Abstract
Purpose
Inhibiting exaggerated wound healing responses, which are primarily mediated by human Tenon’s fibroblast (HTF) migration and proliferation, has become the major determining factor for a successful trabeculectomy. Antivascular endothelial growth factor (anti-VEGF) has showed promising results as a potential antifibrotic candidate for use concurrently in trabeculectomy. Preliminary cohort studies have revealed improved bleb morphology following trabeculectomy augmented with ranibizumab. However, the effects on HTFs remain unclear. This study was conducted to understand the effects of ranibizumab on transforming growth factor (TGF)-β1 and transforming growth factor (TGF)-β2 expression by HTFs.
Methods
The effect of ranibizumab on HTF proliferation and cell viability was determined using 3-(4,5-dimethylthiazone-2-yl)-2,5-diphenyl tetrazolium (MTT) assay. Ranibizumab at concentrations ranging from 0.01 to 0.5 mg/ml were administered for 24, 48, and 72 h in serum and serum-free conditions. Supernatants and cell lysates from samples were assessed for TGF-β1 and TGF-β2 mRNA and protein levels using quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA).
Results
At 48 h, 0.5 mg/ml of ranibizumab significantly induced cell death under serum-free culture conditions (p<0.05). Ranibizumab caused a significant reduction in TGF-β1 mRNA, but not for TGF-β2. However, the total protein production of TGF-β1 and TGF-β2 was unaffected by this anti-VEGF treatment.
Conclusions
Exposure of HTFs to an intravitreal dose of ranibizumab significantly suppresses cell viability in vitro; however, the application seemed unable to affect the ultimate production of TGF-β. Therefore, we highlighted ranibizumab as a potential antiscarring agent that acts via a different mechanism when used synergistically with another antifibrotic agent. Understanding the mechanism of actions of ranibizumab offers an additional view of a possible new rational therapeutic strategy.
Introduction
Extreme tissue scarring and wound healing processes at the filtering bleb serve as the major cause of trabeculectomy failure [1]. In trabeculectomy, a filtering bleb is created under the conjunctival and subconjunctival area to assist the outflow of aqueous humor from the anterior chamber of the eye, therefore maintaining the ideal intraocular pressure (IOP). Aberrant deposition of extracellular matrix components that result from human Tenon’s fibroblast (HTF) activities contribute to the significant surgical failure. Although antimetabolites such as mitomycin-C (MMC) and 5-flurouracil (5-FU) effectively prevent post-surgical scarring, their application is associated with devastating and potentially blinding complications [2-4].
Scarring at the filtering bleb involves HTF proliferation and migration. This series of events is highly influenced by various cytokines and growth factors [5,6]. Transforming growth factor (TGF)-β is a multifunctional growth factor that acts as a potent inducer of scarring throughout the body [7,8]. There are three isoforms of TGF-β in humans (TGF-β1, TGF-β2, and TGF-β3). TGF-β2 is the predominant isoform, and its presence has been identified in normal and diseased eyes [9] and in several ocular scarring processes particularly post-trabeculectomy [10-12]. In addition, an in vitro investigation by Tripathi et al. revealed that TGF-β1 but not TGF-β2 mRNA and protein were significantly expressed by primary cultured HTFs [13]. Although cultured conjunctival fibroblasts were shown to express all three TGF-β isoforms, the expression of TGF-β1 was the strongest and most upregulated [14].
Vascular endothelial growth factors (VEGFs) have been implicated as proangiogenic agents responsible for vascular endothelial cell growth and increased vascular permeability [15]. The involvement of VEGF as a primary inducer of neovascularization in age-related macular degeneration (AMD), diabetic retinopathy, and neovascular glaucoma has been well-established [16-18]. Data have shown that VEGF inhibition effectively suppresses scar formation at the filtering bleb and thus increases the success of trabeculectomy [19,20].
The discovery of antivascular endothelial growth factor (anti-VEGF) offers an alternative as a safer and effective mediator in preventing scarring post-trabeculectomy. Bevacizumab (Avastin; Genetech, Inc., San Francisco, CA) is a full-length, humanized monoclonal antibody designed against all types of VEGF. Bevacizumab is approved by the U.S. Food and Drug Administration (FDA) for the treatment of metastasic colorectal cancer [21]. Several studies have demonstrated the efficacy of bevacizumab in the management of neovascular AMD and pathologic myopia [22,23]. In vivo and in vitro studies of bevacizumab have demonstrated significant decrease in the IOP with minimal bleb vascularity through the reduction of viable HTFs, which induces cell death at a low level and inhibits cell-mediated gel contraction [24-26]. Ranibizumab (Lucentis; Genetech, Inc.) is a recombinant, humanized monoclonal antibody Fab derived from the same precursor of bevacizumab. Ranibizumab has been approved by the FDA for the treatment of choroidal neovascularization (CNV) due to AMD. Intravitreal ranibizumab has a good safety profile and is effective as an antiangiogenic mediator in AMD [27], and synergistic action with MMC in trabeculectomy causes a more diffuse bleb with less vascularity [28].
Although many clinical studies have deliberately described the significance of using anti-VEGF in trabeculectomy, the mechanism of action of anti-VEGF is not well understood [28,29]. Recently, Park et al. reported the direct effect of VEGF on myofibroblast transformation via induction of TGF-β1 synthesis [30]. This finding verified the parallel effect between VEGF, TGF-β1 synthesis, and myofibroblast deposition at the filtering bleb. The role of ranibizumab (anti-VEGF) in this antifibrotic strategy seems important. Thus, we investigated the role of ranibizumab in TGF-β1 and TGF-β2 expression by HTFs.
Methods
Cell culture
Primary HTFs were propagated from Tenon’s capsule explanted from patients with primary open-angle glaucoma (POAG) undergoing trabeculectomy as previously described [31]. This study adhered to tenets of the Declaration of Helsinki, ARVO statement on human subjects, and our local government institution review board (Medical Research and Ethics Committee of The Ministry of Health Malaysia). Patients gave their written informed consent. Primary HTFs were harvested as an expansion culture of the human Tenon’s explants and were propagated in Roswell Park Memorial Institution (RPMI) culture media (Gibco; Life Technology, Grand Island, NY). Culture media were supplemented with fetal bovine serum (FBS), 20% of the final volume (Gibco, Life Technology), penicillin 100,000 U/I, and streptomycin 10 ml/l (Gibco). The cells were maintained at 37 °C in 5% CO2 in a humidified atmosphere. Only HTFs between the third and sixth passages were used for all experiments. Cells at the higher passage indicated altered morphology compared to the ones at the lower passage, and this might affect the response to treatment.
Immunofluorescence for vimentin antibody staining
HTFs were cultured in a four-chamber culture chamber at the concentration of 10×103 cells/well in complete culture media and incubated at 37 °C, 5% CO2 for 24 h. The slides were fixed with 100% acetone (R&M, Essex, UK) and were left to dry. The slides then were washed with PBS (1X 0.02 mol/l NaPO4, 0.15 mol/l NaCl, pH 7.0; DAKO; Agilent Technologies, Glostrup, Denmark) and subsequently incubated with anti-vimentin antibody [V9] (ab8069) from (Abcam; Cambridge, UK) with 1:100 dilution in a wet chamber. Then the slides were washed again with PBS and were further incubated with goat polyclonal secondary antibody to mouse (Abcam, Ab96879) in 1:100 dilution in a wet chamber. These steps were performed in a dark room. After the final washing step, the slides were mounted with 4’6-diamidino-2-phenylindole-dilactate (DAPI; DAKO) and fluorescence mounting medium and covered with coverslips. The slides then were viewed immediately. HTF cytoplasm stained positively with anti-vimentin antibody appeared green, and the nucleus appeared blue.
Ranibizumab effects on HTF viability
The cell viability test was based on the ready-to-use cell viability reagent 4,5-dimethylthiazone-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Promega, Madison, WI). HTFs were seeded at a concentration of 5×103 cells/well in a 96-well plate (Thermo Scientific, Waltham, MA) and incubated overnight in 100 µl complete culture media. The fibroblast monolayers were then washed to remove serum and replaced with serum-free culture media overnight for starvation. After treatment for 24, 48, and 72 h with various concentrations of ranibizumab (starting concentration (0.5 mg/ml), 1/2 (0.25 mg/ml), 1/10 (0.05 mg/ml), and 1/50 (0.01 mg/ml) of the human intravitreal dose) in 5% FBS media and serum-free media, 10 µl of the MTT reagent was added to the medium in each well and incubated in a humidified atmosphere at 37 °C in 5% CO2 for 4 h. MTT assay was performed only up to 72 h due to the cells’ susceptibility to prolonged starvation and exposure to treatment. Then, all the media in the well were discarded, and 100 µl of dimethyl sulfoxide (DMSO) was added in each well and further incubated for 1 h in the same environment. The optical density was determined with the Victor X5, Multilabel Reader Spectrophotomer (Perkin Elmer, Waltham, MA) at 570 nm, and cell viability data were obtained from at least three experiments with at least six wells at each concentration. Viability experiments were repeated that compared ranibizumab with non-humanized control immunoglobulin G (IgG) isotype (Invitrogen, Life Technology). The mRNA and protein studies were conducted according to the optimum concentration and condition (time and culture media) of ranibizumab on the HTFs obtained from this assay.
mRNA extraction and quantification from HTFs
HTFs were seeded in six-well plates at a density of 3×105 cells/well and were treated with 0.5 mg/ml of ranibizumab for 48 h. In the control wells, cells were treated with control IgG antibody with the same concentration. Total mRNA was isolated from the cultured cells by using the NucleoSpin RNA isolation kit (Macherey-Nagel, Duren, Germany). Briefly, 353 µl of buffer and β-mercaptoethanol (β-ME) was added to the cell pellet and vigorously vortexed for 30 s. The mixture then was filtered through the NucleoSpin filter, and the lysate was centrifuged for 1 min at 11,000 ×g. The homogenized lysate then was added with 350 µl ethanol and mixed thoroughly. The lysate was loaded into the column and centrifuged for 30 s at 11,000 ×g. Then 350 µl of membrane desalting buffer (DMB) was added to the lysate and centrifuged at 11,000 ×g for 1 min. The lysate was incubated with 95 µl of the DNase reaction mixture at room temperature for 15 min. After three steps of washing and centrifuge, the lysate finally was eluted with 40 µl of RNase-free H2O and centrifuged at 11,000 × g for 1 min. Total mRNA was quantified using the Agilent 2100 Bioanalyzer (Santa Clara, CA). Each sample contained approximately 200 pg/µl.
RT–PCR
cDNA was synthesized from mRNA with iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA). Amplification and analysis of the cDNA fragments were performed with CFX96 Real-Time PCR. Cycling conditions were initial denaturation at 95 °C for 5 min, followed by 44 cycles consisting of 10 s annealing and extension at 60 °C. The PCR primers for TGF-β1, TGF-β2, GAPDH, and ACTIN were designed from published human gene sequences (Table 1). These two housekeeping genes were chosen because they were the most established and reliable genes used in PCR analysis particularly in HTFs [32-34]. Each sample was analyzed in triplicate. TGF-β1 and TGF-β2 mRNA levels were measured as threshold cycle (CT) values. Data generated from each PCR were analyzed with CFX Manager (Bio-Rad).
Table 1. Human primer sequences used for RT–PCR.
| Gene | Accession number | Primers (5’-3’) | nM |
|---|---|---|---|
| TGF-β1 |
NM_000660.5 |
F: CTCGCCAGAGTGGTTATCTT |
50 |
| R: AGTGTGTTATCCCTGCTGTCA |
|||
| TGF-β2 |
NM_003238.3 |
F: GAGGGATCTAGGGTGGAA |
50 |
| R: GCTGTGCTGAGTGTCTGAA |
|||
| GAPDH |
NM_002046.5 |
F: GAAGGTGAAGGTCGGAGTC |
50 |
| R: GAAGATGGTGATGGGATTTC |
|||
| ACTIN |
AK225414.1 |
F: CATGTACGTTGCTATCCAGGC |
50 |
| R: CTCCTTAATGTCACGCACGAT |
GenBank accession numbers are available at NCBI.
Protein extraction and quantification of HTFs
HTFs were seeded in six-well plates at a density of 3×105 cells/well and were treated with 0.5 mg/ml of ranibizumab for 48 h. The wells that served as the control were treated with control IgG antibody with the same concentration. Supernatants were collected after 48 h incubation in serum-free culture media and spun at 1000 ×g for 15 min in 15 °C to remove particulates. Total soluble proteins in samples were quantified with the NanoDrop 1000 Spectrophotometer (Thermo Scientific) and immediately stored at −80 °C until use. Samples were standardized to 1 mg/ml to normalize the enzyme-linked immunosorbent assay (ELISA) results.
Activation on latent TGF-β1 and TGF-β2
To activate latent TGF-β1 and TGF-β2 to the immunoreactive forms detectable by the assay, the activation procedure was conducted. For 100 µl of sample, 20 μl of 1 N HC was added, mixed well, and incubated for 10 min. Then, 13 μl of 1.2 N NaOH/0.5 M HEPES was added to neutralize the acidified samples. The mixture was then mixed well and assayed immediately.
ELISA for TGF-β1 and TGF-β2
The supernatants were analyzed in triplicate for TGF-β1 and TGF-β2 protein with solid phase sandwich ELISAs (Cusabio Biotech Co, Wuhan, China). Purified human TGF-β1 and TGF-β2 (Cusabio Biotech Co) were used as standards. Standards and samples were incubated in a high-binding 96-well microtiter plate precoated with antibody specific to TGF-β1 and TGF-β2 for 2 h at 37 °C. Subsequently, liquid from each well was removed, and the biotin antibody that had been diluted 1:100 in biotin antibody diluent was added to each well. Plates were incubated for 1 h at 37 °C. Then, the plates were washed with wash buffer for three cycles. Horseradish peroxidase (HRP)-avidin working solution that had been diluted 1:100 with HRP diluent was added to each well, and the plate was incubated for 1 h at 37 °C. After the final washing steps, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added to each well, and the plates were incubated for 15–30 min at 37 °C in the dark. Finally, stop solution was added to each well, and within 5 min, the optical density was determined with Victor X5, Multilabel Reader Spectrophotomer (Perkin Elmer) at 450 nm.
Statistical analysis
Data were presented as means ± standard deviation (SD). Statistical evaluation of significant differences was performed using the Kruskal–Wallis test for mean comparison and Mann–Whitney U test for multiple comparisons. P values less than 0.05 were considered statistically significant. The statistical analysis was performed with SPSS version 16.0.
Results
Immunofluorescence for vimentin antibody staining
Immunocytochemistry assay of vimentin, a special cell marker of HTF, was used in our study to identify HTFs. As shown in Figure 1, the fibroblasts isolated from Tenon’s capsule expressed vimentin in the cytoplasm, which indicated HTFs in vitro. Fibroblast produced vimentin stained positively green. Nuclei stained with DAPI were blue.
Figure 1.
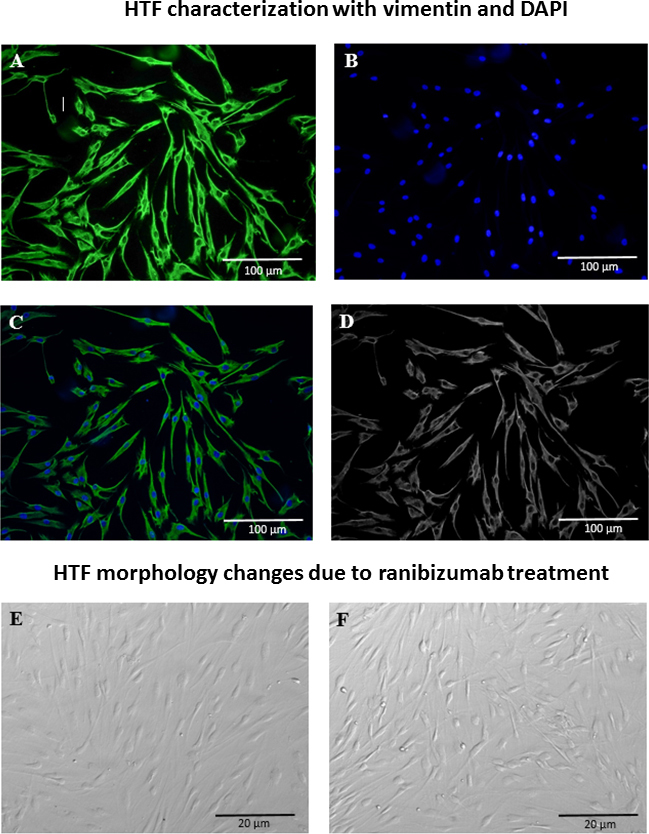
Characterization of human Tenon’s fibroblast by vimentin and DAPI staining and cells morphological changes due to ranibizumab treatment. A: Cytoplasm stained in green (Vimentin). B: Nucleus stained in blue (DAPI). C: Merge. D: Monochrome. E: Untreated HTF. F: HTF treated with Ranibizumab 0.5 mg/ml.
Effects of ranibizumab on HTF viability
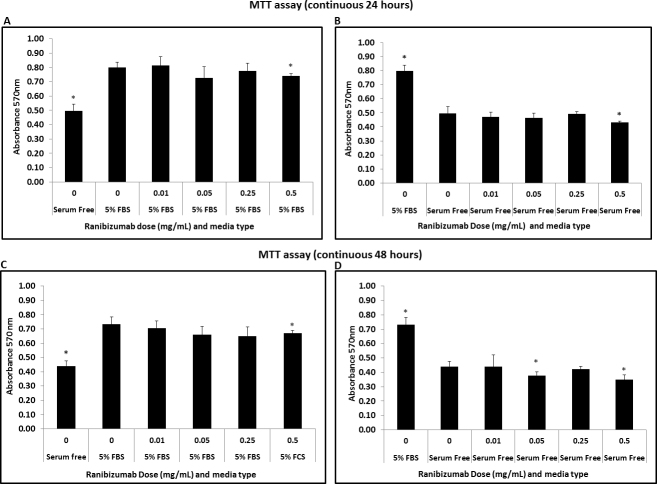
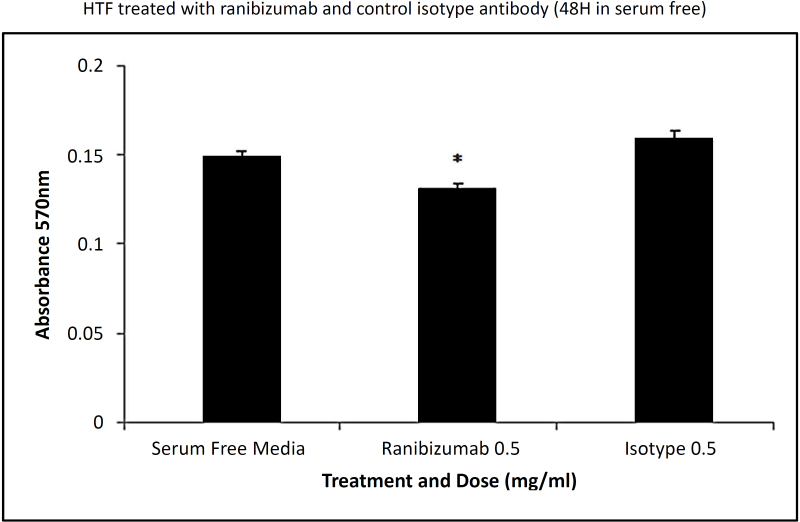
The untreated HTFs appeared as spindle-shaped cells (Figure 1E) as observed in the phase contrast pictures. This shape did not change after treatment with ranibizumab (Figure 1F). The effect of ranibizumab on Tenon’s fibroblast viability was quantified with the MTT assay to determine the number of viable cells (Figure 2). Application of ranibizumab caused a significant decrease in HTF viability only at 24 and 48 h. At 72 h, HTF showed a non-specific proliferation trend following the treatment due to prolong starvation and exposure to ranibizumab. For all concentrations, cell viability was significantly reduced at the human intravitreal dose of 0.5 mg/ml. At 24 h incubation, a statistically significant decrease in the number of fibroblasts was observed with 0.5 mg/ml of ranibizumab (p<0.05) in cells with 5% FBS; see Figure 2A. In the serum-free conditions, a significant reduction in the ranibizumab-treated fibroblasts was observed at the 0.5 mg/ml concentration (p<0.05); Figure 2B. At 48 h, a statistically significant decrease in HTF viability was observed at the 0.5 mg/ml concentration (p<0.05) in cells with 5% FBS; see Figure 2C. Meanwhile in the serum-free condition, a relevant reduction in viable HTFs was observed at 0.05 mg/ml and 0.5 mg/ml (p<0.05); see Figure 2D. In this investigation, we found that ranibizumab caused a significant reduction in HTF viability at 24 and 48 h incubation, but not at 72 h. However, the degree of reduction was much higher at 48 h compared to 24 h. This showed that ranibizumab exerts its optimum antiproliferative property at 48 h. With regard to serum and serum-free media, a greater impact of ranibizumab on HTF viability was observed in the serum-free media. To confirm that the fibroblast death was specific to ranibizumab and not due to a nonspecific consequence of high antibody concentration, we compared the viability assays of ranibizumab with those of an isotype control antibody. Experiments were performed in serum-free conditions that compared the effect of 0.5 mg/ml ranibizumab with 0.5 mg/ml of the isotype control antibody. The isotype control antibody had no significant effect on the number of viable cells. There was a statistically significant decrease in the number of viable fibroblasts with the ranibizumab-treated cells only (p<0.05); see Figure 3.
Figure 2.
Ranibizumab effects on HTF’s viability in different concentrations and conditions (incubation time and culture media). A: Reduction in MTT absorbance in HTFs treated with ranibizumab at concentration 0.5 mg/ml in 5% FBS media conditions (continuous 24 h; p <0.05; n=3) *p <0.05 with respect to cells in media with 5% FBS with no ranibizumab treatment. B: Reduction in MTT absorbance in HTFs treated with ranibizumab at concentration 0.5 mg/ml in cells in serum-free media (continuous 24 h; p <0.05; n=3).*p<0.05 with respect to cells in serum-free media with no ranibizumab treatment. C: Reduction in MTT absorbance at 0.5 mg/ml ranibizumab in cells in media with 5% FBS. (continuous 48 h; p <0.05; n=3) *p<0.05 with respect to cells in media with 5% FBS with no ranibizumab treatment. D: Reduction in MTT absorbance at ranibizumab concentration of 0.05 mg/ml and 0.5 mg/ml in cells in serum-free media (continuous 48 h; p<0.05; n=3).*p<0.05 with respect to cells in serum-free media with no ranibizumab treatment.
Figure 3.
Comparison between the effects of ranibizumab and control antibody on HTFs (continuous 48 h) in serum-free condition. Ranibizumab 0.5 mg/ml induced significant human Tenon’s fibroblast (HTF) death at 48 h in serum-free conditions compared to the control isotype (n=3).
Effect of ranibizumab on TGF-β1 and TGF-β2 mRNA expression
Due to the important role of TGF-β1 and TGF-β2 in scar formation, we further evaluated the quantitative mRNA level of these growth factors using SYBR Green PCR technology. TGF-β1 mRNA was significantly downregulated in cultures treated with ranibizumab at 0.5 mg/ml for 48 h. However, ranibizumab treatment showed no significant impact on the TGF-β2 level (p<0.05); see Figure 4.
Figure 4.
TGF-β1 and TGF-β2 mRNA investigated with RT–PCR (continuous 48 h) in serum-free conditions. Ranibizumab regulated transforming growth factor-β1 (TGF-β1) mRNA expression in vitro. Human Tenon’s fibroblasts (HTFs) treated with ranibizumab concentration 0.5 mg/ml demonstrated significant decrease in the mRNA level of TGF-β1 (p<0.05; n=3). However no significant changes distinguished the mRNA level of TGF-β2 (p<0.05; n=3).
Effect on TGF-β1 and TGF-β2 protein expression
A relevant increase in the production of TGF-β1 and TGF-β2 was observed. Protein expression of TGF-β1 in ranibizumab increased from 2.10 ng/ml ± 0.01211 (p<0.01) in the control cultures to 2.20 ng/ml ± 0.0179 in the treated cultures; see Figure 5A. Additionally, the TGF-β2 level in the ranibizumab-treated cultures increased from 1.48 pg/ml ± 0.02811 (p<0.05) in the control cultures to 1.56 ng/ml ± 0.01452 in the treated cultures; see Figure 5B.
Figure 5.
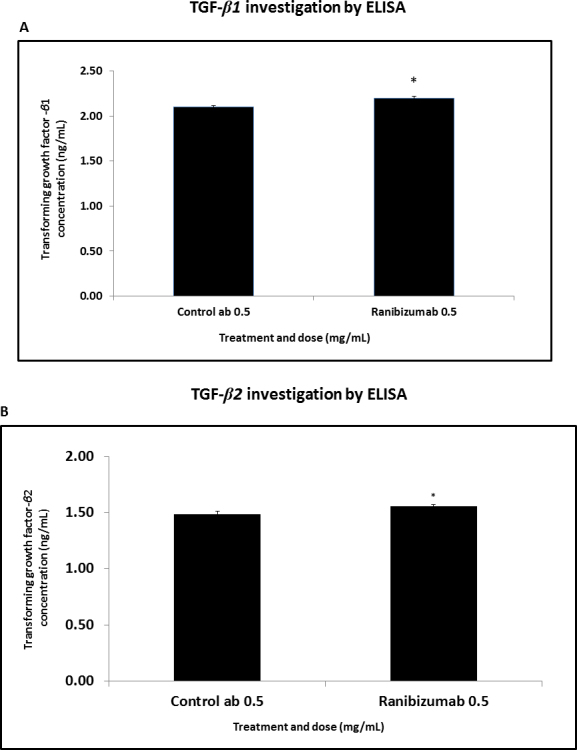
TGF-β1 and TGF-β2 protein evaluated with ELISA (continuous 48 h) in serum-free condition. A: Human Tenon’s fibroblasts (HTFs) treated with the 0.5 mg/ml ranibizumab concentration demonstrated significant increase in transforming growth factor- β 1 (TGF-β1) levels compared to the control antibody (p<0.01; n=3). B: HTFs treated with the 0.5 mg/ml ranibizumab concentration for 48 h in serum-free conditions demonstrated a significant increase in the TGF-β2 levels compared to the control antibody (p<0.05; n=3).
Discussion
Our study was driven by the need to find effective antifibrotic agents with less toxicity to prevent scarring after glaucoma filtration surgery. We explored the antifibrotic effect of ranibizumab on cultured human Tenon’s fibroblasts. The use of anti-VEGF as a wound modulator agent has increased the overall success of trabeculectomy. However, to date, anti-VEGF has been routinely applied with other known antimetabolites such as MMC and 5-FU. The success of a single application of ranibizumab thus far has not been elucidated. By understanding the actual influence of ranibizumab on cell behavior, we will possibly offer an appropriate adjustment of the treatment.
Ranibizumab is anti-VEGF agent that exhibits an inhibitory effect on various cells in vitro. Although ranibizumab is widely used as an antiangiogenic and antiproliferative agent, the underlying mechanism is relatively unknown. Deissler et al. described that ranibizumab significantly reverses cell proliferation and migration stimulated by VEGF in immortalized bovine retinal endothelial cells (iBRECs) [35]. Meanwhile, Lowe et al. examined the capability of ranibizumab to bind to recombinant human VEGF165, VEGF121, and VEGF110, and therefore inhibit VEGF-A induced human umbilical vein endothelial cells (HUVECs) [36]. Apart from in vitro analyses, ranibizumab has been applied in a prospective randomized pilot study and showed effectiveness in lessening bleb vascularity with more diffuse blebs [28].
Wound healing consists of a series of events that is highly influenced by numerous cytokines and growth factors. To the best of our knowledge, we are the first to describe the potential underlying mechanism of ranibizumab on HTFs, specifically the effects on cell viability and TGF-β1 and TGF-β2 production. Our in vitro analysis revealed that ranibizumab significantly reduces HTF viability at an intravitreal dose. Since TGF-β isoforms are known as a key mediator in fibrosis and are critically involved in post-operative scarring [37], we assume that ranibizumab may somehow have a direct effect on TGF-β production. Unfortunately, we found that a single application of ranibizumab is inadequate to arrest the production of TGF-β1 and TGF-β2 inclusively. Thus, based on this evidence, we pointed out ranibizumab as a potential anti-VEGF that can reduce HTF viability; however, the combinatory regime with another antimetabolite seem imperative to inhibit the wound healing process. We believe that the synergistic effect between ranibizumab and the other agent triggers an alternative distinct mechanism in minimizing the extracellular matrix accumulation at the filtering bleb. These findings are consistent with published data that describe the importance of anti-VEGF and adjunctive therapy. Kahook reported that a combination of ranibizumab with MMC resulted in diffuse blebs with less vascularity compared to MMC alone [28]. Meanwhile, How et al. reported that a combination of bevacizumab with 5-FU offers a superior antifibrotic effect compared to monotherapy [38]. We believe that the synergistic action between anti-VEGF with other antimetabolites generates a different antiscarring mechanism that could offer safer and more effective therapeutic approaches.
TGF-β is a cytokine with many roles, as well as being a key player in the wound healing response and fibrosis. In tissue injury, TGF-β is released mainly by macrophages and by circulating lymphocytes, platelets, and fibroblasts [39,40]. Other proinflammatory and profibrogenic soluble factors, for example, platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), insulin-like growth factor-1 and -2 may be also locally released from damaged blood vessels or subconjunctival fibroblasts during the inflammatory phase of wound healing. Later, in the proliferative phase fibroblasts are activated to lay down extracellular matrix components. Tenon’s fibroblasts under autocrine regulation release several other growth factors including VEGF, basic fibroblast growth factor (bFGF), and PDGF [13, 19]. Therefore, targeting VEGF has opened a new window in the scar limitation strategy deprived of the importance of other growth factors.
The antimetabolites 5-FU and MMC continue to be the backbone of antiscarring treatments. Alastair et al. described that these antimetabolites may possibly act directly on circulating macrophages and resident fibroblasts [41]. Meanwhile, ranibizumab is an anti-VEGF targeted directly to arrest fibroblast angiogenesis and proliferation. Xiong et al. suggested in a meta-analysis study that antimetabolites are more effective in lowering IOP due to the nonselective cell death and apoptosis effects compared to anti-VEGF alone although application of anti-VEGF with antimetabolites demonstrated greater antifibrotic effects compared with monotherapy with anti-VEGF or antimetabolite alone [42]. This emphasized that a single application of anti-VEGF does not carry much weight in reducing scarring at the filtering bleb. Therefore, the synergistic effect between anti-VEGF and antimetabolites seems important to produce a great antifibrotic impact post-trabeculectomy. More work is needed to improve our understanding of the related pathway.
Conclusion
In summary, our study demonstrated that ranibizumab had inhibitory effects on HTF viability. However, the wound healing response is dynamic, with cytokines and growth factors appearing and disappearing at different time points. This is certainly a challenge for us to identify and understand their effects individually, including all the possible interactions of the multiple cytokines in the wound healing milieu. Due to that reason, we suggest synergistic therapy is the best antiscarring approach for enhancing the success of trabeculectomy.
Acknowledgments
This research was funded by The Ministry of Science, Technology and Innovation (MOSTI), Malaysia. Project code: 100-RMI/SF16/6/2 in collaboration with Universiti Malaya HIR grant: H-20001-00-E000058.
References
- 1.Hitchings RA, Grierson I. Clinico pathological correlation in eyes with failed fistulizing surgery. Trans Ophthalmol Soc U K. 1983;103:84–8. [PubMed] [Google Scholar]
- 2.Stamper RL, McMenemy M, Lieberman M. Hypotonous maculopathy after trabeculectomy with subconjunctival 5-fluorouracil. Am J Ophthalmol. 1992;114:544–53. doi: 10.1016/s0002-9394(14)74481-2. [DOI] [PubMed] [Google Scholar]
- 3.Parrish R, Minckler D. Late endophthalmitis”–filtering surgery time bomb? Ophthalmology. 1996;103:1167–8. doi: 10.1016/s0161-6420(96)30527-7. [DOI] [PubMed] [Google Scholar]
- 4.Jampel HD, Pasquale LR, Dibernardo C. Hypotony maculopathy following trabeculectomy with mitomycin C. Arch Ophthalmol. 1992;110:1049–50. doi: 10.1001/archopht.1992.01080200029011. [DOI] [PubMed] [Google Scholar]
- 5.Costa VP, Spaeth G, Eiferman R, Orengo-Nania S. Wound healing modulation in glaucoma filtration surgery. Ophthalmic Surg. 1993;24:152–70. [PubMed] [Google Scholar]
- 6.Daniels JT, Occleston NL, Crowston JG, Cordeiro MF, Alexander RA, Wilkins M, Porter R, Brown R, Khaw PT. Understanding and controlling the scarring response: the contribution of histology and microscopy. Microsc Res Tech. 1998;42:317–33. doi: 10.1002/(SICI)1097-0029(19980901)42:5<317::AID-JEMT3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Shah M, Foreman DM, Ferguson M. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 8.Levine JH, Moses HL, Gold LI, Nanney LB. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993;143:368–80. [PMC free article] [PubMed] [Google Scholar]
- 9.Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-beta in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993;34:477–87. [PubMed] [Google Scholar]
- 10.Connor TB, Jr, Roberts AB, Sporn M, Danielpour D, Dart LL, Michels RG, Bustros S, Enger C, Kato H, Lansing M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989;83:1661–6. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khaw PT, Occleston N, Schultz G, Grierson I, Sherwood M, Larkin G. Activation and suppression of fibroblast function. Eye (Lond) 1994;8:188–95. doi: 10.1038/eye.1994.44. [DOI] [PubMed] [Google Scholar]
- 12.Kay EP, Lee HK, Park KS, Lee SC. Indirect mitogenic effect of transforming growth factor-beta on cell proliferation of subconjunctival fibroblasts. Invest Ophthalmol Vis Sci. 1998;39:481–6. [PubMed] [Google Scholar]
- 13.Tripathi RC LI J, Chalam K, TRIPATHI BJ. Expression of growth factor mRNAs by human Tenon's capsule fibroblasts. Exp Eye Res. 1996;63:339–46. doi: 10.1006/exer.1996.0123. [DOI] [PubMed] [Google Scholar]
- 14.Li DQ, Lee S, Tseng S. Differential expression and regulation of TGF-beta1, TGF-beta2, TGF-beta3, TGF-betaRI, TGF-betaRII and TGF-betaRIII in cultured human corneal, limbal, and conjunctival fibroblasts. Curr Eye Res. 1999;19:154–61. doi: 10.1076/ceyr.19.2.154.5321. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 16.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–8. doi: 10.1097/00006982-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 18.Tolentino MJ, Miller JW, Gragoudas ES, Chatzistefanou K, Ferrara N, Adamis AP. Vascular endothelial growth factor is sufficient to produce iris neovascularization and neovascular glaucoma in a nonhuman primate. Arch Ophthalmol. 1996;114:964–70. doi: 10.1001/archopht.1996.01100140172010. [DOI] [PubMed] [Google Scholar]
- 19.Van Bergen T, Vandewalle E, Van de Veire S, Dewerchin M, Stassen J-M, Moons L, Stalmans I. The role of different VEGF isoforms in scar formation after glaucoma filtration surgery. Exp Eye Res. 2011;93:689–99. doi: 10.1016/j.exer.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Van Bergen T, Van de Veire S, Van de Vel I, Moreau H, Dewerchin M, Maudgal PC, Zeyen T, Spileers W, Moons L. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009;50:5217–25. doi: 10.1167/iovs.08-2662. [DOI] [PubMed] [Google Scholar]
- 21.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 22.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–47. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen QD, Shah S, Tatlipinar S, Do DV, Anden EV, Campochiaro PA. Bevacizumab suppresses choroidal neovascularisation caused by pathological myopia. Br J Ophthalmol. 2005;89:1368–70. [PMC free article] [PubMed] [Google Scholar]
- 24.O'Neill EC, Qin Q, Van Bergen NJ, Connell PP, Vasudevan S, Coote MA, Trounce IA, Wong TL, Crowston JG. Antifibrotic activity of bevacizumab on human Tenon's fibroblasts in vitro. Invest Ophthalmol Vis Sci. 2010;51:6524–32. doi: 10.1167/iovs.10-5669. [DOI] [PubMed] [Google Scholar]
- 25.Kapetansky F, Pappa K, Krasnow M, Baker N, Francis C. Subconjunctival injection (s) of bevacizumab for failing filtering blebs. Invest Ophthalmol Vis Sci. 2007;Vol 48.837 [Google Scholar]
- 26.Grewal DS, Jain R, Kumar H, Grewal SPS. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy: a pilot study. Ophthalmology. 2008;115:2141–5. doi: 10.1016/j.ophtha.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 28.Kahook MY. Bleb morphology and vascularity after trabeculectomy with intravitreal ranibizumab: a pilot study. Am J Ophthalmol. 2010;150:399–403. doi: 10.1016/j.ajo.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Nilforushan N, Yadgari M, Kish SK, Nassiri N. Subconjunctival bevacizumab versus mitomycin C adjunctive to trabeculectomy. Am J Ophthalmol. 2012;153:352–7. doi: 10.1016/j.ajo.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Park H-YL, Kim JH, Park CK. VEGF induces TGF-β1 expression and myofibroblast transformation after glaucoma surgery. Am J Pathol. 2013;182:2147–54. doi: 10.1016/j.ajpath.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Khaw PT, Ward S, Porter A, Grierson I, Hitchings R, Rice N. The long-term effects of 5-fluorouracil and sodium butyrate on human Tenon's fibroblasts. Invest Ophthalmol Vis Sci. 1992;33:2043–52. [PubMed] [Google Scholar]
- 32.Kottler UB, Jünemann AG, Aigner T, Zenkel M, Rummelt C, Schlötzer-Schrehardt U. Comparative effects of TGF-β1 and TGF-β2 on extracellular matrix production, proliferation, migration, and collagen contraction of human Tenon's capsule fibroblasts in pseudoexfoliation and primary open-angle glaucoma. Exp Eye Res. 2005;80:121–34. doi: 10.1016/j.exer.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Seet LF, Su R, Toh LZ, Wong TT. In vitro analyses of the anti‐fibrotic effect of SPARC silencing in human Tenon’s fibroblasts: comparisons with mitomycin C. J Cell Mol Med. 2012;16:1245–59. doi: 10.1111/j.1582-4934.2011.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jing J, Li P, Li T, Sun Y, Guan H. RNA Interference Targeting Connective Tissue Growth Factor Inhibits the Transforming Growth Factor-β 2 Induced Proliferation in Human Tenon Capsule Fibroblasts. J Ophthalmol. 2013;2013:354798. doi: 10.1155/2013/354798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deissler H, Lang S, Lang G. VEGF-induced effects on proliferation, migration and tight junctions are restored by ranibizumab (Lucentis) in microvascular retinal endothelial cells. Br J Ophthalmol. 2008;92:839–43. doi: 10.1136/bjo.2007.135640. [DOI] [PubMed] [Google Scholar]
- 36.Lowe J, Araujo J, Yang J, Reich M, Oldendorp A, Shiu V, Quarmby V, Lowman H, Lie S, Gaudreault J. Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitro and in vivo. Exp Eye Res. 2007;85:425–30. doi: 10.1016/j.exer.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Cordeiro MF, Siriwardena D, Chang L, Khaw PT. Wound healing modulation after glaucoma surgery. Curr Opin Ophthalmol. 2000;11:121–6. doi: 10.1097/00055735-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 38.How A, Chua JLL, Charlton A, Su R, Lim M, Kumar RS, Crowston JG, Wong TT. Combined treatment with bevacizumab and 5-fluorouracil attenuates the postoperative scarring response after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2010;51:928–32. doi: 10.1167/iovs.09-3949. [DOI] [PubMed] [Google Scholar]
- 39.Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–4. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–60. [PubMed] [Google Scholar]
- 41.Lockwood A, Brocchini S, Khaw PT. New developments in the pharmacological modulation of wound healing after glaucoma filtration surgery. Curr Opin Pharmacol. 2013;13:65–71. doi: 10.1016/j.coph.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Chua BE, Nguyen DQ, Qin Q, Ruddle JB, Wells AP, Niyadurupola N, Gupta V, Wong TT, Coote MA, Crowston JG. Bleb vascularity following post‐trabeculectomy subconjunctival bevacizumab: a pilot study. Clin Experiment Ophthalmol. 2012;40:773–9. doi: 10.1111/j.1442-9071.2012.02798.x. [DOI] [PubMed] [Google Scholar]