Abstract
For the 365 children diagnosed with acute myeloid leukemia in the US annually, 5-year survival for patients on COG trials with low, intermediate, and high risk disease is 83%, 62%, and 23%, respectively. Recent advances include improved therapeutic stratification, improved survival with dose intensification, and further elucidation of the heterogeneity specific to childhood AML. These discoveries now guide current strategy incorporating targeted agents to pathways specific to childhood AML as well as evaluating methods to increase the sensitivity of the leukemic stem cell, first in Phase II feasibility trials followed by Phase III efficacy trials of the most promising agents. Acute myeloid leukemia in children, though with similar subgroups to adults, remains uniquely different based upon quite different prevalence of subtypes as well as overall response to therapy. The Children's Oncology Group's research agenda builds upon earlier efforts to better elucidate the leukemogenic steps distinct to childhood AML in order to more scientifically develop and test novel therapeutic approaches to the treatment and ultimate cure for children with this disorder.
Keywords: acute myeloid leukemia, children's oncology group
STATE OF DISEASE—CLINICAL
Overview
Myeloid neoplasms, constituting 20% of childhood leukemias annually of which the majority are AML, can be subdivided into three differing biologically driven approaches of treatment. Two smaller groups include (1) PML-RARA acute promyelocytic leukemia (APL) in which the unique sensitivity to two agents, all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), is leveraged and (2) the myeloid leukemias of Down syndrome (MLDS) in which the leukemogenic sentinel mutation, GATA1, confers its unique clinical spectrum and contributes to its increased chemosensitivity.
The majority of pediatric patients have AML that, although present in quite different subtype proportions from adults with AML, have many of the same biologic etiologies and often similar risk impact seen in adult AML. The key differences, as also described in the recent international panel consensus statement with participants from all the major childhood cancer cooperative groups [1], lie firstly in the prevalence of subtypes which in turn impacts prioritization of therapeutic approaches. Secondly, host differences are critically important for quite different reasons including (1) the child's improved tolerance of intensive therapies as compared to adults and (2) the child's greater risk of long-term sequelae and their many years of potential impact. Though childhood AML constitutes just 6% of all AML cases [2], the high clinical trial accrual rate and uniformity of care across COG institutions has allowed the conduct of large clinical trials and further clarified treatment efficacy and tolerability in both children and young adults ≤29 years of age. Further distinguishing the children with AML from adults is the high prevalence of MLL-related leukemias, particularly in the <2 year of age group, and the lower prevalence of adverse factors such as cytogenetic (e.g., abnormal 3, 5qdel, monosomy 7) [3] and molecular risk characteristics (e.g., FLT3-ITD, IDH1, DNMT3) [4–6]. While similarly prognostic in children, the degree and overall outcome does vary from adults and for a variety of reasons including better intensive therapy tolerance.
Staging/Stratification
Childhood AML, excluding APL and MLDS, has an evolving risk group stratification based upon leukemic cell biology (cytogenetic and molecular abnormalities) and response to therapy [7]. Treatment stratification based upon risk is a recent step in AML therapy, and as such, definitions of risk groups vary among cooperative groups although some basic principles are found in most. Common to the low risk (LR) classifications are the core-binding factor (CBF) leukemias (t(8;21) & Inv16) and to high risk (HR) chromosomal deletions, for example, –5/5qdel and monosomy 7. Intermediate risk (IR) includes all others. Several groups incorporate APL t(15;17) patients into their low risk outcomes as opposed to their exclusion by the COG. Recently added to HR designations is the FLT3 internal tandem duplication (FLT3/ITD). Induction response has been added to many risk classifications, primarily using morphologic criteria; COG has now included minimal residual disease (MRD) by flow cytometric immunophenotyping [8]. COG has recently added two molecularly detected mutations, CEBPa and NPM, to the LR group. Other molecularly and cytogenetically identified mutations have a variable prognostic impact and seem often to be therapy and/or age dependent.
Current Outcome
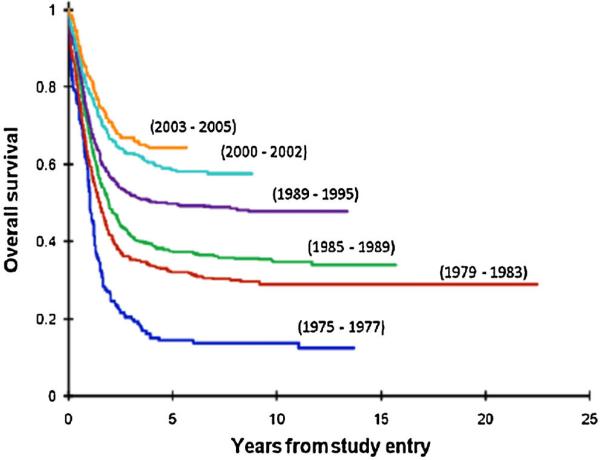
Survival of patients with AML has improved over the past 20–30 years with childhood overall survival (OS) now 60–70% and event-free survival (EFS) exceeding 50% [3,9] (Fig. 1) similar to that seen in other recent pediatric AML trials [10–13]. This exceeds that seen in recent adult AML trials including the SWOG study S0106 (OS 45–50%) [14], the ECOG study (35–45%) [15], and the MRC AML15 study (41–43%) [16]. Treatment-related mortality (TRM), primarily infectious, is a significant problem in patients with AML. Reports from COG AAML03P1 and the MRC AML12 show this to range from 9% to 10% in general [3,17] but significantly higher in AYA patients treated with pediatric intensive AML regimens [18]. However, relapse remains the greatest cause of failure as seen in COG trials, with 38% for CCG-2961 post-suspension and 34% for AAML03P1 [19]. For the survivors, anthracycline related cardiotoxicity is the most prevalent long-term sequelae [20–22].
Fig. 1.
Overal survival–incremental improvements over the last 40 years in COG and legacy trials in childhood AML.
STATE OF DISEASE—BIOLOGICAL
Molecular Targets
Numerous genomic alterations are implicated in disease pathogenesis and clinical outcome; however, only a minority have been exploited for therapeutic interventions. Activating mutations of the FLT3 gene as a result of internal tandem duplication (FLT3/ITD) of the dimerization domain or the missense mutation of the activation loop domain (FLT3/ALM) are common in AML though only FLT3/ITD is prognostic [4]. This prognostic factor has greater sensitivity to pro-apoptotic effects of numerous FLT3 inhibitors including sorafenib under study in AAML1031. However, accumulating data suggest the emergence of resistance to FLT3 inhibitors in the patient with FLT3/ITD may be due to the evolution of secondary FLT3/ALM mutations such as D835Y [23], and thus identifies a need to gather and compare diagnostic and relapse leukemic specimens.
Recent COG work has demonstrated that 30% of patients with CBF AML harbor one of the common variants of the KIT mutations [24]. Though not prognostic in children, these mutations lead to autonomous activation of the kinase moiety, are directly inhibited by newly emerging inhibitors [24,25], and thus remain a viable molecular target. Other mutations, including DNMT3A and IDH1/2, have been identified in older patients with AML. However, we have demonstrated that these are rare in children with AML [5,6], illustrating not all discovery from adult studies can be translated to children. Nevertheless, there remains a large patient cohort in whom no mutations are detectable. In addition, few prognostic markers are fool-proof emphasizing the need to detect additional interacting mutations. Ongoing studies, including whole genome sequencing efforts through the NCI and COG joint TARGET initiative, are aimed at defining novel molecular targets useful for outcome prediction or as therapeutic targets. Preliminary data from this initiative identified numerous novel, recurrent somatic mutations [26]. Mutations that are highly likely to be prevalent and clinically impactful are undergoing verification, frequency validation and outcome correlation in COG specimens. Further, evolution of a significant number of novel mutations were noted in relapse specimens, demonstrating that resistance may be due to either evolution of, or selection of cells with secondary mutations. Identification of mutations associated with therapeutic resistance may allow monitoring for early emergence of relapse and directed intervention. Merging of the genomic data with functional studies would help identify altered pathways and networks that can be used for therapeutic targeting.
RECENT FINDINGS
Overview
Within COG specifically, we have seen the transition from intensive-timing to dose-intensive therapy for de novo AML [3,9], the success of adding ATRA to APL therapy [27], the reduction of therapy for MLDS [28], the elucidation of transient myeloproliferative disorder (TMD) in DS [29], and the benefit of imatinib in children with CML [30]. Risk classification incorporating response by multi-dimensional flow (MDF)-based MRD was validated and is now incorporated for treatment stratification in Phase III trials [8]. The prognostic impact of the molecular mutations, CEBPA, NPM, and FLT3/ITD allelic ratio were validated [4,31,32] and also incorporated into treatment stratification. Further biologic discovery includes prevalence and prognostic impact of molecular mutations and their polymorphic variability that impacts their prognostic significance.
Clinical Achievements
For patients with de novo AML two phase III trials have been completed (AAML03P1, AAML0531) and one recently opened this past year (AAML1031). AAML03P1, piloting the intensive dosing MRC-based protocol along with feasibility testing of Gemtuzumab ozogamicin (GO), identified continued improvement trends for OS, DFS, and reduced TRM compared to prior CCG & POG trials (CCG-2891, CCG-2961, POG 9421) [3,9,33]. AAML0531 validated the benefits of response-based therapy upon remission and the greater sensitivity and specificity of MRD over the use of morphology [8,34,35]. AAML0531's randomization of GO outcome data have not yet been released, but concurrent with COG's CD33 targeting approach have been several adult groups’ similar efforts [14,16,36–38]. These saw benefit with CD33 targeting in the intermediate and favorable risk groups, predominantly composed of AML subtypes arising from more mature cells of origin, but none in patients with HR AML whose leukemias are of a more immature cell of origin.
Overall survival has improved OS through both reduction in TRM and in relapse risk (RR) leading to an improved EFS in newly updated analyses (Table I, Fig. 2). Outcomes from other groups with similar approaches include the MRC AML12 trial [10] with 10 year OS—61%, EFS 51%, RR 38%, TRM 10%, and DFS 56%, and the BFM AML 2004 trial [11] with 5 year OS and EFS 72% and 54%, respectively. When comparison is made between CCG-2961 and COG AAML03P1 outcomes, improvement trends are seen in the LR and IR groups (cytogenetic risk group only as response-based classification was not incorporated until AAML0531; Table II), consistent with the adult trials investigating GO [14,16,36–38].
TABLE I.
Overall Outcomes Over Three Consecutive Children's Oncology Group Phase III Trials
| COG AML study in de novo AML | OS | EFS | RRa | TRMa | DFS from EOI | RR from EOI | TRM from EOI |
|---|---|---|---|---|---|---|---|
| POG-9421 [29] | 50 ± 4% (n = 565) | 37 ± 4% (n = 565) | 55 ± 4% (n = 565) | 8 ± 2% (n = 565) | 43 ± 5% (n = 475) | 51 ± 5% (n = 475) | 6 ± 2% (n = 475) |
| CCG-2961 post-susp [9] | 58 ± 5% (n = 406) | 46 ± 5% (n = 406) | 41 ± 5% (n = 406) | 12 ± 3% (n = 406) | 56 ± 6% (n = 269) | 38 ± 6% (n = 269) | 6 ± 3% (n = 269) |
| COG AAML03P1 [3] | 64 ± 5% (n = 340) | 51 ± 6% (n = 340) | 40 ± 5% (n = 340) | 9 ± 3% (n = 340) | 58 ± 6% (n = 267) | 34 ± 6% (n = 267) | 8 ± 3% (n = 267) |
EOI, end of induction.
From time of study entry.
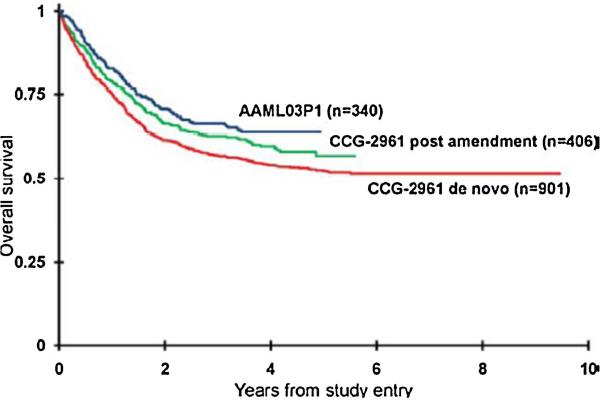
Fig. 2.
Overall survival for patients enrolled on two consecutive COG AML trials for newly diagnosed patients. CCG-2961 (pre- and post-supportive care amendments) and AAML03P1. CCG-2961 utilized the intensive timing approach and AAML03P1 applied the dose-intensive approach for induction treatment (This research was originally published in Cancer [3]).
TABLE II.
Outcomes by Cytogenetic Risk Groups in CCG-2961 and COG AAML03P1
| De novo patients | OS LR | EFS LR | DFS LR | OS IR | EFS IR | DFS IR |
|---|---|---|---|---|---|---|
| CCG-2961 post-susp [9] | 72 ± 12% (n = 60) | 60 ± 13% (n = 60) | 62 ± 14% (n = 47) | 51 ± 7% (n = 190) | 39 ± 7% (n = 190) | 48 ± 9% (n = 125) |
| COG AAML03P1 [3] | 83 ± 9% (n = 78) | 67 ± 11% (n = 78) | 66 ± 11% (n = 70) | 62 ± 7% (n = 225) | 49 ± 7% (n = 225) | 57 ± 8% (n = 171) |
Similar to adult AML trials and other pediatric trials with comparable HR definitions, HR outcome on AAML03P1 remains poor, OS 23 ± 23%, EFS 15 ± 20%, and DFS 25 ± 31%. The MRC AML12 cytogenetic HR 10 year OS outcomes [39] were within a comparable range for patients with abnormal 5q, 27%, and monosomy 7, 32%.
MRD and Outcome
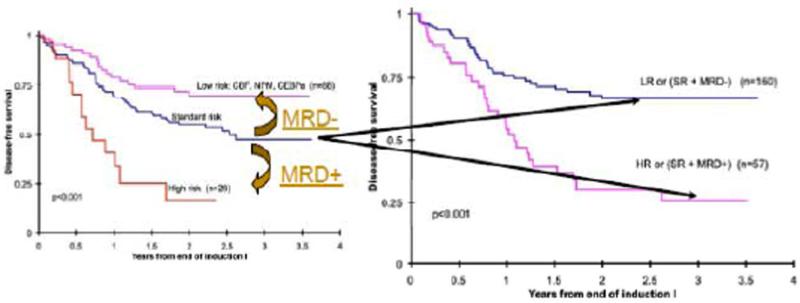
COG has examined the combined use of cytogenetics, molecular genetics, and treatment response for treatment stratification to balance relapse-free survival (RFS) with TRM building upon its own and others’ work in MRD detection and use in AML therapy [12,40–42]. This alternative risk classification reduces the risk groups to two, HR and LR, with a clear delineation of RR [8,43]. Within the previously defined IR group (the largest group in most classification schemes) MRD determination identified two distinct subgroups with quite divergent RFS (Figs. 3 and 4). The BFM group, whether due to size of the trial, risk cohort definitions, therapy used, or MRD methods did not find this same effect [42]. This impact of MRD at the end of induction 1 in COG overshadowed later marrow exams that became MRD negative and supporting its critical role in risk assignment and stratification. These risk groups are now utilized for treatment stratification in AAML1031 (Supplemental Table).
Fig. 3.
Risk group reclassification incorporating minimal residual disease assessment after induction therapy. This was found to only be significantly prognostic in the previously defined intermediate risk group and now allows improved prognostic classification and improved therapeutic stratification in the current COG trail, AAML1031.
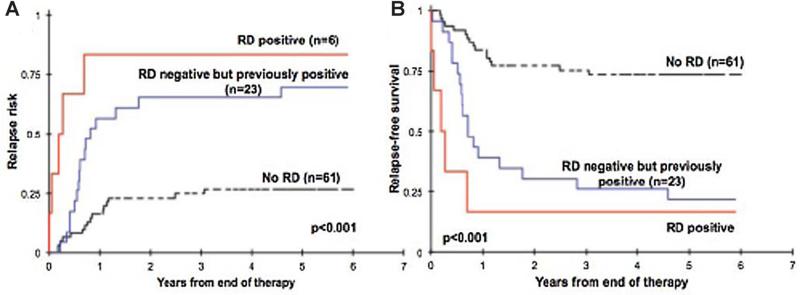
Fig. 4.
Relapse risk and relapse-free survival curves illustrate that regardless of the timing of minimal residual disease (MRD) detection, once found it persists as a prognostic factor regardless of whether it later is negative (This research was originally published in Blood [8]). Residual disease (RD) groups: RD positive–MRD detected at end of therapy. NO RD–MRD not detected at end of induction, during therapy, nor at end of therapy. RD negative but previously positive—Negative at the end of therapy but among the regimen-specified monitoring points during therapy, there was at least one positive MRD finding.
Biological Achievements
Through the use of integrated correlative biologic investigations embedded into the clinical trials combined with a robust mechanism to further investigate the expansive AML Reference lab's collection of leukemic samples from enrolled patients, efforts towards target discovery have been pursued to help prioritize new agents evaluated in COG trials.
From the latter, there are over 40 ongoing biology studies. These include defining the correlation of CD33 expression with CD33-conjugated therapy outcomes [44], defining the role of the adhesion antigen VLA-4 expression with outcome and identifying a potential novel therapeutic target [45], studies evaluating the prevalence and clinical significance of genomic/transcript alterations [5,6,24,26,32,43,46–55], and functional alterations of signal transduction pathways in AML [56,57] illuminating potential therapeutic targets and prognostic biomarkers. These and similar efforts continue in the current phase III trial.
Myeloid Leukemias of Down Syndrome (MLDS)
Despite reduction of therapy, COG DS trial A2971 maintained a high CR, 92.7 ± 6%, 5 year OS of 84 ± 6% with EFS of 79 ± 7%, and DFS 89 ± 6% [28], similar to other groups’ results for DS patients [58–63]. However, older age remained an adverse factor for those ≥4 years (EFS of 33 ± 38%). The ensuing AAML0431 attempted to improve DFS by intensifying induction II while reducing anthracycline exposure (240 mg from 350 mg/m2). Outcomes from this now closed trial await further data maturation. While TRM was not seen, 41% experienced grade 3–4 adverse events and 7% required ICU admissions suggesting a need to further reduce therapy. Findings from A2971 [64] identify that rapidity of response (d14 BM <5% blasts, 5y DFS: 86% vs. 72%, P = 0.12), and MRD clearance on d28 of induction (d28 BM <1% blasts, 5y DFS: 90% vs. 73%, P = 0.015) are strong prognostic factors in DS AML [19], and this will be validated with AAML0431. COG and others have shown that DS children with recurrent or refractory AML have a 10–25% OS [65,66], identifying a strong need to prevent relapse by improved therapy for DS patients with HR AML. A2971 further clarified the natural history of TMD in the newborn [29]. For TMD, three risk groups were derived based upon hepatomegaly and/or life-threatening symptoms, which significantly predicted 3y OS (92% vs. 77% vs. 51%) and 3y TMD-related mortality (2% vs. 0% vs. 45%).
Chronic Myeloid Leukemia (CML)
AAML0123 identified the successful control of CML in children utilizing imatinib similar to outcomes in adults (3y PFS 72 ± 6.4% and OS 92 ± 3.9%). This has resulted in a shift in the standard of care for children with CML [30].
Acute Promyelocytic Leukemia (APL)
Children in COG were enrolled on CALGB's C9710 to evaluate whether the addition of all-trans retinoic acid and an extended maintenance improved outcome. The 3 year DFS, EFS, and OS was 63, 53, and 87%, respectively, with 4/56 experiencing an early death [27]. Subsequently, analysis of these children revealed an association between FLT3 mutations and high risk APL which was highly predictive of early death (30% vs. 3%) [67]. Results from Italy (AIDA 0493) [68] showed improved survival in children with APL (CR 96%, 10 year EFS 76% and OS 89%) and led to the most recent COG APL trial which added arsenic trioxide to the modified AIDA with less anthracycline.
STRATEGIC APPROACHES TO THERAPY—FOCUS UPON COG EFFORTS
Overview
The COG Myeloid clinical trials, leveraging its ability to perform large Phase III trials in children, will continue to study and incorporate effective targeted and/or unique broadly applicable new agents onto proven backbones of therapy, while also working to reduce the complications of therapy. COG's biologic research of AML that is elucidating the mutations in the numerous altered hematopoietic pathways will be leveraged to prioritize evaluations of targeted agents that are relevant to the AML sub-types most common and/or resistant to current therapy in children. Additionally, laboratory investigation within COG is examining the underlying combination of mutations present at diagnosis coupled with ensuing clonal evolution of mutations in those who relapse to address the hypothesis that acquired mutations to a primitive leukemia stem/progenitor cell (LSC) permits the development and dominance of a resistant subclone that leads to treatment failure. Finally, efforts continue to focus upon the refinement and incorporation of MRD monitoring with greater sensitivity and specificity for relapse. These will in turn be incorporated to further improve the stratification of therapeutic consolidation approaches and permit a re-exploration of the use of maintenance and/or pre-emptive therapies.
Unique Myeloid Leukemia Subtypes—MLDS and APL
Before examining the strategic approaches for the majority of children with AML, de novo or relapsed, it is important to first distinguish the two unique forms of myeloid leukemia now treated distinctly different.
For children with MLDS (exclusive of TMD) in whom an approach to reduce therapy has been pursued over the past decade, recent COG trials now suggest a role for the incorporation of risk-stratification for therapy. For patients who are MRD negative, further therapy reduction for the 80% with a favorable projected outcome of 90% is being investigated. For the 20% with HR disease (age >4 years or monosomy 7 or persistent MRD), RR is significantly higher and if relapse occurs there is little chance of salvage. Therefore intensification to post-induction therapies similar to non-DS patients with AML will be pursued with the premise the approach will reduce relapse.
For the pediatric population with APL, the COG APL trial AAML0631 builds on the ATRA success and has recently tested ATO with the contemporary AIDA based regimen in order to further reduce anthracycline exposure; it is identical to the concurrent I-BFM APL trial without ATO which should allow further comparison. Subsequent trials look to further reduce chemotherapy utilizing the ATO and ATRA combinations as well as examine ways to reduce early mortality.
De novo and Relapsed AML
For the vast majority of children with AML, the strategic approach guiding current research focuses upon two broad areas of exploration, (1) the overcoming of the LSC inherent chemo-therapy resistance through the use of pro-apoptotic sensitizing agents (e.g., bortezomib) and (2) the direct targeting of prevalent mutations in childhood AML. This is exemplified in the open AAML1031, COG's primary trial for patients with de novo AML. This trial, a randomization of bortezomib, postulated to increase chemotherapeutic induced apoptosis in the LSC population [69,70], presumes that constitutive activation of cell-signaling pathways in the LSC population is a critical factor allowing emergence of refractory leukemic clones in AML, and particularly HR AML. Additionally, for those with FLT3/ITD mutations, adding the targeted agent, sorafenib, in place of bortezomib is being evaluated based upon promising recent work in adult AML [71]. The eventual possible combination of CD33 targeting with both or either of these awaits data release from AAML0531. Thus, the continuing pursuit and incorporation of biologic investigations in this and future clinical trials will be critical to better prioritize targets based upon their prevalence (both at diagnosis and relapse), their impact upon relapse, and the availability of agents targeting the mutations of interest. Feasibility evaluation of the new agents upon known AML retrieval backbones first in this population most in need of new approaches is intended to further permit prioritization of new agents that will then go on to Phase III evaluation in newly diagnosed children with AML.
Inherent in this strategy is the pursuit of an improved understanding of the biology of childhood AML. With the increasingly recognized heterogeneity of AML at diagnosis and the clonal evolution of AML with relapse, it is apparent that more integral and integrated approaches to biologic discovery are critical to future therapeutic design. Despite cataloguing of numerous somatic alterations in AML, very few have been utilized for clinical intervention in part due to the paucity of clinically relevant biomarkers. Significant efforts have been put forth for identification of novel alterations associated with disease outcome. Heterogeneity of outcome in those with HR (e.g., FLT3/ITD, monosomy 7) or LR (e.g., CBF AML, NPM) genomic alterations indicates the need to identify additional disease and risk modifiers that lead to these variable outcomes. This is exemplified by the variation in LSC involvement of somatic events leading to resistance variation. This is demonstrated using monosomy 7 and FLT3/ITD as markers of high risk disease where the presence of LSC heterogeneity involvement significantly correlates with outcome [72,73]. Thus a central hypothesis is that the diversity of LSC stage(s) of maturation in which somatic events occur determines disease response, where patients with somatic alterations limited to the committed myeloid precursors may have a better outcome as compared to those with involvement of more primitive LSC.
Full integration of functional genomics into clinical trial design has been hampered in part by the paucity of clinically relevant somatic alterations as well as the ability to accurately isolate and study LSC. Emerging technologies have allowed full interrogation of the genomic/epigenomic make-up of childhood AML and define the extent of LSC involvement in these somatic events. The NCI TARGET AML initiative has supported whole genome sequencing (WGS) in 230 cases of pediatric AML (nearly 600 genomes) to define novel somatic events associated with outcome as well as to identify therapeutic targets [26]. Early results have been extremely valuable identifying numerous promising targets that are undergoing frequency validation en route to clinical implementation. These novel markers will be utilized to more accurately classify risk. In addition, to fully study LSC heterogeneity, we have developed a robust in vitro culture system for the long-term growth of LSC contained in primary human AML specimens, allowing comparison of mutations in the more mature progenitor population derived following short-term culture [74], with the more primitive precursors detected giving rise to LSC only after longer term culture. Combination of discovered genomic/epigenomic events with LSC interrogation may clarify the functional genomic pathogenesis of AML and better identify high and low risk cohorts using novel biomarkers, and within risk cohorts define the response likelihood based on LSC involvement.
Identification of complementary events contributing to leukemic pathogenesis is critical for future improvements in therapeutic targeting. Despite identification of numerous genomic alterations, no single event has been shown to be causally associated with the evolution of AML [75–78]. A current model inferred from the in vivo complementation studies suggests that evolution of a mutation leading to a proliferative process (e.g., FLT3/ITD) in cells combined with a primary event that causes maturation arrest (e.g., t(8;21)) leads to an AML phenotype. Whereas early genomic events are not sufficient for evolution or maintenance of an AML phenotype, it is hypothesized that elimination of the leukemic population harboring the complementing event (Type 1 mutation) may be sufficient for long-term remission. Additionally there is emerging data that the maturational stage in which the secondary, proliferative event occurs is the determining factor in long term response, such that complementary mutations in the committed progenitor population are more responsive to conventional chemotherapy or targeted agents to the mature hematopoietic cells (e.g., anti-CD33), whereas those where both mutation types occur in the more primitive LSC are less responsive and will need LSC directed therapy (e.g., bortezomib, SCT) [79]. The NCI sponsored COG TARGET AML initiative is designed to shed light on the known and novel genomic/epigenomic events. Similar studies performed in European cooperative groups (DCOG, BFM, etc.) are pursuing similar somatic events. Overlap between different translocations, mutations, or epigenetic alterations are being investigated so as to identify potential candidate events to be further selected for verification and validation in larger patient subsets. These complementing mutations can then be used for interrogation of the LSC to ascertain the presence of each event in different progenitor cells.
AML relapse is associated with selection or evolution of novel genomic events potentially causally associated with resistance [80,81]. Identification of additional acquired genomic alterations which lead to relapse may allow monitoring and pre-emptive intervention prior to morphologic relapse. COG trial AAML1031 is treating patients with FLT3/ITD with the multi-kinase inhibitor, sorafenib and performing complete genotyping of the FLT3 gene in diagnostic and relapse specimens from these patients which will allow the identification and confirmation of mutational evolution with relapse. Substantial efforts are directed at identification of novel mutations that may contribute to disease re-emergence. The TARGET AML initiative will provide WGS data from relapse specimens, identifying novel somatic mutations absent at diagnosis, or present as a rare clone and selected through chemotherapy exposure. Initial focus on patients with known primary genomic alterations (e.g., translocations, NPM) will try to identify relapse-associated events. As part of discovery phase efforts in those lacking known primary alteration, comparison of diagnostic and relapse data will provide potential new events associated with relapse.
Initial response to chemotherapy is one of the strongest predictors of survival in AML [8] and therefore identification of prognostic factors for induction failure (IF) are critical. Patients with IF constitute the highest risk population in AML [74]. Although some genomic events are associated with higher IF risk, no predictive model yet identifies those who would be better served with target directed or experimental therapies than standard induction chemotherapy. Ongoing studies are targeting patients with IF to define the genomic make up of this high risk cohort, and define specific genomic/epigenomic changes that may be associated with IF. Identification of such biomarkers can aid in targeting those destined for IF prior to exposure to standard chemotherapy and be offered alternate therapeutic strategies, such as targeted agents directed at a specific activating mutation or more intensive or experimental chemotherapy in those without an appropriate target.
Strategy development for disease monitoring and pre-emptive therapy is an important approach to explore, as despite efforts to minimize relapse in childhood AML, nearly half of patients achieving an initial remission relapse and die of their disease. In patients who achieve remission, the incidence of disease emergence during therapy is low as most relapse in the 12–24 months following therapy. Standard surveillance utilizes serial peripheral blood counts to detect morphologic relapse. Although sensitive methodologies such as MDF and molecular assays are utilized to assess initial remission, they have not been routinely used for monitoring for disease emergence following therapy. In APL, quantitative RT-PCR is utilized during and after therapy allowing intervention upon molecular emergence of disease prior to morphologic relapse [82–85]. Such pre-emptive interventions have improved survival in patients with APL. Data utilizing molecular MRD for post-therapy monitoring in CBF AML has demonstrated that nearly all patients who have molecular MRD detected after therapy relapse within 48 months [86]. It is apparent that it will be critical to develop strategies to monitor for emergence of submorphologic disease by established and novel methodologies. In addition to the MDF assay used for response evaluation, molecular assays for disease monitoring in patients have been established including those with CBF, MLL, FLT3/ITD [87–90]. Recent sequencing technology advances is allowing development of highly sensitive assays to detect low levels of disease [91]. In development are highly sensitive genomic MRD assays using next generation sequencing methodologies to identify early markers of disease emergence prior to morphologic relapse. Use of these methods to assess for the emergence of disease at the end of therapy as well as at pre-determined intervals (e.g., every 4 months) by conventional (MDF, RT-PCR) and by high throughput sequencing would permit the employment of early intervention. Such early intervention may circumvent emergence of morphologic disease, obviating intensive interventions and may lead to improved survival as seen in APL. Exemplification of this concept has been seen when sorafenib used alone resulted in sustained remissions following the re-emergence of the leukemic clone after SCT [92], and is now leading to efforts to further study sorafenib post-SCT by utilizing MRD monitoring and early pre-emptive therapy.
Trial design strategies need to be modernized to incorporate these methods and discoveries. Novel clinical trial designs should more rapidly study promising new agents while minimizing the excess study development time and later administrative burdens upon the cooperative groups and their participating institutions. The AML phase III trial, AAML1031, has a two stage design: (1) a feasibility pilot stage confirming the ability to add new agents or revised regimens, followed by (2) the efficacy stage determining impact upon OS. Combining these into one trial eliminates administrative burdens of both a pilot and a definitive Phase III trial, and lost time between trials. The basic premise this design builds upon is that efficacy is best determined in the de novo setting before emergence of resistant dominant clones at relapse. This is further supported by recent discoveries showing the dynamic nature of AML which acquires or loses subclones between initial diagnosis and relapse [26,93]. Critical therefore is a robust and flexible early feasibility trial in the retrieval setting that can best prioritize the potential new agents to be tested in the de novo setting. Utilization of a single umbrella trial design that serially evaluates promising new agents and is devised to identify their ideal dosing when added to the most effective AML retrieval chemotherapy backbones will further hasten therapeutic advances in childhood AML. Selection of new agents to incorporate into this retrieval AML trial design should be based upon early Phase II efficacy in adult AML coupled with dose-finding Phase I results in adults and children. Leveraging discovery of prevalent resistant subclones in childhood AML, in combination with discovery of new agents found in pre-clinical or adult early phase trials, would aid in the prioritization of available new agents. Novel approaches in target identification, agent evaluation, and trial design will facilitate the more rapid evaluation of new clinical modalities and allow the earlier adoption of effective treatment to improve the overall survival and quality of life for children with myeloid leukemias.
Supplementary Material
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Creutzig U, van den Heuvel-Eibrink M, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: Recommendations from an international expert panel. Blood. 2012;120:3187–3205. doi: 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- 2.Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children adults in the United States, 2001–2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper TM, Franklin J, Gerbing R, et al. AAML03P1, a pilot study of the safety of Gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 4.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108:3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho PA, Alonzo TA, Kopecky KJ, et al. Molecular alterations of the IDH1 gene in AML: A Children's Oncology Group and Southwest Oncology Group study. Leukemia. 2010;24:909–913. doi: 10.1038/leu.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho PA, Kutny MA, Alonzo TA, et al. Leukemic mutations in the methylation-associated genes DNMT3A and IDH2 are rare events in pediatric AML: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2011;57:204–209. doi: 10.1002/pbc.23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radhi M, Meshinchi S, Gamis A. Prognostic factors in pediatric acute myeloid leukemia. Curr Hematol Malign Rep. 2010;5:200–206. doi: 10.1007/s11899-010-0060-z. [DOI] [PubMed] [Google Scholar]
- 8.Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: A report from Children's Oncology Group. Blood. 2012;120:1581–1588. doi: 10.1182/blood-2012-02-408336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children's Oncology Group Phase 3 Trial for untreated pediatric acute myeloid leukemia: A report from the Children's Oncology Group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson BE, Webb DK, Howman AJ, et al. Results of a randomized trial in children with acute myeloid leukaemia: Medical Research Council AML12 trial. Br J Haematol. 2011;155:366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 11.Creutzig U, Zimmerman M, Dworzak M, et al. Study AML-BFM 2004: Improved survival in childhood acute myeloid leukemia without increased toxicity. Blood. 2010;116:181. [Google Scholar]
- 12.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: Results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–315. doi: 10.1200/JCO.2010.30.6829. [DOI] [PubMed] [Google Scholar]
- 14.Petersdorf S, Kopecky K, Stuart R, et al. Preliminary results of Southwest Oncology Group study S0106: An international intergroup phase 3 randomized trial comparing the addition of Gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation Gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 2009;114:790. [Google Scholar]
- 15.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of Gemtuzumab ozogamicin: Results of the MRC AML15 trial. J Clin Oncol. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 17.Gibson B. Personal communication. 2012 [Google Scholar]
- 18.Canner JA, Alonzo TA, Franklin J, et al. Treatment outcomes in older adolescent and young adult (AYA) patients with newly diagnosed AML. J Clin Oncol. 2011;29(suppl) abstr 9506. [Google Scholar]
- 19.Alonzo TA. COG statistician [Google Scholar]
- 20.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. Br Med J. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 23.Man CH, Fung TK, Ho C, et al. Sorafenib treatment of FLT3-ITD_acute myeloid leukemia: Favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119:5133–5143. doi: 10.1182/blood-2011-06-363960. [DOI] [PubMed] [Google Scholar]
- 24.Pollard JA, Alonzo TA, Gerbing RB, et al. Prevalence and prognostic significance of KIT mutations in pediatric patients with core binding factor AML enrolled on serial pediatric cooperative trials for de novo AML. Blood. 2010;115:2372–2379. doi: 10.1182/blood-2009-09-241075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goemans BF, Zwaan CM, Miller M, et al. Mutations in KIT and RAS are frequent events in pediatric core-binding factor acute myeloid leukemia. Leukemia. 2005;19:1536–1542. doi: 10.1038/sj.leu.2403870. [DOI] [PubMed] [Google Scholar]
- 26.Meshinchi S, Ries RE, Farrar J, et al. Demonstration of significant clonal evolution from diagnosis to relapse in childhood AML determined by exome capture sequencing—An NCI/COG TARGET AML Study. Cancer Res. 2012;72:LB–93. [Google Scholar]
- 27.Feusner JH, Gregory J, Moser BK, et al. Dose-intensified daunorubicin induction and consolidation plus combined modality maintenance therapy for children with newly diagnosed acute promyelocytic leukemia (APL): North American Intergroup Study C9710. J Clin Oncol. 2010;28:15s. [Google Scholar]
- 28.Sorrell AD, Alonzo TA, Hilden JM, et al. Favorable survival maintained in children who have myeloid leukemia associated with Down syndrome using reduced-dose chemotherapy on Children's Oncology Group Trial A2971. Cancer. 2012;118:4806–4814. doi: 10.1002/cncr.27484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamis AS, Alonzo TA, Gerbing RB, et al. Natural history of transient myeloproliferative disorder clinically diagnosed in Down syndrome neonates: A report from the Children's Oncology Group Study A2971. Blood. 2011;118:6752–6759. doi: 10.1182/blood-2011-04-350017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champagne MA, Fu CH, Chang M, et al. Higher dose imatinib for children with de novo chronic phase chronic myelogenous leukemia: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2011;57:56–62. doi: 10.1002/pbc.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho PA, Alonzo TA, Gerbing RB, et al. Prevalence and prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia (AML): A report from the Children's Oncology Group. Blood. 2009;113:6558–6566. doi: 10.1182/blood-2008-10-184747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown P, McIntyre E, Rau R, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007;110:979–985. doi: 10.1182/blood-2007-02-076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becton D, Dahl GV, Ravindranath Y, et al. Randomized use of cyclosporinA(CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood. 2006;107:1315–1324. doi: 10.1182/blood-2004-08-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gamis AS, Alonzo TA, Gerbing RB, et al. Remission rates in childhood acute myeloid leukemia (AML) utilizing a dose-intensive induction regimen with or without Gemtuzumab ozogamicin (GO): Initial results from the Children's Oncology Group Phase III Trial, AAML0531. Blood. 2010;116:182. [Google Scholar]
- 35.Meshinchi S, Alonzo TA, Gerbing RB, et al. Identification of post-induction minimal residual disease by multidimensional flow cytometry identifies patients with AML at high risk of relapse and poor outcome-a report from the Children's Oncology Group. Blood. 2010;116:1702. [Google Scholar]
- 36.Prebet T, Etienne A, Devillier R, et al. Improved outcome of patients with low- and intermediate-risk cytogenetics acute myeloid leukemia (AML) in first relapse with Gemtuzumab and cytarabine versus cytarabine. Cancer. 2011;117:974–981. doi: 10.1002/cncr.25554. [DOI] [PubMed] [Google Scholar]
- 37.Castaigne S, Pautus C, Terre C, et al. Effect of Gemtuzumab ozogamicin on survival of adult patients with denovo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 38.Burnett AK, Russell NH, Hills RK, et al. Addition of Gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30:3924–3931. doi: 10.1200/JCO.2012.42.2964. [DOI] [PubMed] [Google Scholar]
- 39.Harrison CJ, Hills RK, Moorman AV, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment Trials AML 10 and 12. J Clin Oncol. 2010;28:2674–2681. doi: 10.1200/JCO.2009.24.8997. [DOI] [PubMed] [Google Scholar]
- 40.Sievers EL, Lange BJ, Alonzo TA, et al. Immunophenotypic evidence of leukemia after induction therapy predicts relapse: Results from a prospective Children's Cancer Group study of 252 patients with acute myeloid leukemia. Blood. 2003;101:3398–3406. doi: 10.1182/blood-2002-10-3064. [DOI] [PubMed] [Google Scholar]
- 41.van der Velden VHJ, van der Sluijis-Geling A, Gibson BES, et al. Clinical significance of flowcyto-metric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010;24:1599–1606. doi: 10.1038/leu.2010.153. [DOI] [PubMed] [Google Scholar]
- 42.Langebrake C, Creutzig U, Dworzak M, et al. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: The MRD-AML-BFM Study Group. J Clin Oncol. 2006;24:3686–3692. doi: 10.1200/JCO.2005.05.4312. [DOI] [PubMed] [Google Scholar]
- 43.Alonzo TA, Ho PA, Gerbing RB, et al. Conventional cytogenetics, molecular profiling, and flow cytometric response data allow the creation of a two-tiered risk-group system for risk-based therapy allocation in childhood AML—A report from the Children's Oncology Group. Blood. 2010;116:761. [Google Scholar]
- 44.Pollard JA, Alonzo T, Gerbing R, et al. Correlation of CD 33 expression level with disease characteristics and response to Gemtuzumab ozogamycin-containing chemotherapy in childhood AML. Blood. 2008;112:148. doi: 10.1182/blood-2011-12-398370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter RB, Alonzo TA, Gerbing RB, et al. High expression of the very late antigen-4 integrin independently predicts reduced risk of relapse and improved outcome in pediatric acute myeloid leukemia: a report from the children's oncology group. J Clin Oncol. 2010;28:2831–2838. doi: 10.1200/JCO.2009.27.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacayo NJ, Alonzo TA, Gayko U, et al. Sincle cell network profiling (SCNP)-based classifier to predict response to induction therapy in pediatric patients with de novo acute myeloid leukemia (AML): Validation study results. Blood. 2011;118:3544. doi: 10.1111/bjh.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kutny MA, Alonzo TA, Gerbing RB, et al. TET2 SNP rs2454206 (I1762V) correlates with improved survival in pediatric acute myelogenous leukemia, a report from the Children's Oncology Group. Blood. 2010;116:949. [Google Scholar]
- 48.Meshinchi S. FLT3 is a stratification factor for targeted therapy treatments in adult and pediatric AML-Phase III trials. Eur J Cancer. 2011;47:SP 126. [Google Scholar]
- 49.Kutny MA, Alonzo TA, Gerbing RB, et al. RUNX1 mutations in pediatric AML: A report from the Children's Oncology Group. Blood. 2009;114:2614. [Google Scholar]
- 50.Ho PA, Zeng R, Alonzo TA, et al. Prevalence and prognostic implications of WT1 mutations in pediatric acute myeloid leukemia (AML): A report from the Children's Oncology Group. Blood. 2010;116:702–710. doi: 10.1182/blood-2010-02-268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pulikkan JA, Peramangalam PS, Dengler V, et al. C/EBP alpha regulated microRNA-34a targets E2F3 during granulopoiesis and is down-regulated in AML with CEBPA mutations. Blood. 2010;116:5638–5649. doi: 10.1182/blood-2010-04-281600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho PA, Kuhn J, Gerbing RB, et al. WT1 synonymous single nucleotide polymorphism rs16754 correlates with higher mRNA expression and predicts significantly improved outcome in favorable-risk pediatric acute myeloid leukemia: A report from the children's oncology group. J Clin Oncol. 2011;29:704–711. doi: 10.1200/JCO.2010.31.9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berman JN, Gerbing RB, Alonzo TA, et al. Prevalence and clinical implications of NRAS mutations in childhood AML: A report from the Children's Oncology Group. Leukemia. 2011;25:1039–1042. doi: 10.1038/leu.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho PA, Kopecky KJ, Alonzo TA, et al. Prognostic implications of the IDH1 synonymous SNP rs11554137 in pediatric/adult AML: A report from the Children's Oncology Group and SWOG. Blood. 2011;118:4561–4566. doi: 10.1182/blood-2011-04-348888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen Q, Goldenson B, Silver S, et al. Integrative screening approach to identify regulators of polyploidization and targets for acute megakaryocytic leukemia. Cell. 2012;150:1–15. [Google Scholar]
- 56.Lacayo NJ, Cohen A, Westfall M, et al. Single cell network profiling (SCNP) signatures predict response to induction therapy and relapse risk in pediatric patients with acute myeloid leukemia: Children's Oncology Group (COG) Study POG-9421. Blood. 2010;116:954. [Google Scholar]
- 57.Redell MS, Ruiz MJ, Gerbing RB, et al. FACS analysis of Stat3/5 signaling reveals ligand sensitivity as a significant prognostic factor in pediatric AML: A Children's Oncology Group Report. Blood. 2011;118:938. doi: 10.1182/blood-2012-04-421925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taga T, Shimomura Y, Horikoshi Y, et al. Continuous and high-dose cytarabine combined chemotherapy in children with Down syndrome and acute myeloid leukemia: Report from the Japanese Children's Cancer and Leukemia Study Group (JCCLSG) AML 9805 Down Study. Pediatr Blood Cancer. 2011;57:36–40. doi: 10.1002/pbc.22943. [DOI] [PubMed] [Google Scholar]
- 59.Tandonnet J, Clavel J, Baruchel A, et al. Myeloid leukaemia in children with Down syndrome: Report of the registry-based French experience between 1990 and 2003. Pediatr Blood Cancer. 2010;54:927–933. doi: 10.1002/pbc.22515. [DOI] [PubMed] [Google Scholar]
- 60.Abildggaard L, Ellebaek E, Gustafsson G, et al. Optimal treatment intensity in children with Down syndrome and myeloid leukaemia: Data from 56 children treated on NOPHO-AML protocols and a review of the literature. Ann Hematol. 2006;85:275–280. doi: 10.1007/s00277-005-0045-5. [DOI] [PubMed] [Google Scholar]
- 61.Kudo K, Kojima S, Tabuchi K, et al. Prospective Study of a pirarubicin, intermediate-dose cytarabine, and etoposide regimen in children with Down syndrome and acute myeloid leukemia: The Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2007;25:5442–5447. doi: 10.1200/JCO.2007.12.3687. [DOI] [PubMed] [Google Scholar]
- 62.Creutzig U, Reinhardt D, Diekamp S, et al. AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia. 2005;19:1355–1360. doi: 10.1038/sj.leu.2403814. [DOI] [PubMed] [Google Scholar]
- 63.Rao A, Hills RK, Stiller C, et al. Treatment for myeloid leukaemia of Down syndrome: Population-based experience in the UK and results from the Medical Research Council AML 10 and AML 12 trials. Br J Haematol. 2005;132:576–583. doi: 10.1111/j.1365-2141.2005.05906.x. [DOI] [PubMed] [Google Scholar]
- 64.Sorrell A, Alonzo TA, Gerbing RB, et al. Remission marrow blast percentage predicts relapse risk in children with myeloid leukemia associated with Down syndrome. Pediatr Blood Cancer. 2012;58:1039. [Google Scholar]
- 65.Loew T, Gamis A, Smith F, et al. Down syndrome patients with relapsed acute myelogenous leukemia. Blood. 2004;104:215b. [Google Scholar]
- 66.Taga T, Saito AM, Kudo K, et al. Clinical characteristics and outcome of refractory/relapsed myeloid leukemia in children with Down syndrome. Blood. 2012;120:1810–1815. doi: 10.1182/blood-2012-03-414755. [DOI] [PubMed] [Google Scholar]
- 67.Kutny MA, Moser BK, Laumann K, et al. FLT3 mutation status is a predictor of early death in pediatric acute promyelocytic leukemia: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59:662–667. doi: 10.1002/pbc.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOP AIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005;106:447–453. doi: 10.1182/blood-2004-05-1971. [DOI] [PubMed] [Google Scholar]
- 69.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-{kappa}B is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 70.Stapnes C, Doskeland AP, Hatfield K, et al. The proteasome inhibitors bortezomib and PR-171 have antiproliferative and proapoptotic effects on primary human acute myeloid leukaemia cells. Br J Haematol. 2007;136:814–828. doi: 10.1111/j.1365-2141.2007.06504.x. [DOI] [PubMed] [Google Scholar]
- 71.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnston DL, Meshinchi S, Opheim KE, et al. Progenitor cell involvement is predictive of response to induction chemotherapy in paediatric acute myeloid leukaemia. Br J Haematol. 2003;123:431–435. doi: 10.1046/j.1365-2141.2003.04633.x. [DOI] [PubMed] [Google Scholar]
- 73.Pollard JA, Alonzo TA, Gerbing RB, et al. FLT3 internal tandem duplication in CD34+/CD33−precursors predicts poor outcome in acute myeloid leukemia. Blood. 2006;108:2764–2769. doi: 10.1182/blood-2006-04-012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loken MR, Alonzo TA, Pardo L, et al. Multidimensional flow cytometry significantly improves upon the morphologic assessment of post-induction marrow remission status—Comparison of morphology and multidimensional flow cytometry: A report from the Children's Oncology Group AML Protocol AAML0531. Blood. 2011;118:939. [Google Scholar]
- 75.Caligiuri MA, Strout MP, Gilliland DG. Molecular biology of acute myeloid leukemia. Semin Oncol. 1997;24:32–44. [PubMed] [Google Scholar]
- 76.Dash A, Gilliland DG. Molecular genetics of acute myeloid leukaemia. Best Pract Res Clin Haematol. 2001;14:49–64. doi: 10.1053/beha.2000.0115. [DOI] [PubMed] [Google Scholar]
- 77.Kelly LM, Kutok JL, Williams IR, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci USA. 2002;99:8283–8288. doi: 10.1073/pnas.122233699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelly LM, Gilliland DG. Genetics of myeloid leukemias. Annu Rev Genomics Hum Genet. 2002;3:179–198. doi: 10.1146/annurev.genom.3.032802.115046. [DOI] [PubMed] [Google Scholar]
- 79.Walter RB, Applebaum FR, Estey EH, et al. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119:6198–6208. doi: 10.1182/blood-2011-11-325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore AS, Faisal A, de Castro DG, et al. Selective FLT3 inhibition of FLT3-ITD(+) acute myeloid leukaemia resulting in secondary D835Y mutation: A model for emerging clinical resistance patterns. Leukemia. 2012;26:1462–1470. doi: 10.1038/leu.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grimwade D, Lo-Coco F. Acute promyelocytic leukemia: A model for the role of molecular diagnosis and residual disease monitoring in directing treatment approach in acute myeloid leukemia. Leukemia. 2002;16:1959–1973. doi: 10.1038/sj.leu.2402721. [DOI] [PubMed] [Google Scholar]
- 83.Jurcic JG, Nimer SD, DeBlasio T, et al. Prognostic significance of minimal residual disease detection and PML/RAR-alpha isoform type: Long-term follow-up in acute promyelocytic leukemia. Blood. 2001;98:2651–2656. doi: 10.1182/blood.v98.9.2651. [DOI] [PubMed] [Google Scholar]
- 84.Lo-Coco F, Breccia M, Diverio D. The importance of molecular monitoring in acute promyelocytic leukaemia. Best Pract Res Clin Haematol. 2003;16:503–520. doi: 10.1016/s1521-6926(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 85.Miller WH, Jr, Levine K, DeBlasio A, et al. Detection of minimal residual disease in acute promyelocytic leukemia by a reverse transcription polymerase chain reaction assay for the PML/RAR-alpha fusion mRNA. Blood. 1993;82:1689–1694. [PubMed] [Google Scholar]
- 86.Liu Yin JA, O'Brien MA, Hills RK, et al. Minimal residual disease monitoring by RT-qPCR in core-binding factor AML allows risk-stratification and predicts relapse: Results of the UK MRC AML-15 trial. Blood. 2012;120:2826–2835. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 87.Buonamici S, Ottaviani E, Testoni N, et al. Real-time quantitation of minimal residual disease in inv(16)-positive acute myeloid leukemia may indicate risk for clinical relapse and may identify patients in a curable state. Blood. 2002;99:443–449. doi: 10.1182/blood.v99.2.443. [DOI] [PubMed] [Google Scholar]
- 88.Hughes MD, Zeng R, Miller KL, et al. Augmentation of sensitivity of FLT3/ITD assay allows detection of minimal residual disease in stem cell transplant recipients-correlation with flow cytometric MRD assessment. Blood. 2010;116:1717. [Google Scholar]
- 89.Radich JP. The use of PCR technology for detecting minimal residual disease in patients with leukemia. Rev Immunogenet. 1999;1:265–278. [PubMed] [Google Scholar]
- 90.Tobal K, Newton J, Macheta M, et al. Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse. Blood. 2000;95:815–819. [PubMed] [Google Scholar]
- 91.Wu D, Sherwood A, Fromm JR, et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Translat Med. 2012;4:134ra163. doi: 10.1126/scitranslmed.3003656. [DOI] [PubMed] [Google Scholar]
- 92.Metzelder S, Shroeder T, Finck A, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia (AML) synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26:2353–2359. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 93.Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.