Abstract
Background
The discovery of effective re-induction regimens for children with more than one relapse of acute lymphoblastic leukemia (ALL) remains elusive. The novel nucleoside analog clofarabine exhibits modest single agent efficacy in relapsed ALL, though optimal combinations of this agent with other active chemotherapy drugs have not yet been defined. Herein we report the response rates of relapsed ALL patients treated on Children’s Oncology Group study AAML0523, a Phase I/II study of the combination of clofarabine and cytarabine.
Procedure
AAML0523 enrolled 21 children with ALL in second or third relapse, or those refractory to re-induction therapy. The study consisted of two phases: a dose finding phase and an efficacy phase. The dose finding portion consisted of a single dose escalation/de-escalation of clofarabine for 5 days in combination with a fixed dose of cytarabine (1 g/m2/day for 5 days). Eight patients received clofarabine at 40 mg/m2/day and 13 patients at 52 mg/m2/day.
Results
Toxicities observed at all doses of clofarabine were typical of intensive chemotherapy regimens for leukemia, with infection being the most common. We did not observe significant hepatotoxicity as reported in other clofarabine combination regimens. The recommended pediatric Phase II dose of clofarabine in combination with cytarabine for the efficacy portion of AAML0523 was 52 mg/m2. Of 21 patients with ALL, 3 (14%) achieved a complete response (CR). Based on the two-stage design definition of first-stage inactivity, the therapy was deemed ineffective.
Conclusion
The combination of clofarabine and cytarabine in relapsed/refractory childhood ALL does not warrant further clinical investigation.
Keywords: acute lymphoblastic leukemia, clofarabine, cytarabine, pediatric
INTRODUCTION
Cure rates for children with acute lymphoblastic leukemia (ALL) have dramatically improved over the last several decades with the advent of intensified chemotherapy regimens and improvements in supportive care [1–3]. Despite these improvements, many children still relapse with resistant disease. It is critical to develop novel re-induction regimens that will allow for curative therapy with hematopoietic stem cell transplantation (HSCT). Multiple studies have indicated that the depth of remission prior to HSCT in ALL is a strong predictor of sustained remission, and the development of novel retrieval regimens that reduce or eliminate pre-transplant minimal residual disease (MRD) is a high priority for the management of ALL relapse. Clofarabine is a second-generation purine nucleoside analog designed to overcome the limitations and integrate the mechanistic properties of fludarabine and cladribine [4]. Clofarabine requires intracellular phosphorylation by deoxycytidine kinase (dCK) to the active triphosphate form (clo-CTP) prior to inhibition of DNA polymerase and ribonucleotide reductase. It also affects mitochondria directly, resulting in release of cytochrome c and proapoptotic proteins [5–7]. Clofarabine has shown single agent activity in Phase I and II studies in pediatric patients with relapsed or refractory ALL [8,9]. In 2005, the US Food and Drug Administration approved its use for the treatment of children aged 1–21 years with relapsed or refractory ALL treated with at least two prior treatment regimens. Subsequent development efforts have focused on defining combinations for relapsed acute leukemia.
Pre-clinical investigations have led to the development of a clofarabine/cytarabine combination regimen in acute leukemia. As a potent inhibitor of ribonucleotide reductase, clofarabine can be used to increase the accumulation of the cytotoxic triphosphate form of cytarabine (ara-CTP) in leukemia cells. Inhibition of ribonucleotide reductase by clofarabine results in a decrease of deoxynucleotide production, causing a decrease in the feedback inhibition of dCK—also the rate limiting step in the synthesis of ara-CTP. This biochemical modulation of cytarabine by clofara-bine is well established in vitro and has been studied in clinical trials in adults with relapsed acute myeloid leukemia (AML) [10,11].
Here we describe the findings of the ALL stratum of Children’s Oncology Group Phase I/II study AAML0523. The primary objective of this study was to define the overall response rate to clofarabine in combination with cytarabine in children with relapsed or refractory AML or ALL. This manuscript reports only results for children with relapsed ALL; the findings for children with AML will be described separately.
METHODS
Patients
AAML0523 opened to accrual for ALL patients on March 12, 2007 and closed October 22, 2010. Data analyses for patients with ALL are current as of June 30, 2011. Patients eligible for the ALL strata were required to be between 1 and 21 years of age, in second or third relapse or refractory to re-induction in first relapse. Relapsed patients were allowed to have no more than three prior induction regimens. Patients were required to have histologically proven ALL according to the French-American-British (FAB) classification system and >25% bone marrow blasts. Other requirements included adequate liver (serum bilirubin ≤1.5 times upper limit of normal (ULN) for age, ALT ≤2.5 times ULN for age), renal (based on age/gender derived from the Schwartz formula), cardiac (echocardiogram with shortening fraction ≥27%), and pancreatic function (serum amylase and lipase ≤1.5 times ULN), and adequate performance status (Karnofsky or Lansky performance status of ≥50%). Exclusion criteria included prior clofarabine treatment, uncontrolled systemic infection, and active central nervous system involvement (CNS3). Due to severe hepatotoxicity observed in a concurrent pediatric clinical trial using concurrent clofarabine, cyclophosphamide, and etoposide [16], AAML0523 was amended to exclude ALL patients that had received HSCT within 12 months of study entry. Three patients with ALL who had received prior HSCT were enrolled prior to the amendment, and two patients with prior HSCT were enrolled after the amendment. Institutional review boards at participating centers approved the study, and participating patients or their parents signed written informed consent. The original clinical trial was registered at www.clinicaltrials.gov as NCT00372619.
Study Design
The study was conducted in two phases: a dose finding phase and an efficacy phase. The dose finding phase of the study consisted of a single dose escalation/de-escalation of clofarabine in combination with a fixed dose of cytarabine (1 g/m2/day for 5 days). For each dose level, 10 patients were enrolled and consisted of both ALL and AML patients. The first cohort of patients received clofarabine at 40 mg/m2/day for 5 days (the adult MTD). Based on safety data on the first cohort, the dose of clofarabine would either be escalated to 52 mg/m2/day (single-agent pediatric MTD) or de-escalated to a dose of 30 mg/m2/day. The recommended Phase II dose was used in the efficacy portion of the study with separate ALL and AML cohorts. Previous studies of multi-agent regimens in ALL patients in second relapse report 30–40% complete response rate [12–14]. Therefore, a two-stage design was implemented to test the null hypothesis that the complete response rate (CR only) is ≤30% versus the alternative hypothesis that the response rate is ≥50%. Patients who received therapy at recommended Phase II dose in the first phase were included in the efficacy phase.
Treatment Plan
Induction therapy consisted of up to two cycles. If a bone marrow aspirate (BMA) performed between Days 14 and 21 of Cycle 1 revealed ≥5% blasts, Cycle 2 of induction was administered without waiting for count recovery. In the event of a hypocellular BMA, marrow evaluation was repeated not less than once every 14 days until response assessment was possible. If the Days 14–21 BMA revealed <5% blasts, patients received therapy once attaining adequate peripheral blood count recovery. Adequate peripheral blood count recovery in patients with ALL was defined as absolute neutrophil count (ANC) >750/μl and platelet count >75,000/μl. Patients without adequate peripheral blood count recovery by Day 42 were to proceed to Cycle 2 if there was no bone marrow aplasia. In the dose finding phase, Cycle 1 consisted of clofarabine on Days 2–6 and cytarabine on Days 1–5 for correlative study purposes. Correlative biology studies will be described in a separate manuscript. In the Phase II activity assessment part of the study, both clofarabine and cytarabine were administered intravenously (IV) over 2 hours on Days 1–5. Cytarabine was administered as a 2-hour IV infusion beginning 4 hours after the start of clofarabine to optimize the biochemical modulation of ara-CTP. Systemic inflammatory response is a known side effect of clofarabine [8,9]. Patients experiencing respiratory distress, unexplained hypotension or tachycardia with clofarabine were to receive intravenous hydrocortisone pre-treatment for up to 3 days for the remainder of that cycle. Prophylactic intrathecal cytarabine (IT) was administered to all patients at the time of diagnostic lumbar puncture or on Day 0 of Cycle 1 (at least 24 hours prior to administration of IV cytarabine). On Day 1 of Cycle 2 and all subsequent cycles patients received intrathecal methotrexate dosed according to age.
Response Criteria
The overall response rate (OR) consisted only of patients who achieved a complete remission (CR), and did not include those with incomplete platelet or neutrophil count recovery (CRp or CRi, respectively). CR was defined as attainment of an M1 bone marrow (<5% blasts) with an ANC >750/μl and platelet count >75,000/μl; partial remission (PR) as complete disappearance of circulating blasts and achievement of M2 marrow status (≥5% and <25%) and adequate cellularity with recovery of peripheral counts (ANC >750/μl and platelet count >75,000/μl); partial remission cytolytic (PRCL) as disappearance of peripheral blasts and at least 50% reduction of marrow blasts from baseline; minimal response cytolytic (MRCL) as ≥50% reduction in peripheral blasts from baseline without an increased peripheral WBC count; and stable disease (SD) in patients who did not qualify for either CR, PR, PRCL, MRCL, or progressive disease (PD).
Statistical Analysis
The Kaplan–Meier method was used to estimate overall survival (OS, defined as time from study entry to death). Patients were censored for OS analyses at the date of last contact. Patients defined as responders (those with a best response of CR) were compared with patients who were non-responders (best response not CR). The significance of observed difference in proportions was tested using the Chi-squared test and Fisher’s exact test when data were sparse comparing groups of patients. The Kruskal–Wallis test was used to determine the significance between differences in medians of groups.
RESULTS
Study Population
In total, 21 patients with ALL were enrolled on AAML0523—including eight patients treated with clofarabine at 40 mg/m2 and 13 patients at 52 mg/m2. All patients were determined to be eligible for study. Table I lists the characteristics of these patients. Eighteen patients were in second or third relapse, while three patients were refractory to re-induction therapy at first relapse.
TABLE I.
Patient Characteristics
| AAML0523 (ALL only)
|
AAML0523 (Dose: 40 mg/m2)
|
AAML0523 (Dose: 52 mg/m2)
|
P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| ALL patients | 21 | 8 | 38% | 13 | 62% | ||
| Precursor B-ALL | 15 | ||||||
| Precursor T-ALL | 1 | ||||||
| Unspecified | 5 | ||||||
| Gender | |||||||
| Male | 10 | 48% | 3 | 38% | 7 | 54% | 0.659 |
| Female | 11 | 52% | 5 | 63% | 6 | 46% | |
| Refractory to re-induction in first relapse | 3 | 14% | 1 | 13% | 2 | 15% | 1.000 |
| Second or third relapse | 18 | 86% | 7 | 88% | 11 | 85% | |
| Prior hematopoietic stem cell transplant | |||||||
| No | 15 | 71% | 5 | 63% | 10 | 77% | 0.631 |
| Yes | 6 | 29% | 3 | 38% | 3 | 23% | |
| Age at diagnosis in years (median, range) | 6.2 | 0.27–21.8 | 6.0 | 1.5–13.2 | 6.2 | 0.27–21.8 | 0.942 |
| Age at study entry in years (median, range) | 11.8 | 1.2–25.7 | 12.3 | 4.3–25.7 | 10.9 | 1.2–22.5 | 0.426 |
| WBC (×103/μl) (median, range) | 6.1 | 1.0–2,962 | 4.6 | 1.9–1,440 | 7.3 | 1.0–2,962 | 0.562 |
Toxicity
Non-hematologic toxicities Grade 3 and higher as defined in Common Terminology Criteria for Adverse Events (CTCAE), version 3 were collected on all patients. Those occurring in more than one individual at clofarabine doses of 40 and 52 mg/ m2 are included in Tables II and III. AML patients treated concurrently on the dose-finding portion of the study are also included in these tables. In the dose finding portion of the study, eight eligible ALL patients and two eligible AML patients were enrolled to the first strata at a dose of 40 mg/m2 of clofarabine. Two ALL patients with prior HSCT (22 and 5 months prior to enrollment on study) had dose-limiting toxicities (DLT’s): one with Grade 4 fungal infection and pneumonitis and another with Grade 4 fungal infection. Neither patient received antifungal prophylaxis. As two or fewer patients exhibited a DLT at 40 mg/m2 of clofarabine, 10 additional patients (three ALL and seven AML) were enrolled at 52 mg/m2 in the second portion of the dose finding study. None of the ALL patients enrolled at 52 mg/m2 experienced a DLT, while one patient with AML experienced a DLT (bone marrow aplasia, Grade 4 hypokalemia, Grade 3 nausea, Grade 3 dehydration), therefore the recommended dose of clofarabine for the efficacy portion of AAML0523 was 52 mg/m2. The most common toxicities were anorexia, fever/neutropenia, transaminitis, and hypokalemia, consistent with prior studies of clofarabine.
TABLE II.
Non-Hematologic Adverse Events of Grade 3 and Higher for Patients With ALL and AML Treated at Clofarabine Dose of 40 mg/m2
| Toxicity site | Toxicity type | CTCAE
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL (n = 8)
|
AML (n = 2)
|
||||||||
| Induction, Cycle I
|
Induction, Cycle II
|
Induction, Cycle I
|
Induction, Cycle II
|
||||||
| N | % | N | % | N | % | N | % | ||
| Gastrointestinal | |||||||||
| Anorexia | 1 | 13% | 0 | 0% | 1 | 50% | 0 | 0% | |
| Diarrhea | 2 | 25% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Infection | |||||||||
| Febrile neutropenia (fever of unknown origin without clinically or microbiologically documented infection; ANC <1.0 × 109/L, fever ≥38.5 days) | 5 | 63% | 1 | 25% | 1 | 50% | 0 | 0% | |
| Infection (documented clinically or microbiologically) with Grade 3 or 4 neutrophils (ANC <1.0 × 109/L) lung (pneumonia) | 2 | 25% | 0 | 0% | 2 | 100% | 0 | 0% | |
| Infection (documented clinically or microbiologically) with Grade 3 or 4 neutrophils (ANC <1.0 × 109/L) blood | 2 | 25% | 1 | 25% | 1 | 50% | 0 | 0% | |
| Infection, other | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 100% | |
| Metabolic/laboratory | |||||||||
| ALT, SGPT (serum glutamic pyruvic transaminase) | 2 | 25% | 0 | 0% | 1 | 50% | 0 | 0% | |
| AST, SGOT (serum glutamic oxaloacetic transaminase) | 3 | 38% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Potassium, serum-low (hypokalemia) | 3 | 38% | 0 | 0% | 1 | 50% | 0 | 0% | |
| Pain | |||||||||
| Abdomen NOS | 3 | 38% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Head/headache | 2 | 25% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Pulmonary/upper respiratory | |||||||||
| Hypoxia | 2 | 25% | 1 | 25% | 0 | 0% | 0 | 0% | |
| Pneumonitis/pulmonary infiltrates | 2 | 25% | 0 | 0% | 1 | 50% | 0 | 0% | |
| Total patients | 8 | 4 | 2 | 1 | |||||
| Patients with toxicity | 7 | 88% | 2 | 50% | 2 | 100% | 1 | 100% | |
| Patients without toxicity | 1 | 13% | 2 | 50% | 0 | 0% | 0 | 0% | |
CTCAE = Common Terminology Criteria for Adverse Events (version 3.0). Listed are toxicities occurring in more than one patient, and are listed regardless of relationship to study drug.
TABLE III.
Non-Hematologic Adverse Events of Grade 3 and Higher for Patients With ALL at Clofarabine Dose of 52 mg/m2 AML Patients Treated at This Dose on the Dose-Finding Portion of the Study are Also Included
| Toxicity site | Toxicity type | CTCAE
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ALL (n = 13)
|
AML (n = 7)
|
||||||||
| Induction, Cycle I
|
Induction, Cycle II
|
Induction, Cycle I
|
Induction, Cycle II
|
||||||
| N | % | N | % | N | % | N | % | ||
| Gastrointestinal | |||||||||
| Anorexia | 3 | 23% | 2 | 29% | 1 | 14% | 2 | 40% | |
| Diarrhea | 1 | 8% | 0 | 0% | 2 | 29% | 2 | 40% | |
| Nausea | 0 | 0% | 0 | 0% | 2 | 29% | 2 | 40% | |
| Infection | |||||||||
| Febrile neutropenia (fever of unknown origin without clinically or microbiologically documented infection; ANC <1.0 × 109/L, fever ≥38.5 days) | 6 | 46% | 3 | 43% | 3 | 43% | 2 | 40% | |
| Infection (documented clinically or microbiologically) with Grade 3 or 4 neutrophils (ANC <1.0 × 109/L) catheter-related | 2 | 15% | 0 | 0% | 1 | 14% | 0 | 0% | |
| Infection (documented clinically or microbiologically) with Grade 3 or 4 neutrophils (ANC <1.0 × 109/L) skin (cellulites) | 1 | 8% | 0 | 0% | 1 | 14% | 0 | 0% | |
| Infection (documented clinically or microbiologically) with Grade 3 or 4 neutrophils (ANC <1.0 × 109/L) blood | 2 | 15% | 2 | 29% | 4 | 57% | 2 | 40% | |
| Metabolic/laboratory | ALT, SGPT (serum glutamic pyruvic transaminase) | 5 | 38% | 1 | 14% | 0 | 0% | 0 | 0% |
| AST, SGOT (serum glutamic oxaloacetic transaminase) | 4 | 31% | 1 | 14% | 0 | 0% | 0 | 0% | |
| Calcium, serum-high (hypercalcemia) | 1 | 8% | 2 | 29% | 0 | 0% | 0 | 0% | |
| GGT (gamma-glutamyl transpeptidase) | 2 | 15% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Glucose, serum-high (hyperglycemia) | 1 | 8% | 2 | 29% | 2 | 29% | 1 | 20% | |
| Potassium, serum-low (hypokalemia) | 4 | 31% | 2 | 29% | 2 | 29% | 2 | 40% | |
| Pain | |||||||||
| Abdomen NOS | 2 | 15% | 0 | 0% | 1 | 14% | 1 | 20% | |
| Pulmonary/upper respiratory | |||||||||
| Dyspnea (shortness of breath) | 2 | 15% | 0 | 0% | 0 | 0% | 0 | 0% | |
| Hypoxia | 1 | 8% | 1 | 14% | 0 | 0% | 0 | 0% | |
| Total patients | 13 | 7 | 7 | 5 | |||||
| Patients with toxicity | 12 | 92% | 6 | 86% | 7 | 100% | 5 | 100% | |
| Patients without toxicity | 1 | 8% | 1 | 14% | 0 | 0% | 0 | 0% | |
CTCAE, Common Terminology Criteria for Adverse Events (version 3.0). Listed are toxicities occurring in more than one patient, and are listed regardless of relationship to study drug.
There did not appear to be a significant difference in time to recovery between Cycles 1 and 2 in either dose level. The median cycle length for patients without ANC and platelet recovery at the 40 mg/m2 level was 22 and 29 days, respectively after one course and 19 and 17.5 days, respectively after two courses. At the 52 mg/m2 dose level, the median cycle length for patients without ANC and platelet recovery was 20 and 17 days, respectively after one course and approximately 17 days after two courses. These findings reflect that patients without marrow recovery had PD, received alternate chemotherapy, or proceeded to HSCT prior to count recovery, rather than drug-induced prolonged bone marrow aplasia.
Response
Table IV shows the response of patients to protocol therapy. Of 21 patients, three achieved CR—one at a clofarabine dose of 40 mg/m2 and two at 52 mg/m2. All three patients with CR achieved this response after one course of therapy and went on to receive a second course; one died during the second course due to complications arising from capillary leak and multi-organ failure. Ten patients went off study after the first course: three for PD, three with SD, two with PRCL, and two with MRCL. Reasons for study withdrawal included: physician decision (six patients), unacceptable toxicity (two patients), and parental refusal of further protocol therapy (two patients). Based on the two-stage design definition of first-stage inactivity as four or fewer CRs in the first 13 ALL patients treated at 52 mg/m2, the therapy was deemed ineffective.
TABLE IV.
Response Summary for Patients With ALL
| AAML0523 (ALL only) n = 21
|
AAML0523 (Dose: 40 mg/m2) n = 8
|
AAML0523 (Dose: 52 mg/m2) n = 13
|
P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Course 1 response | |||||||
| CR: complete response | 3 | 14% | 1 | 13% | 2 | 15% | 1.000 |
| SD: stable disease | 6 | 29% | 2 | 25% | 4 | 38% | 0.197 |
| PD: progressive disease | 3 | 14% | 2 | 25% | 1 | 8% | 0.531 |
| PRCL: partial remission—cytolytic | 4 | 19% | 1 | 13% | 3 | 23% | 1.000 |
| MRCL: minimal response—cytolytic | 5 | 24% | 2 | 25% | 3 | 15% | 0.618 |
| Is patient off protocol therapy at end of course 1? | |||||||
| No | 11 | 52% | 4 | 50% | 7 | 54% | 1.000 |
| Yes | 10 | 48% | 4 | 50% | 6 | 46% | |
| Course 2 response | |||||||
| CR: complete response | 2 | 18% | 1 | 25% | 1 | 14% | 1.000 |
| Death (in CR) | 1 | 9% | 0 | 0% | 1 | 14% | 1.000 |
| PR: partial response | 1 | 9% | 1 | 25% | 0 | 0% | 0.381 |
| PD: progressive disease | 2 | 18% | 1 | 25% | 1 | 14% | 1.000 |
| SD: stable disease | 4 | 36% | 1 | 25% | 3 | 43% | 1.000 |
| MRCL: minimal response—cytolytic | 1 | 9% | 0 | 0% | 1 | 14% | 1.000 |
| Best response | |||||||
| CR: complete response | 3 | 14% | 1 | 13% | 2 | 15% | 1.000 |
| PR: partial response | 1 | 5% | 1 | 13% | 0 | 0% | 0.381 |
| PRCL: partial remission—cytolytic | 4 | 19% | 1 | 13% | 3 | 23% | 1.000 |
| MRCL: minimal response—cytolytic | 4 | 19% | 1 | 13% | 3 | 23% | 1.000 |
| SD: stable disease | 6 | 29% | 2 | 25% | 4 | 31% | 1.000 |
| PD: progressive disease | 3 | 14% | 2 | 25% | 1 | 8% | 0.531 |
DISCUSSION
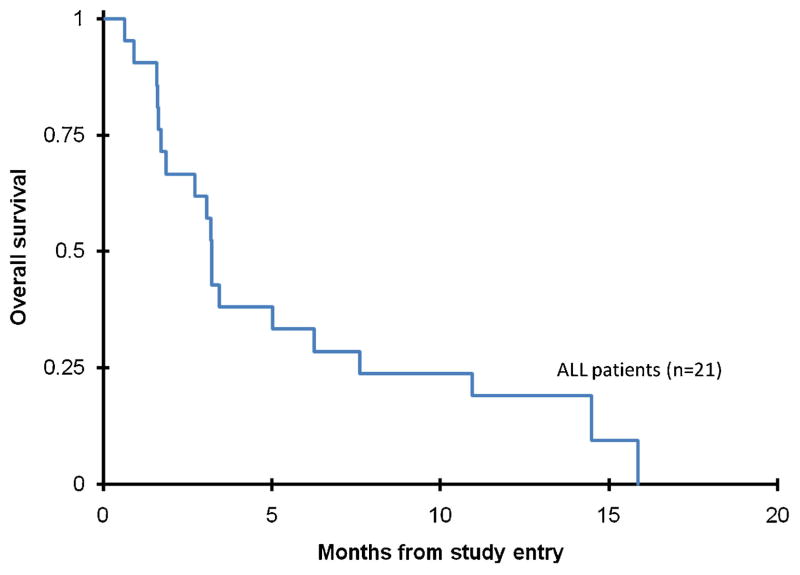
Cure following multiple relapses in children and adolescents with ALL is very difficult. With current therapies, approximately 40% of patients in second relapse achieve a third remission of which only about 15% of those achieve disease-free survival over 5 years [15]. Clearly, novel therapies are needed. In our study of clofarabine/cytarabine in children with second or greater relapse, response rates and OS were poor (Fig. 1). The CR rate in our study (14%) was not significantly better than that seen with clofarabine alone in a pediatric Phase II trial (12%) [9]. A contributing factor may be that almost half of the patients on this study received only one induction course, while the median number of courses received on the pediatric Phase II trial was two courses. Moreover, many patients in this trial proceeded to other forms of therapy before waiting for peripheral blood count recovery, thus negating formal response assessment. Finally, accumulation of ara-CTP by biochemical modulation may not be a significant factor in ALL, as compared to AML.
Fig. 1.
Overall survival from study entry for ALL patients on AAML0523.
The toxicity profile of this combination was consistent with other cytotoxic acute leukemia re-induction regimens, with febrile neutropenia, transaminitis, and hypokalemia being the most common. As described, DLT’s during the dose-finding portion of the study included fungal infection. It is recommended that patients receiving clofarabine receive anti-fungal prophylaxis, consistent with intensive AML-type chemotherapy regimens. No veno-occlusive disease (VOD) was observed, including five patients that received HSCT prior to study entry. In addition to AAML0523, clinical trials utilizing clofarabine and cytarabine in adult AML therapy also do not demonstrate an increased incidence of VOD [16]. One death on study was felt to be treatment-related; a patient achieving CR after one course developed a systemic inflammatory response during course 2, with capillary leak leading to multi-organ failure and death, despite protocol-recommended steroid pre-treatment. Similar events have been reported with clofarabine as a single agent [8,9]. Due to this acceptable toxicity profile, five of seven patients who had some measurable response to therapy went on to receive HSCT. A total of six patients went on to receive HSCT after protocol therapy. Of these six patients, two achieved CR on study, and three achieved CR with further therapy. One patient received HSCT without achieving CR.
Hijiya et al. recently reported the activity of clofarabine (40 mg/m2), cyclophosphamide (440 mg/m2), and etoposide (100 mg/m2) IV daily for 5 days in a Phase II study for children with second or greater relapse of acute leukemia. The trial demonstrated an overall response rate (ORR defined as CR + CRp) in children with ALL of 44%. The regimen was a successful bridge to HSCT in 40% of patients. However, this trial also demonstrated significant toxicity. Among the first eight patients enrolled, four experienced severe hepatotoxicity in the setting of infection/sepsis and/or capillary leak syndrome. Three patients, including two with prior HSCT, were diagnosed with VOD and died of multi-organ failure, leading to both an amendment to exclude patients with prior HSCT in that study and an AAML0523 amendment excluding patients with prior HSCT within 1 year [17]. Locatelli et al. [18] reported a 56% ORR with a single course of clofarabine (40 mg/m2) combined with lower daily doses of cyclophosphamide (400 mg/m2), and higher daily doses of etoposide (150 mg/m2). In that study, no treatment-related deaths or cases of VOD were reported, although reversible liver toxicity did occur. The Children’s Oncology Group is evaluating clofarabine/cyclophosphamide/etoposide in an arm of AALL1131 in children with de novo very high risk ALL.
Based on the results of this clinical trial, further study of this combination and schedule of clofarabine and cytarabine is not warranted in children with relapsed ALL.
Acknowledgments
Grant sponsor: National Institute of Health; Grant number: NIH U10 CA98543; Grant sponsor: Children’s Oncology Group Chair’s; Grant number: SDC U10 CA98413.
The work was supported by National Institute of Health Grants: Children’s Oncology Group Chair’s grant NIH U10 CA98543 and SDC U10 CA98413. Clofarabine was supplied by Genzyme Oncology/Sanofi.
Footnotes
Conflict of interest: Nothing to declare.
References
- 1.Pui CH. Childhood leukemias. N Engl J Med. 1995;332:1618–1630. doi: 10.1056/NEJM199506153322407. [DOI] [PubMed] [Google Scholar]
- 2.Kersey JH. Fifty years of studies of the biology and therapy of childhood leukemia. Blood. 1997;90:4243–4251. [PubMed] [Google Scholar]
- 3.Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J Clin Oncol. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery JA, Shortnacy-Fowler AT, Clayton SD, et al. Synthesis and biologic activity of 2′-fluoro-2-halo derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- 5.Mansson E, Flordal E, Liliemark J, et al. Down-regulation of deoxycytidine kinase in human leukemic cell lines resistant to cladribine and clofarabine and increased ribonucleotide reductase activity contributes to fludarabine resistance. Biochem Pharmacol. 2003;65:237–247. doi: 10.1016/s0006-2952(02)01484-3. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T, Nowak BJ, Keating MJ, et al. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroperoxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin Cancer Res. 2001;7:3580–3589. [PubMed] [Google Scholar]
- 7.Parker WB, Shaddix SC, Rose LM, et al. Comparison of the mechanism of cytotoxicity of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine, 2-chloro-9-(2-deoxy-2-fluoro-beta-D-ribofuranosyl )adenine, and 2-chloro-9-(2-deoxy-2,2-difluoro-beta-D-ribofuranosyl)adenine in CEM cells. Mol Phar-macol. 1999;55:515–520. [PubMed] [Google Scholar]
- 8.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 9.Jeha S, Gaynon PS, Razzouk BI, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24:1917–1923. doi: 10.1200/JCO.2005.03.8554. [DOI] [PubMed] [Google Scholar]
- 10.Cooper T, Ayres M, Nowak B, et al. Biochemical modulation of cytarabine triphosphate by clofar-abine. Cancer Chemother Pharmacol. 2005;55:361–368. doi: 10.1007/s00280-004-0906-y. [DOI] [PubMed] [Google Scholar]
- 11.Faderl S, Gandhi V, O’Brien S, et al. Results of a phase 1–2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein ML, Abshire TC, Pollock BH, et al. Idarubicin and cytosine arabinoside reinduction therapy for children with multiple recurrent or refractory acute lymphoblastic leukemia: A pediatric oncology group study. J Pediatr Hematol Oncol. 1997;19:68–72. doi: 10.1097/00043426-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Crooks GM, Sato JK. Ifosfamide and etoposide in recurrent childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1995;17:34–38. doi: 10.1097/00043426-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kolb EA, Steinherz PG. A new multidrug reinduction protocol with topotecan, vinorelbine, thiotepa, dexamethasone, and gemcitabine for relapsed or refractory acute leukemia. Leukemia. 2003;17:1967–1972. doi: 10.1038/sj.leu.2403097. [DOI] [PubMed] [Google Scholar]
- 15.Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lympho-blastic leukemia: A therapeutic advances in childhood leukemia consortium study. J Clin Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: Results from the CLASSIC I trial. J Clin Oncol. 2012;30:2492–2499. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hijiya N, Thomson B, Isakoff MS, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118:6043–6049. doi: 10.1182/blood-2011-08-374710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locatelli F, Testi AM, Bernardo ME, et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol. 2009;147:371–378. doi: 10.1111/j.1365-2141.2009.07882.x. [DOI] [PubMed] [Google Scholar]