Abstract
Phenylpyrazole insecticides such as fipronil have been used as replacements for organophosphates. The wide application of fipronil raises concern about environmental contamination and risk for fish, birds, other non-targeted beings and human health. A sensitive, competitive indirect heterologous enzyme-linked immunosorbent assay (ELISA) was developed. Antibodies with different specificities to fipronil and its metabolites were produced. Two ELISAs having IC50 values of 0.58 ± 0.06 and 2.6 ± 0.4 ng/mL were developed. Design of different haptens and coating antigens resulted in two assays with distinct cross-reactivity patterns for structurally related compounds: 96%, 38% and 101% vs 39%, 1.4% and 25% for fipronil-sulfide, fipronil-detrifluoromethylsulfonyl and fipronil-desulfinyl, respectively. Performance of the immunoassays was demonstrated by a recovery study from spiked water, human serum and urine matrices, giving recovery values in the range of 85–111% for different concentrations. The assays demonstrated good correlation in fipronil recovery with conventional LC-MS/MS analysis. The generic assay 2265 has the sensitivity to measure fipronil and its analogs in serum at levels relevant for exposure monitoring. The assays were used to analyze human urine samples obtained from exposure studies and serum samples from rats treated with fipronil-containing diet.
Keywords: Fipronil, ELISA, polyclonal antibody, cross-reactivity, environmental contamination, human health, GABA inhibitor
INTRODUCTION
Fipronil is a highly effective broad-spectrum insecticide widely used for agricultural and non-agricultural purposes: soil injection,1–3 use on fruits, vegetables, coffee, rice and other crops, as well as for treatment of seeds.2,4 It is registered for use by lawn care and pest control operators to treat golf courses and food handling establishments. It has also found application in topical pet care products. Acting as a neurotoxic GABAergic insecticide, fipronil disrupts normal nerve function by blocking the inhibitory gamma-aminobutyric acid type A (GABAA) receptor system of insects. Such inhibition results in excessive neuronal stimulation and death of the target insect.5 It has been shown that fipronil has higher affinity toward the insect GABAA receptor than to the human or other mammalian receptors.6–9 Despite the lower fipronil affinity to the native mammalian heterooligomeric receptor, a recent report showed a similar high affinity of fipronil to the human receptor subunit β3 as to the insect GABAA receptors.6,10 In turn, the human β3 GABAA receptor is linked to neurodevelopmental disorders such as autism,11,12 Angleman syndrome,13 and epilepsy.13 Fipronil can induce some cytochrome P450s, and the in vitro cytotoxic effects of fipronil and its metabolites at high concentrations suggest the possibility of toxicity by non-neural mechanisms.7,14–16 Fipronil metabolites have also been shown to maintain bioactivity and toxicity in mammals, having 10-fold higher potency (for fipronil-desulfinyl, an environmental metabolite) at the mammalian GABA-gated chloride channel, narrowing the selectivity between insects and mammals.7 Therefore, there is theoretical evidence of possible fipronil toxicity in humans independent of its neural target.
Human exposure may occur though interactions with pets both in pet industry and at home. The principal risk to human health is likely to the brain and nervous system of young children and fetuses since exposures to the toxicants can alter organizational events in the developing brain.17–20 General symptoms of fipronil exposure are similar in rats and humans and include increased excitability, headache, dizziness, seizures, reduced food consumption, nausea and vomiting in humans.21,22 US EPA classified fipronil as moderately toxic possible human carcinogen, with negligible risk for residential application1. According to the national survey about 40% of American homes tested positive for the presence of fipronil (0.16 ng/cm2 of the floor).23 A number of case studies were registered with incidents generally of low to moderate severity, with few severe and lethal cases.1 In contrast to possible risk from human exposure, the US EPA identified a number of significant risks for the environment including acute and chronic risks to freshwater and marine invertebrates and fish species, acute lethal and reproduction risks to birds, and reproductive effects in insectivorous mammals.1 To mitigate ecological risks routine environmental monitoring could help in timely detection of environmental contamination thus preventing at-risk species from exposure. Rapid detection tools could also be applied to monitoring of population exposure occurring at their homes, thus preventing undesirable consequences of fipronil exposure.
Detection of fipronil residues (fipronil and metabolites) in environmental samples24,25 and body fluids26–29 is usually performed by well established analytical techniques, high-performance liquid chromatography (HPLC) or gas chromatography (GC) coupled to sensitive and highly selective mass detectors. Despite the advantage of being highly sensitive (LOQ 0.18–2.5 µg/L)26,28,29 and selective, instrumental methods require extensive sample preparation and clean-up procedures, that become laborious, time-consuming and expensive when a large number of samples have to be analyzed in monitoring studies. Immunoassay methods have been proven to be quantitative, relatively inexpensive, high throughput methods of choice for large screening studies of environmental contaminants,30,31 pesticides,32–34 their degradation products and biological metabolites.35,36 In the literature, only one publication37 and one patent3 have been found on the development of a fipronil assay. However, in those studies authors used only one hapten to raise the antibody and assay development. The reported assays were not characterized for their robustness to matrix variables, such as pH, ionic strength or effect of organic solvent on assay performance. In addition, they tested the cross-reactivity in their assays with different insecticides and pesticides, but these were only distantly structurally similar to fipronil.
In this study, we developed an immunoassay to fipronil and the class of fipronil metabolites. Our effort was directed to improve the sensitivity of the immunoassay compared to one published by Liu et al.,37 by applying careful design of immunizing and coating antigen haptens. We also studied the selectivity of resulting assays by testing not only compounds generally similar to fipronil, but with close structurally related molecules, chiefly environmental and biological metabolites. The resulting assays were optimized, characterized, and validated with spike-recovery studies from fortified water, human serum and urine matrices. The recovery values were also compared to conventional LC-MS/MS analysis. Finally, the developed assay was applied to the analysis of real urine samples from a human exposure study.
MATERIALS AND METHODS
Information concerning chemicals and instruments, buffers, hapten synthesis, immunization and antiserum preparation, reagents and assay buffer optimization, cross-reactivity, human serum matrix effect is detailed in the Supporting Information (SI).
Preparation of imunogens and coating antigens
Haptens with a reactive carboxylic acid group were conjugated to proteins by a sulfo-N-hydroxysuccinimide (NHS) (Haptens 1–4) method and haptens with an amine group (–NH2), by the diazotization method (Haptens 5–6). Haptens 1–4 (Table S1) were conjugated to thyroglobulin (Thy) for immunogen preparation. Haptens 1–6 were conjugated to bovine serum albumin (BSA) and conalbumin (CON) for coating antigen screening (Table S1). The conjugation protocols are detailed in the SI.
Indirect competitive ELISA
Plates were coated with the optimal concentration of antigen diluted in coating buffer (100 µL/well). After incubation for 1h at room temperature (RT), the solution was replaced with blocking buffer (200 µL/well) and plates were incubated over night at 4 °C or for 1–4 hours at RT. Plates were washed with PBST 3 times prior to sample loading. Sample solutions in assay buffer were loaded in the first row of the coated plate (in duplicates or triplicates) and diluted in subsequent rows preloaded with assay buffer (50 µL/well). An equal volume of anti-fipronil antiserum diluted in PBS was added. The plate was incubated for 1h at RT and then washed 5 times with wash buffer. Goat anti-rabbit IgG-HRP conjugate was added at 100 µL/well in a 1:10000 dilution as instructed by manufacturer. The plate was incubated for 1h at RT and washed 5 times. Substrate solution was added (100 µL/well) and was left to develop color for about 10 min. The reaction was stopped by addition of 2M H2SO4 (50 µL/well) and absorbance was read at 450 nm. SigmaPlot 11.0 software was used for curve fitting and data analysis.
Immunoassay validation
To evaluate the performance of the fipronil immunoassays three series of experiments were performed. A) Recovery from fortified samples measured by immunoassay (from industrial tap water, human serum and urine). B) Correlation of recovery values obtained by immunoassay and LC-MS/MS. C) Immunoassay application to analysis of real samples. An extensive validation was performed only for the generic assay #2265 since it could be applied for the detection of fipronil-like analytes in environmental samples, as well as in human biofluids. The cross-reactivity of the assay #2268 for fipronil-sulfide-amide makes this assay more suitable for environmental analysis. Thus #2268 was only characterized for a water matrix. The immunoassay was used to analyze urine samples obtained from people exposed to fipronil during application of flea treatment to their companion animals. Urine samples were kindly provided by Dr. Krieger from the University of California, Riverside and detailed information on the exposure study is available from Dyk et al.38 The immunoassays were also used for quantification of total concentration of fipronil and its metabolites in the serum of rats treated with fipronil-containing diet. Serum samples were provided by Dr. Strynar from the Environmental Protection Agency, and details on animal experiments are available from McMahen et al.39 Sample preparation for validation studies and analysis of samples from exposure studies are detailed in the SI.
RESULTS AND DISCUSSION
Hapten design
Fipronil is a small molecule, thus it does not elicit an immune response by itself. To be immunogenic, it is conjugated to a carrier protein of high molecular weight (i.e. Thy) in a particular orientation so that key functional groups of the target molecule are most effectively exposed. Antibodies are generally formed to the part of the molecule that is the most distal from the protein.40 It is generally accepted that a linker arm of 3–5 carbon atoms41 is the most efficient distance of hapten from carrier protein: neither too short for the hapten to be shielded by the protein, nor too long that would allow the hapten to fold back into the lipophilic core of the protein.
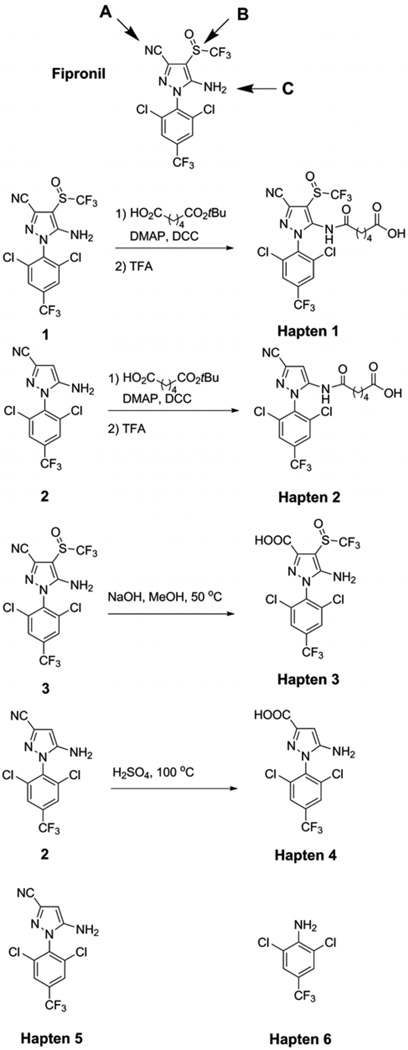
Immunizing haptens were designed to expose structural determinants A, B, C (Scheme 1) of the fipronil molecule to produce antibodies selective to these particular patterns of analyte. Haptens were synthesized from fipronil through modification of the amine group by attachment of a linker arm containing a carboxylic group (Hapten 1) and by hydrolysis of the nitrile group to carboxylic group (Hapten 3, Scheme 1). In this way, pairs of structural determinants A and B or B and C were exposed for recognition and antibody production. Two other immunizing haptens (Haptens 2 and 4) were synthesized by similar reactions starting from the fipronil analogue missing the SOCF3 group (compound 2, Scheme 1). Haptens 1 and 3 were designed to produce fipronil-selective antibodies, while haptens 2 and 4 targeted more class-selective recognition.
Scheme 1.
Synthetic routes of designed haptens. Haptens 1 and 2 were obtained by linker attachment to the amine group. Haptens 3 and 4 were obtained by hydrolysis of the nitrile group. A, B, and C refer to structural determinants exposed for antibody production.
A number of publications have shown that in competitive immunoassays the sensitivity of the assay is greatly increased when the hapten in the coating antigen is different from the immunogen.40,42–44 Therefore, additional haptens were used in the development of heterologous immunoassays (Haptens 5and 6). Fipronil analogue, compound 2 (Scheme 1), and 2,6-dichloro-4-(trifluoromethyl)aniline were attached to protein without additional modification through the amine group. Thereby, heterology was achieved by altering parent structure (Haptens 2, 4, 5 and 6) or by altering parent structural determinants (Haptens 1, 3). Haptens 5 and 6 also used a different coupling chemistry (diazotization).
Coating antigen screening
Each immunogen was used for immunization of three rabbits. Sera from 12 rabbits were screened in a three-point competitive format against 6 haptens conjugated with BSA and CON (data not shown). The serum/coating antigen pairs showing good inhibition with fipronil were then tested in a full competitive format (Table S2, selected data). As expected, the IC50 values for homologous assays were generally higher than the IC50 values for heterologous assays. For example, in the homologous competitive assay with serum 2265, the IC50 value was 54.2 µg/L (2-BSA), whereas the IC50 was 2.1 µg/L in the heterologous assay (5-CON). Among the most successful combinations of sera/coating antigens, having high assay sensitivity, sufficiently high maximum signal, good signal-to-noise ratio and slope values around 0.5–1, the pairs of #2265/5-CON and #2268/1-CON were chosen for the following studies because these assays had the highest sensitivity.
Assay optimization
Since fipronil has only moderate solubility in aqueous solutions (about 2 mg/L), the influence of organic solvent concentration in assay buffer on assay sensitivity was evaluated. The organic solvent in assay buffer is also necessary to keep hydrophobic analytes in solution and prevent their non-specific binding on the plastic containers. Only methanol was assessed because methanol is often used in sample preparation: it is less volatile than acetonitrile, yet is still easy to evaporate. It is an appropriate solvent when downstream LC/MS analysis is required. PBS buffer containing an increasing amount of methanol was tested in both assays with serum 2265 and 2268 (Fig. S1A and Fig S2A). There was no significant effect on the serum 2268 based assay sensitivity with IC50 around 3.5 µg/L. However, the maximum of absorbance was concentration dependent and a 20% decrease in maximum absorbance value was observed in the assay with 40% methanol in the buffer. In the 2265 assay, the IC50 values were very close, 0.49 and 0.51 µg/L respectively, when methanol was present at 10% or 20% in PBS buffer (prior antibody addition). However, in PBS containing 40% of methanol the sensitivity of the assay decreased dramatically with an IC50 at 9.0 µg/L, probably due to protein denaturation by the organic solvent.
An increase in ionic strength resulted in a slight improvement of the 2268 assay sensitivity, but the maximum absorbance was negatively affected (Fig. S2B). In contrast, ionic strength had a dramatic effect on the 2265 assay leading to a 10 fold increase in IC50 value and about a 50% decrease in maximum absorbance (Fig. S1B). These results suggest that the binding interaction between antibody and analyte/coating antigen is gradually suppressed in solutions with high ionic strength.
There was a slight change in sensitivity upon pH change in the range from 6.5 to 9.5, with lowest IC50 values at pH 8.5 for both the 2265 and the 2268 assays (Fig. S1C, S2C). Similar to IC50 values, the maximum absorbance also decreased constantly as pH increased for the 2265 assay, but no remarkable change was observed for the 2268 assay. The pH was retained at 7.5 for the following experiments.
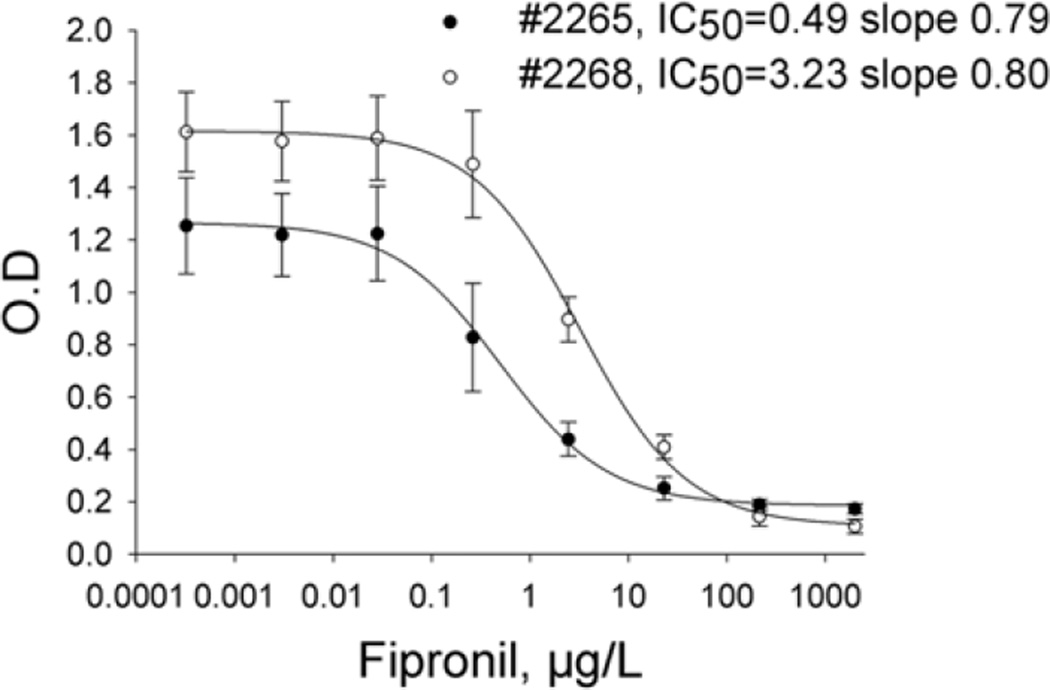
The optimized ELISAs used coating antigen-antibody pairs 5-CON/2265 and 1-CON/2268 at coating concentrations of 1 µg/mL. The coated plate was blocked with 1% BSA. The analyte was loaded in assay buffer containing 10% MeOH in PBS, pH 7.5. Sera dilutions were 1:6000 and 1:8000 in PBS, respectively after addition to the plate. The heterologous assay had a linear range (IC20–80) of 0.14–2.22 µg/L of fipronil in assay buffer and IC50 value of 0.58±0.06 µg/L (tested in triplicate for 8 days) for #2265 (Fig. 1); and a linear range (IC20–80) of 0.54–12.6 µg/L of fipronil in assay buffer and IC50 value of 2.6±0.4 µg/L (tested in triplicate for 8 days) for #2268 (Fig. 1). The LOD in the buffer was determined from the IC10 value, and estimated at 0.06 µg/L and 0.22 µg/L for 2265 and 2268, respectively. The sensitivity of the assays is comparable with those of instrumental LC- and GC/MS/MS methods being in the range of 0.18–2.5 µg/L.26,29
Figure 1.
Fipronil competition curves with serum from rabbit 2265 and 2268 in 10mM PBS assay buffer at pH 7.5 containing 10% MeOH. Reagent concentrations: coating antigen 1µg/mL (5-CON/2265, 1-CON/2268); anti-fipronil serum (1/6000); goat anti-rabbit IgG-HRP (1/10000). Data points are the mean and standard deviation over 3 days.
The overall assay optimization data suggest that the binding properties of the serum obtained from rabbit 2268 are less affected by changes of sample matrix, thus giving a more robust assay. The two assays significantly differ in metrological characteristics, where assay 2265 gives a very low limit of detection and high sensitivity, while the 2268 assay has a wider linear analytical range and better signal to noise ratio.
Only a few references to a fipronil immunoassay could be found in the literature. Liu et al.37 developed poly- and monoclonal antibodies using a homologous hapten, a derivative of fipronil-sulfone, for preparation of the immunogen and coating antigen. The assay had lower sensitivity compared to those described in this paper, having IC50 values of 18.0 µg/L and 6.0 µg/L for polyclonal (pAb) and monoclonal (mAb) antibodies, respectively. However, the linear range of detection for the reported assays was wider. Based on IC10-IC90 the assay was linear between 0.07–203 µg/L for the pAb and 0.07–485 µg/L for the mAb. Another assay based on a mAb aimed to detect the active ingredients of a termite insecticide, including fipronil, was patented by Miyake et al.3 They demonstrated that fipronil detection occurs almost linearly through the range of concentrations from 5–80 µg/L. Based on comparison with these two publications it appears that the assay described here demonstrates very good sensitivity to fipronil. Careful design of immunogen and a heterologous approach for coating antigen selection allowed the development of a high sensitivity assay using polyclonal serum that is much easier to obtain compared to the laborious procedure of monoclonal antibody production.
Cross-reactivity (CR)
A range of fipronil analogues with modified substituents were purchased or synthesized to be used in cross-reactivity studies to determine to which specific epitope of hapten the antibody was raised to; and how the structure of immunogen may alter the selectivity of the developed antiserum. The main reactions to prepare fipronil congeners were based on methods previously described. Analytical data of the resulting compounds conformed to published information.45–47
Antiserum 2265 was raised against a hapten exposing the nitrile group while attached to the protein through the amine moiety (Hapten 2). Thus, resulting antibodies are very selective to the presence of nitrile group in the analyte. All fipronil analogues containing the nitrile group strongly inhibited the assay giving CR in the range of 50–100% compared to fipronil (compounds 2–5, Table 1). In contrast the analytes missing the nitrile group poorly competed with coating antigen and their cross-reactivity hardly exceeded 4%. This remaining activity may come from antibody selectivity to the skeletal structure of the substituted phenylpyrazole.
Table 1.
Cross Reactivity of Fipronil Antiserum to Structurally Related Compounds
| Compound | Structure | #2265, % (n) a |
#2268, % (n) |
Compound | Structure | #2265, % |
#2268, % |
|---|---|---|---|---|---|---|---|
| 1. Fipronilb |  |
100 (19) | 100 (7) | 6. Ethiprole (insecticide) |
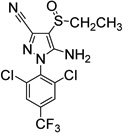 |
61±15 | 43±13 |
| 2. Fipronil- sulfide (environment. photoproduct) |
 |
96±49 (6) |
39±11 (4) |
7. Imidacloprid (insecticide) |
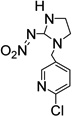 |
<1 | <1 |
| 3. Fipronil- sulfone (environment. photoproduct, biometabolite) |
 |
60±26 (8) |
71±14 (4) |
8. Fipronil-acid (environment. metabolite) |
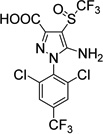 |
4.2±1.5 | 13±2.1 |
| 4. Fipronil- deSOCF3c (environment. photoproduct, biometabolite) |
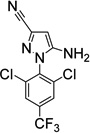 |
38±14 (6) |
1.4±0.2 (4) |
9. Fipronil- sulfide-acid (environment. metabolite) |
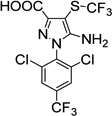 |
4.4±2.4 | 6.9±3.3 |
| 5. Fipronil- desulfinyl (environment. photoproduct) |
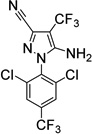 |
101±23 (7) |
25±3 (4) |
10. Fipronil- sulfide-amide (environment. metabolite) |
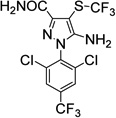 |
2.6±0.7 | 157±109 |
| 11. Fipronil- deSOCF3-acid (environment. metabolite) |
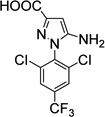 |
<1 | <1 |
Cross-reactivity was calculated as (IC50 of fipronil /IC50 of the tested compound)x100.
IC50 of fipronil was 1.4±0.5 nmol/L (#2265) and 6.8±1.6 nmol/L (#2268).
deSOCF3 refers to detrifluoromethylsulfinyl. (n), number of days CR experiment was performed, in triplicate each. For compounds 6–11: n=3 days, in triplicate each.
The binding pocket seems to be more complex in antibodies from serum 2268 raised against hapten having SOCF3 and the amine group exposed, while attached to the protein through – CONH-linkage of fipronil-acid (Hapten 3). The developed serum selectively recognized SOCF3. This hypothesis is supported by the decrease in CR of compounds with modified substituents at position B of the fipronil molecule: SOCF3>SO2CF3>SOC2H5≈SCF3>CF3>H (compounds 1>3>6≈2>5>4, Table 1) corresponding to 100>71>43≈39>25>1.4%. However, the serum had even stronger selectivity toward the amide group at the adjacent position since specificity to fipronil-sulfide-amide was higher than to fipronil (CR 157%, compound 10, Table 1), despite having a sulfide group instead of a SOCF3. Interestingly, assay 2268 was only slightly inhibited by compounds mimicking fipronil and fipronil-sulfide with the nitrile group substituted by a carboxyl group (compounds 8, 9). Taking into account that the nitrile group was not exposed for recognition and antibody production, the results obtained suggest that nitrogen, as a part of either –CONH- or nitrile group, occupies a specific place in the binding pocket of the antibody used in the assay. The role of the fipronil amine group (structural determinant C) in antiserum recognition was not explored.
These effects demonstrate that hapten design had a significant impact on the resulting antiserum selectivity. The careful hapten design and successful organic synthesis allow construction of a library of diverse but closely related chemicals that could be manipulated to produce antibodies with desired characteristics: target, group or class specific reagents. It is not always necessary to have highly selective assays. For example, a number of organophosphorus pesticides are used for residential and agricultural purposes. These compounds have led to numerous poisonings of non-target species, including human fatalities. The specific assays for individual compounds of this large class are less efficient for pesticide control and screening.48,49 In contrast to selective tests, the antibodies used in screening assays should be cross-reactive in order to detect the analyte in a risk cup. In the literature, it has been shown that considered manipulation of the hapten structure gives useful highly cross-reactive assays for rapid screening of dangerous substances and related compounds.50,51 High CR is usually achieved when the immunizing antigen structure combines the analyte main body and some of its structural determinants common to all compounds aimed to be recognized. In our study, the serum 2265 recognizes not only fipronil, but also metabolites of fipronil which contain the nitrile group and variable substituents at the adjacent position. Indeed, such broad selectivity appears since the antibodies were raised against hapten 2, where the nitrile group was preserved in the structure, carrier protein was attached to the amine moiety, and the SOCF3 structural determinant was missing. The results obtained were in strong accordance with literature reports.48,51
The serum 2265 having broad-selectivity properties for closely related compounds can be used for construction of unique biosensors or ELISA assays that will be able to detect fipronil-sulfone and fipronil in the blood of humans and animals to assess their exposure to the insecticide. The same assay/sensor could also be applied for rapid on-site monitoring of fipronil and fipronil-desulfinyl in environmental samples, thus assuring the real-time monitoring of environmental contamination.
In addition to fipronil analogues we also tested a number of GABA-antagonists (Table S3). There are cases where the antibody raised to biologically active molecules will detect a group of different structures all of which bind to the same receptor. In these cases the antibody can act as a surrogate receptor for screening.52,53 In our case the immunoassay failed to detect a variety of insecticides and cage convulsants acting on the GABA channel. Since these compounds bind to a series of diverse sites on the GABA-gated chloride channel of arthropods and vertebrate, cross-reactivity was not expected.
Matrix effect
Sample preparation is an important step in complex sample analysis influencing accuracy and reliable determination in many analytical methods. However, sample clean-up procedures are often time consuming and laborious. Similarly to other analytical techniques, immunoreactions employed in an ELISA may be altered by multiple components present in complex media. However, depending on the nature of the matrix and immunoassay characteristics the interference could be minimal, so that preparation of a calibration curve in a similar matrix may decrease the error of analysis. The interference in ELISA could also be diminished by simple sample dilution. The effect of matrix on assay 2265 performance was evaluated in human serum (Fig. S3). In this study fipronil-sulfone was chosen as the analyte since it is the major metabolite of fipronil identified in serum. Since we wanted to use the generic assay for both analytes, other parameters of the assay were used as optimized for fipronil. Serum matrix had variable effects on the competition curve. When the assay was performed in 100% human serum (prior to antibody addition), the sensitivity did not change dramatically compared to the assay conducted in buffer, with IC50 values 4.11 and 4.95 µg/L (Fig. S3), respectively. However, a constant decrease in maximum signal was observed when the content of serum matrix increased in assay buffer. Interestingly, the sensitivity of the assay increased in buffer containing serum matrix at 10% (IC50 at 2.47 µg/L), followed by a subsequent decrease in sensitivity with an increase of serum matrix portion in the assay buffer (IC50 3.37 µg/L at 50% matrix). It is possible that proteins present in the serum matrix help to decrease non-specific binding in the assay thus improving assay characteristics without affecting the desired immuno-recognition and binding. Since matrix suppressed the maximum signal even at 10-fold dilution, we decided to prepare the calibration curve in a 10% blank matrix of human serum.
Similar analysis was performed with urine matrix for sera 2265 and 2268. There was no significant effect of urine matrix on assays sensitivity. However, the signal intensity was again suppressed with increasing amount of urine matrix (data not shown). A 10-fold dilution of sample with assay buffer was chosen for further validation studies.
Validation in various samples
To evaluate the performance of the fipronil immunoassays developed to detect quantitatively the analyte in complex samples, we performed a spike-recovery analysis from different matrixes, including industrial water, human serum and urine. In case report studies the concentration of fipronil and fipronil-sulfone in the serum of humans intoxicated with fipronil were reported to be up to 4000 µg/L of plasma.21 Taking into account these data, human serum was fortified with known concentrations of fipronil, or fipronil-sulfone at 10–50 µM range (0.5–2.5 µg/L in the well after dilutions). We aimed to study the recovery of low concentrations to estimate the influence of the matrix on the accuracy of quantification. Otherwise, with higher spiked concentrations the assay would require higher dilution of the sample decreasing the amount of interfering matrix and facilitating quantitative analysis. We also evaluated concentrations of fipronil over a narrow range to validate the accuracy of the assay to distinguish close but different concentrations of the analyte in the matrix. Table 2 presents good recoveries ranging from 93 to 118% for both analytes at all concentrations tested.
Table 2.
Recovery of fipronil and fipronil metabolites in spiked samples of industrial water, human serum and urine measured by immunoassay.
| Spiked conc* µg/L |
Fipronil, µg/L (% recovered) |
|||
|---|---|---|---|---|
| industrial water |
serum | urine | ||
| 2.5 | 1.8±0.25 (73.3±9.6) |
2.3±0.6 (93.1±22.7) |
2.14±0.28 (85±11) |
|
| 1 | 0.9±0.05 (85.5±5.5) |
1.0±0.1 (103.4±13.3) |
0.90±0.20 (90±20) |
|
| 0.5 | 0.5±0.0 (91.3±11.6) |
0.6±0.1 (111±11) |
0.48±0.0 (96±9) |
|
| Fipronil metabolites, µg/L (% recovered) |
||||
| Fipronil sulfonea | Fipronil- desulfinyb |
|||
| 2.5 | 2.6±0.4 (105.8±17.3) |
2.4±0.23 (96.4±9.3) |
||
| 1 | 1.2±0.1 (116.8±9.1) |
1.0±0.09 (104.4±9.0) |
||
| 0.5 | 0.6±0.2 (118±47) |
0.5±0.04 (107±8) |
||
Assay conditions: coating antigen (5-CON) 1µg/mL; anti-fipronil serum #2265 (1/6000); goat anti-rabbit IgG-HRP (1/10000).
spiked concentrations indicated in the table are the final concentrations in the well after sample dilution prior to loading onto the plate and after addition of the antibody in the well.
in human serum
in industrial water.
Values are the mean ± standard deviation (n≥3 days).
To our knowledge, there are few reports on fipronil monitoring in the environment. From the US Geological Survey54 it appears that fipronil is present in very low concentration in water and soil in a number of US states. The concentration varies from very low to hundreds of ng/L. However, from the same survey, fipronil concentrations may go up to µg/L in spring, for example when water is released from rice fields. Therefore, an ELISA could be used for monitoring downstream water released from farms. Since its sensitivity is around 0.5–1 µg/L and toxicity of fipronil for aquatic animals is generally above 10 µg/L, immunoassay could be an appropriate environmental screening tool. In addition, the assay with serum 2265 detects environmental metabolites that are even more toxic. Similarly to the serum matrix, water samples were separately spiked with fipronil and fipronil-desulfinyl in the 10–50 µM range and recoveries were assessed with assay 2265. No significant matrix effect was observed from industrial water. Table 2 demonstrates good recoveries ranging from 73 to 91% for fipronil and 96 to 107% for fipronil-desulfinyl.
Finally, the metabolism of fipronil in mammals is being studied and only limited data are available concerning humans. Despite the fact that up to now there are contradictory data on presence of parent compound in urine in animals, human urine remains a possible way of exposure monitoring. Recoveries from human urine matrix fortified with fipronil in the 10–50 µM range were 85–96% (Table 2).
In another series of experiments a comparative study was performed to estimate the accuracy of the immunoassay compared to an instrumental method. Three matrixes, including industrial tap water, urban runoff water and human urine were fortified with fipronil, extracted and analyzed blind by LC-MS/MS and immunoassay. The assay 2265 was used to estimate recovery values from urine and the assay 2268 from water. As seen in Table 3, there was relatively good agreement between ELISA and LC-MS/MS data. In industrial water and human urine extracts fipronil concentrations detected by immunoassay were closer to theoretical spiked values than LC-MS/MS. However, recoveries from urban runoff water were closer to theoretical values when detected by LC-MS/MS, with slight overestimation for certain spikes in immunoassay analysis. Overall, there is a linear correlation between data obtained by LC-MS/MS and ELISA with the ratio varying between 1.1–1.4. These overall data suggest that immunoassay based on antiserum 2265 (and 2268) could be directly used for quantitative monitoring of fipronil and fipronil metabolites in various matrices without any additional sample preparation, thus reducing analysis time, especially in the case of a large screening campaign, and reducing the cost of analysis.
Table 3.
Recoveries of fipronil in spiked industrial and urban water, and in spiked human urine samples: comparison between immunoassay and LC-MS/MS.
| Analyte | Spike | ELISA (A) |
LC-MS/MS (B) |
Ratio (A/B) |
|---|---|---|---|---|
| Industrial water | ||||
| Fipronil | 2.0 | 1.7±0.4 | 1.7±0.4 | 1.0 |
| 29.1 | 30.8±4.6 | 21.6±1.7 | 1.4 | |
| 47.6 | 49.6±7.3 | 35.3±5.6 | 1.4 | |
| 9.9 | 10.2±0.8 | 8.1±0.2 | 1.3 | |
| 4.8 | 5.1±1.0 | 4.2±0.3 | 1.2 | |
| Urban runoff water | ||||
| Fipronil | 29.1 | 39.5±14.0 | 20.0±4.9 | 2.0 |
| 2.0 | 1.9±0.8 | 1.8±0.2 | 1.1 | |
| 47.6 | 65.5±22.8 | 39.2±8.4 | 1.7 | |
| 4.8 | 7.0±2.5 | 4.4±0.9 | 1.6 | |
| 9.9 | 10.6±4.0 | 8.0±1.2 | 1.3 | |
| Urine extract | ||||
| Fipronil | 10 | 9.6±0.9 | 8.5±0.6 | 1.1 |
| 20 | 18.0±3.9 | 14.7±2.5 | 1.2 | |
| 25 | 22.7±2.7 | 18.6±3.0 | 1.2 | |
| 50 | 42.7±5.5 | 38.5±1.1 | 1.1 | |
Assay conditions. For recovery studies from water, assay 2268: coating antigen (1-CON) 1µg/mL; anti-fipronil serum (1/8000); assay buffer with 20% MeOH, goat anti-rabbit IgG-HRP (1/20000). For recovery studies from urine: coating antigen (5-CON) 1µg/mL; anti-fipronil serum (1/6000); goat anti-rabbit IgG-HRP (1/10000). Values are the mean ± standard deviation (n=3 days).
Urine samples from exposure studies
Dyk et al.38 were looking for fipronil and its metabolites in urine of pet owners after they used Frontline® insecticide in companion animals. Many urine samples from pet owners were collected prior and post product application. Authors analyzed samples by LC-MS/MS before and after hydrolysis of possible bioconjugates of fipronil and its metabolites. The selected urine samples were also analyzed by a third independent laboratory using LC-MS/MS. We used selected urine samples to conduct the analysis using the fipronil immunoassay. Assay 2265 was chosen since it was the most sensitive to fipronil and a number of its metabolites. In the literature there are limited data on fipronil metabolism in humans, and metabolites present in human urine have not been well studied. Xenobiotics are excreted in the urine in forms of glucuronide or sulfate conjugates of parent compound or its metabolites. To hydrolyze possible conjugates of fipronil we used an enzymatic solution of β-glucuronidase/sulfatase. This method provides mild conditions for hydrolysis decreasing the possibility of destroying the compound of interest by harsh acidic hydrolysis conditions. The assay showed non-detectable levels of compounds of interest (data not shown) with limits of detection of 0.05±0.02, 0.02±0.01, 0.04±0.02, and 0.07± 0.02 µg/L (n=6 days) for fipronil-sulfone, fipronil-sulfide, fipronil detrifluoromethylsulfinyl and fipronil-desulfinyl, respectively. Our findings are similar to data published by Dyk et al.38 They concluded that levels observed after Frontline® application were not different from levels observed in pre-application urine samples and that a time/concentration trend was not observed.
Serum samples from dosed rats
McMahen et al.39 were identifying serum/urine biomarkers of fipronil exposure from dosed animal samples as potential biomarkers for use in human biomonitoring studies. Authors used LC/QQQ (triple quadrupole) mass spectrometry to identify possible fipronil derivatives present in biofluids, and to quantify fipronil and fipronil-sulfone in rat serum. We used both immunoassays to quantify total concentration of fipronil and its metabolites in the selected serum samples. The data obtained by both assays are in very good correlation with LC/QQQ results (Table 4). The assays gave higher estimates compared to fipronil or fipronil-sulfone concentrations separately, because compounds are cross-reactive in both assays. However, the values obtained by the assays are very close to the total fipronil detected with LC/QQQ. No significant difference was detected between methods using simple T-test analysis with p<0.05.
Table 4.
Detection of fipronil and fipronil-sulfone in rat serum samples: comparison between immunoassays and LC/QQQ-MS.
| Rat sample |
LC/QQQ-MS (µg/mL)£ | Immunoassays (µg/mL) |
|||
|---|---|---|---|---|---|
| Fipronil (A) |
Fipronil- sulfone(B) |
A+B | #2265 | #2268 | |
| 1 (CNTR) |
<LOQ* | <LOQ** | <LOQ | <LOD$ | <LOD§ |
| 2 | 0.5 | 1.9 | 2.4 | 3.9±2.7 | 2.4±0.6 |
| 3 (CNTR) |
<LOQ | <LOQ | <LOQ | <LOD | <LOD |
| 4 | 0.5 | 2.3 | 2.8 | 3.0±1.3 | 2.3±0.8 |
| 5 (CNTR) |
<LOQ | <LOQ | <LOQ | <LOD | <LOD |
| 6 | 0.4 | 1.9 | 2.3 | 1.7±0.4 | 1.9±0.3 |
| 7 (CNTR) |
<LOQ | <LOQ | <LOQ | <LOD | <LOD |
| 8 | 0.3 | 1.4 | 1.7 | 1.5±0.5 | 1.9±0.5 |
| 9 | 0.4 | 0.7 | 1.1 | 0.9±0.1 | 1.3±0.4 |
| 10 | 0.4 | 2.1 | 2.5 | 2.3±0 .4 | 2.5±0.3 |
| 11 (CNTR) |
<LOQ | <LOQ | <LOQ | <LOD | <LOD |
| 12 | 0.3 | 1.3 | 1.6 | 1.6±0.0 | 1.3±0.4 |
Results are provided by McMahen et al.39
LOQ=10 ng/mL
LOQ=10 ng/mL, RSD is <15%.
LOD=0.06 ng/mL
LOD=0.22 ng/mL. For ELISA, prior to addition to the plate, serum samples were diluted 1000 or 100 times for treated and control (CNTR) animals, respectively; n=2 different days, no statistical difference with (A+B) was observed, p<0.05.
In conclusion, two sensitive immunoassays were developed. One assay appeared to be selective to fipronil and its major metabolite fipronil-sulfone. Another assay demonstrated recognition of the class of structures closely related to fipronil. Such difference in recognition behavior of antibodies was achieved by using different haptens exposing either the single nitrile structural determinant of fipronil (for generic assay) or the trifluoromethylsulfonyl and amine structural determinants. A heterologous format has proven to result in more sensitive assays with IC50 of 0.58±0.06 µg/L and 2.6±0.4 µg/L. The assays have the sensitivity to measure fipronil and its analogs relevant for medical screening where fipronil and fipronil-sulfone concentration in serum may be up to 4000 µg/L; and for exposure monitoring (toxicity of fipronil for aquatic animals is above 10 µg/L). The assays successfully demonstrated their accuracy and reliability when applied in spike-recovery studies and compared to established analytical techniques (LC-MS) in different matrices providing a valuable tool for further development of rapid immunochemical screening methods. The developed assay #2265 might be used in screening studies whenever analyte is fipronil-like molecule. The assay #2268 is more convenient for quantitative studies, since the assay is more robust to changing experimental conditions, and thus is the best choice for analysis of environmental samples.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by National Institute of Environmental Health Sciences, Superfund Research Program, P42 ES04699 and the National Institute for Occupational Safety and Health Western Regional Center for Agricultural Health Science U50 OH07550. The research was also supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke, Grant Number U54 NS079202. We are thankful to Dr. McMahen and Dr. Strynar for providing rat serum samples and LC/QQQ data.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
SUPPORTING INFORMATION
Additional information including text, three tables and three figures are available in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.US EPA. Fipronil Summary Document Registration Review: Initial Docket. 2011 Jun; Docket Number: EPA-HQ-OPP-2011-0448. Case No.7423. In 2011. [Google Scholar]
- 2.Watts M. Pesticide Action Network Asia and the Pacific, Highly Hazardous Pesticides: Fipronil. A PAN AP Factsheet Series. 2012 http://archive.panap.net/en/p/post/pesticides-info-database/1209.
- 3.Miyake S, Uchigashima M, Kadowaki A. Kit for measurement of termite insecticide active ingredient by immunoassay method. 2012 Dec 4; US 8,323,904 B2. [Google Scholar]
- 4.National Pesticide Information Center, Technical fact sheet: Fipronil. Oregon State University; http://npic.orst.edu/factsheets/fiptech.pdf. [Google Scholar]
- 5.Cole LM, Nicholson RA, Casida JE. Action of phenylpyrazole insecticides at the GABA-gated chloride channel. Pest. Biochem. Physiol. 1993;46:47–54. [Google Scholar]
- 6.Ratra GS, Kamita SG, Casida JE. Role of human GABA(A) receptor beta3 subunit in insecticide toxicity. Toxicol. Appl. Pharmacol. 2001;172:233–240. doi: 10.1006/taap.2001.9154. [DOI] [PubMed] [Google Scholar]
- 7.Hainzl D, Casida JE. Fipronil insecticide: Novel photochemical desulfinylation with retention of neurotoxicity. PNAS. 1996;93:12764–12767. doi: 10.1073/pnas.93.23.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hainzl D, Cole LM, Casida JE. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem. Res. Toxicol. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, Salgado VL, Yeh JZ, Narahashi T. Differential actions of fipronil and dieldrin insecticides on GABA-gated chloride channels in cockroach neurons. J. Pharmacol. Exp. Ther. 2003;306:914–924. doi: 10.1124/jpet.103.051839. [DOI] [PubMed] [Google Scholar]
- 10.Caboni P, Sammelson RE, Casida JE. Phenylpyrazole insecticide photochemistry, metabolism, and GABAergic action: Ethiprole compared with fipronil. J. Agric. Food Chem. 2003;51:7055–7061. doi: 10.1021/jf030439l. [DOI] [PubMed] [Google Scholar]
- 11.Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EHJ, Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol. Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- 12.Menold MM, Shao Y, Wolpert CM, Donnelly SL, Raiford KL, Martin ER, Ravan SA, Abramson RK, Wright HH, Delong GR, Cuccaro ML, Pericak-Vance MA, Gilbert JR. Association analysis of chromosome 15 GABA(A) receptor subunit genes in autistic disorder. J. Neurogenet. 2001;15:245–259. doi: 10.3109/01677060109167380. [DOI] [PubMed] [Google Scholar]
- 13.DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J. Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vidau C, Brunet JL, Badiou A, Belzunces LP. Phenylpyrazole insecticides induce cytotoxicity by altering mechanisms involved in cellular energy supply in the human epithelial cell model Caco-2. Toxicol. In Vitro. 2009;23:589–597. doi: 10.1016/j.tiv.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Vidau C, Gonzalez-Polo RA, Niso-Santano M, Gomez-Sanchez R, Bravo-San Pedro JM, Pizarro-Estrella E, Blasco R, Brunet JL, Belzunces LP, Fuentes JM. Fipronil is a powerful uncoupler of oxidative phosphorylation that triggers apoptosis in human neuronal cell line SHSY5Y. Neurotoxicology. 2011;32:935–943. doi: 10.1016/j.neuro.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Das PC, Cao Y, Cherrington N, Hodgson E, Rose RL. Fipronil induces CYP isoforms and cytotoxicity in human hepatocytes. Chem. Biol. Interact. 2006;164:200–214. doi: 10.1016/j.cbi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 18.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Godbold JH, Wolff MS. Pesticides and inner-city children: Exposures, risks, and prevention. Environ. Health Perspect. 1999;107:431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bearer CF. Environmental-Health Hazards: How Children Are Different from Adults. Future Child. 1995;5:11–26. [PubMed] [Google Scholar]
- 21.Mohamed F, Senarathna L, Percy A, Abeyewardene M, Eaglesham G, Cheng R, Azher S, Hittarage A, Dissanayake W, Sheriff MH, Davies W, Buckley NA, Eddleston M. Acute human self-poisoning with the N-phenylpyrazole insecticide fipronil - a GABA(A)-gated chloride channel blocker. J. Toxicol. Clin. Toxicol. 2004;42:955–963. doi: 10.1081/clt-200041784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Mulay P, Diebolt-Brown B, Lackovic MJ, Mehler LN, Beckman J, Waltz J, Prado JB, Mitchell YA, Higgins SA, Schwartz A, Calvert GM. Acute illnesses associated with exposure to fipronil--surveillance data from 11 states in the United States, 2001–2007. Clin. Toxicol. 2010;48:737–744. doi: 10.3109/15563650.2010.507548. [DOI] [PubMed] [Google Scholar]
- 23.Stout DM, Bradham KD, Egeghy PP, Jones PA, Croghan CW, Ashley PA, Pinzer E, Friedman W, Brinkman MC, Nishioka MG, Cox DC. American Healthy Homes Survey: A national study of residential pesticides measured from floor wipes. Environ. Sci. Technol. 2009;43:4294–4300. doi: 10.1021/es8030243. [DOI] [PubMed] [Google Scholar]
- 24.Hadjmohammadi MR, Nikou SM, Kamel K. Determination of fipronil residue in soil and water in the rice fields in north of Iran by RP-HPLC method. Acta Chim. Slov. 2006;53:517–520. [Google Scholar]
- 25.Pirard C, Widart J, Nguyen BK, Deleuze C, Heudt L, Haubruge E, De Pauw E, Focant JF. Development and validation of a multi-residue method for pesticide determination in honey using on-column liquid-liquid extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2007;1152:116–123. doi: 10.1016/j.chroma.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix MZ, Puel S, Toutain PL, Viguie C. Quantification of fipronil and its metabolite fipronil sulfone in rat plasma over a wide range of concentrations by LC/UV/MS. J. Chromatogr. B. 2010;878:1934–1938. doi: 10.1016/j.jchromb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Cravedi JP, Delous G, Zalko D, Viguie C, Debrauwer L. Disposition of fipronil in rats. Chemosphere. 2013;93:2276–2283. doi: 10.1016/j.chemosphere.2013.07.083. [DOI] [PubMed] [Google Scholar]
- 28.Cazorla-Reyes R, Fernandez-Moreno JL, Romero-Gonzalez R, Frenich AG, Vidal JL. Single solid phase extraction method for the simultaneous analysis of polar and non-polar pesticides in urine samples by gas chromatography and ultra high pressure liquid chromatography coupled to tandem mass spectrometry. Talanta. 2011;85:183–196. doi: 10.1016/j.talanta.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 29.Bichon E, Richard CA, Le Bizec B. Development and validation of a method for fipronil residue determination in ovine plasma using 96-well plate solid-phase extraction and gas chromatography-tandem mass spectrometry. J. Chromatogr. A. 2008;1201:91–99. doi: 10.1016/j.chroma.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Trindade M, Nording M, Nichkova M, Spinnel E, Haglund P, Last MS, Gee S, Hammock B, Last JA, Gonzalez-Sapienza G, Brena BM. Enzyme-linked immunosorbent assay for screening dioxin soil contamination by uncontrolled combustion during informal recycling in slums. Environ. Toxicol. Chem. 2008;27:2224–2232. doi: 10.1897/07-660.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shelver WL, Parrotta CD, Slawecki R, Li QX, Ikonomou MG, Barcelo D, Lacorte S, Rubio FM. Development of a magnetic particle immunoassay for polybrominated diphenyl ethers and application to environmental and food matrices. Chemosphere. 2008;73:S18–S23. doi: 10.1016/j.chemosphere.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 32.Piao YZ, Kim YJ, Kim YA, Lee HS, Hammock BD, Lee YT. Development of ELISAs for the class-specific determination of organophosphorus pesticides. J. Agric. Food Chem. 2009;57:10004–10013. doi: 10.1021/jf901998y. [DOI] [PubMed] [Google Scholar]
- 33.Nichkova M, Fu X, Yang Z, Zhong P, Sanborn JR, Chang D, Gee SJ, Hammock BD. Immunochemical screening of pesticides (simazine and cypermethrin) in orange oil. J. Agric. Food Chem. 2009;57:5673–5679. doi: 10.1021/jf900652a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Febrero R, Salvador JP, Sanchez-Baeza F, Marco MP. Rapid method based on immunoassay for determination of paraquat residues in wheat, barley and potato. Food Control. 2014;41:193–201. [Google Scholar]
- 35.Thiphom S, Prapamontol T, Chantara S, Mangklabruks A, Suphavilai C, Ahn KC, Gee SJ, Hammock BD. Determination of the pyrethroid insecticide metabolite 3-PBA in plasma and urine samples from farmer and consumer groups in northern Thailand. J. Environ. Sci. Health B. 2014;49:15–22. doi: 10.1080/03601234.2013.836862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang JC, Van Emon JM, Durnford J, Thomas K. Development and evaluation of an enzyme-linked immunosorbent assay (ELISA) method for the measurement of 2,4-dichlorophenoxyacetic acid in human urine. Talanta. 2005;67:658–666. doi: 10.1016/j.talanta.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Yan C, Dong J, Yu X, Xu D. Poly- and monoclonal antibody-based ELISAs for fipronil. J. Agric. Food Chem. 2007;55:226–230. doi: 10.1021/jf062045a. [DOI] [PubMed] [Google Scholar]
- 38.Dyk MB, Liu Y, Chen Z, Vega H, Krieger RI. Fate and distribution of fipronil on companion animals and in their indoor residences following spot-on flea treatments. J. Environ. Sci. Health B. 2012;47:913–924. doi: 10.1080/03601234.2012.706548. [DOI] [PubMed] [Google Scholar]
- 39.McMahen RL, Strynar MJ, Dagnino S, Herr DW, Moser VC, Garantziotis S, Andersen EM, Freeborn DL, McMillan L, Lindstrom AB. Identification of fipronil metabolites by time-of-flight mass spectrometry for application in a human exposure study. Environ. Int. 2015;78:16–23. doi: 10.1016/j.envint.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee N, McAdam DP, Skerritt JH. Development of immunoassays for type II synthetic pyrethroids. 1. Hapten design and application to heterologous and homologous assays. J. Agric. Food Chem. 1998;46:520–534. doi: 10.1021/jf970438r. [DOI] [PubMed] [Google Scholar]
- 41.Tijssen P. Conjugation of haptens. In: Burdon RH, Knippenberg PH, editors. Practice and Theory of Enzyme Immunoassays. Vol. 15. Amsterdam, The Netherlands: Elsevier; 1985. [Google Scholar]
- 42.Wie SI, Hammock BD. Comparison of coating and immunizing antigen structure on the sensitivity and specificity of immunoassays for benzoylphenylurea insecticides. J. Agric. Food Chem. 1984;32:1294–1301. [Google Scholar]
- 43.Liu YH, Xie R, Guo YR, Zhu GN, Tang FB. Comparison of homologous and heterologous formats in nanocolloidal gold-based immunoassays for parathion residue determination. J. Environ. Sci. Health B. 2012;47:475–483. doi: 10.1080/03601234.2012.663613. [DOI] [PubMed] [Google Scholar]
- 44.Cao B, Yang H, Song J, Chang H, Li S, Deng A. Sensitivity and specificity enhanced enzyme-linked immunosorbent assay by rational hapten modification and heterogeneous antibody/coating antigen combinations for the detection of melamine in milk, milk powder and feed samples. Talanta. 2013;116:173–180. doi: 10.1016/j.talanta.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Bastiaans HMM, Donn G, Knittel N, Martel-Letti A, Rees R, Schwall M. Plant growth regulation. 2005 Jul 15; WO 2005/063020 A1. [Google Scholar]
- 46.Tang Y-Z, Liao S-L, Huang S, Deng Y-P, Wen H-R. The hydrolysis of fipronil with Zn2+ ion catalysis and its crystal structure. Youse Jinshu Kexue Yu Gongcheng. 2011;2:19–22. [Google Scholar]
- 47.Wenjun G, Jingli C, Guonian Z, Maojun J. Fipronil artificial antigen, antibody and use thereof. 200710070227. China Patent. 2008 Jan 9;
- 48.Xu ZL, Shen YD, Zheng WX, Beier RC, Xie GM, Dong JX, Yang JY, Wang H, Lei HT, She ZG, Sun YM. Broad-specificity immunoassay for O,O-diethyl organophosphorus pesticides: Application of molecular modeling to improve assay sensitivity and study antibody recognition. Anal. Chem. 2010;82:9314–9321. doi: 10.1021/ac1018414. [DOI] [PubMed] [Google Scholar]
- 49.Banks JN, Chaudhry MQ, Matthews WA, Haverly M, Watkins T, Northway BJ. Production and characterisation of polyclonal antibodies to the common moiety of some organophosphorus pesticides and development of a generic type ELISA. Food Agr. Immunol. 1998;10:349–361. [Google Scholar]
- 50.Julicher P, Mussenbrock E, Renneberg R, Cammann K. Broadening the antibody specificity by hapten design for an enzyme-linked immunoassay as an improved screening method for the determination of nitroaromatic residues in soils. Analyt. Chim. Acta. 1995;315:279–287. [Google Scholar]
- 51.Bucknall S, Silverlight J, Coldham N, Thorne L, Jackman R. Antibodies to the quinolones and fluoroquinolones for the development of generic and specific immunoassays for detection of these residues in animal products. Food Addit. Contam. 2003;20:221–228. doi: 10.1080/0265203021000055388. [DOI] [PubMed] [Google Scholar]
- 52.Bright SW, Gold G, Sage SW, Sportsman JR, Tinsley FC, Dominianni SJ, Schmiegel KK, Kellam ML, Fitch LL, Yen TT. Monoclonal antibodies as surrogate receptors in a high throughput screen for compounds that enhance insulin sensitivity. Life Sci. 1997;61:2305–2315. doi: 10.1016/s0024-3205(97)00934-x. [DOI] [PubMed] [Google Scholar]
- 53.Lomash S, Nagpal S, Salunke DM. An antibody as surrogate receptor reveals determinants of activity of an innate immune peptide antibiotic. J. Biol. Chem. 2010;285:35750–35758. doi: 10.1074/jbc.M110.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunasekara AS, Truong T, Goh KS, Spurlock F, Tjeerdema RS. Environmental fate and toxicology of fipronil. J. Pest. Sci. 2007;32:189–199. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.