et al. Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br J Cancer. 1987;56 (6):814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanchez-Chapado M, Olmedilla G, Cabeza M, et al. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54 (3):238–247. [DOI] [PubMed] [Google Scholar]
- 33. Black WC, Welch HG. Advances in diagnostic imaging and overestimations of disease prevalence and the benefits of therapy. N Engl J Med. 1993;328 (17):1237–1243. [DOI] [PubMed] [Google Scholar]
- 34. Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56 (3):531–538. [DOI] [PubMed] [Google Scholar]
- 35. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144 (5):646–674. [DOI] [PubMed] [Google Scholar]
- 36. Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7 (2):139–147. [DOI] [PubMed] [Google Scholar]
- 37. Baker SG. A cancer theory kerfuffle can lead to new lines of research. J Natl Cancer Inst. 2015;107 (2):dju405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6 (1):24–37. [DOI] [PubMed] [Google Scholar]
- 39. Noonan DM, De Lerma Barbaro A, Vannini N, et al. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27 (1):31–40. [DOI] [PubMed] [Google Scholar]
- 40. Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. 2012;122 (10):3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burgio E, Migliore L. Towards a systemic paradigm in carcinogenesis: linking epigenetics and genetics. Mol Biol Rep. 2015;42 (4):777–790. [DOI] [PubMed] [Google Scholar]
- 42. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12 (4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fu Y, Chang H, Peng X, et al. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS One. 2014;9 (7):e102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mineva ND, Paulson KE, Naber SP, et al. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLoS One. 2013;8 (9):e73464. [DOI] [PMC free article] [PubMed] [Google Scholar]